

14 октября исполнилось 60 лет директору Федерального исследовательского центра «Институт катализа им. Г.К. Борескова СО РАН» Валерию Ивановичу Бухтиярову – действительному члену Российской академии наук, доктору химических наук, профессору, специалисту в области физико-химии поверхности, гетерогенного катализа и функциональных наноматериалов, автору и соавтору более 300 научных работ и 22 патентов на изобретения.
Родился 14 октября 1961 года в Новосибирске. В 1983 году окончил с отличием Новосибирский государственный университет по специальности «химик».
Вся трудовая деятельность Валерия Ивановича связана с Институтом катализа, в который он поступил в 1983 году стажером-исследователем, через два года стал аспирантом, еще через два – младшим научным сотрудником, защитил кандидатскую диссертацию, работал научным, а затем старшим научным сотрудником. С 1995 по 2000 год В.И. Бухтияров был ученым секретарем Института.
В 1999 году защитил докторскую диссертацию по теме «От монокристаллов к наночастицам: молекулярный подход к изучению причин каталитического действия серебра в реакции эпоксидирования этилена».
Руководил Отделом гетерогенного катализа и возглавлял лабораторию исследования поверхности ИК СО РАН. С 2002 по 2015 год Валерий Иванович занимал должность заместителя директора Института по научной работе.
В 2003 году утверждён в звании профессора, в 2008 году избран членом-корреспондентом РАН, с 2013 по 2015 был главным ученым секретарем Сибирского отделения РАН.
С 2015 года Валерий Иванович занимает должность директора Института катализа СО РАН.
В октябре 2016 года избран действительным членом Российской академии наук. В 2020 году избран членом Европейской академии (Academia Europaea).
Основные области научных интересов В.И. Бухтиярова – изучение элементарных химических процессов на поверхности твердых тел, в том числе с использованием современных физических методов in situ, установление взаимосвязи «структура – активность» в гетерогенных катализаторах, разработка способов управляемого синтеза функциональных наноматериалов для каталитических приложений. Цель исследований – определение структуры активных центров катализаторов, их трансформации в условиях протекания каталитической реакции и установление взаимосвязи между строением и составом поверхности гетерогенного катализатора и его каталитическими свойствами в промышленно важных реакциях.
Возглавляемый В.И. Бухтияровым ФИЦ Институт катализа СО РАН входит в категорию ведущих научных организаций России. По итогам рейтингования в 2020 году Центр находится на втором месте среди химических институтов по комплексному баллу публикационной активности. ИК СО РАН активнейшим образом вовлечен в решение актуальных прикладных задач в различных областях промышленности.
По инициативе и под руководством В.И. Бухтиярова начато строительство «Сибирского кольцевого источника фотонов» (ЦКП «СКИФ») в наукограде Кольцово, который планируется запустить в конце 2023 года. На исследовательских станциях СКИФ будут проводиться самые передовые исследования в различных областях науки с использованием синхротронного излучения.
Валерий Иванович Бухтияров уделяет большое внимание международному сотрудничеству. Под его руководством с участием ученых из отечественных и зарубежных академических центров выполнены и выполняются проекты, финансируемые РНФ, РФФИ, CRDF, NWO, DFG. В.И. Бухтияров избирался членом Экспертного совета Берлинского источника синхротронного излучения (BESSY), являлся вице-президентом и членом Совета EFCATS (European Federation of Catalytic Societies). В.И. Бухтияров – действующий представитель от России в Совете Международного конгресса по окислительному катализу (WCOC International Board). Он координирует российскую часть проекта «Развитие методов исследования in situ твердых поверхностей», выполняемого совместно с Институтом Фрица Габера (Общество Макса Планка, Берлин).
В.И. Бухтияров активно занимается подготовкой научных кадров. С 2000 года руководит кафедрой катализа и адсорбции факультета естественных наук НГУ. Под его руководством успешно защищены 11 кандидатских диссертаций.
Валерий Иванович Бухтияров – заместитель председателя Объединенного ученого совета СО РАН по химическим наукам, член диссертационных советов 24.1.222.01 (Д 003.012.01) при Институте катализа и 24.1.086.01 (Д 003.051.01) при Институте неорганической химии СО РАН. Он также является главным редактором журнала «Кинетика и катализ» и членом редакционного совета «Журнала структурной химии».
Награды: Премия М.А. Лаврентьева для молодых ученых в номинации «За выдающийся вклад в развитие Сибири и Дальнего Востока» (2003 г.); Почетное звание «Заслуженный ветеран СО РАН» (2004 г.); Почетная грамота РАН и Профсоюза работников РАН (2007 г.); Почетный знак СО РАН «Серебряная Сигма» (2007 г.); Памятная медаль «За вклад в развитие Новосибирской области» (2012 г.); Благодарность Губернатора Новосибирской области (2014 г.); Юбилейная медаль «80 лет Новосибирской области» (2016 г.); Премия А.А. Баландина за выдающиеся работы в области катализа за серию работ (2016 г.); Почетная грамота ФАНО России (2017 г.); Почетное звание «Заслуженный деятель науки Новосибирской области» (2018 г.); Премия Правительства Российской Федерации в области науки и техники (2019 г.).
Научный совет по катализу ОХНМ РАН и редакция Каталитического бюллетеня сердечно поздравляют Валерия Ивановича с юбилеем, желают ему новых творческих свершений и плодотворной работы на благо российской науки!
Научный руководитель ФИЦ угля и углехимии СО РАН (ФИЦ УУХ СО РАН), главный научный сотрудник ФИЦ Институт катализа им. Г.К. Борескова СО РАН (ФИЦ ИК СО РАН), академик РАН Зинфер Ришатович Исмагилов стал лауреатом международной премии в области энергетики «Глобальная энергия». Она присуждается за выдающиеся научные исследования и научно-технические разработки, которые способствуют повышению эффективности и экологической безопасности источников энергии на Земле в интересах всего человечества.
Зинферу Ришатовичу Исмагилову присуждена премия в номинации «Традиционная энергетика» за фундаментальный вклад в химию углеродных материалов, гетерогенный катализ и борьбу с изменением климата. В июне 2021 года его совместный с академиком РАН Валентином Николаевичем Пармоном проект «Каталитические методы переработки углекислого газа угольной генерации в полезные продукты» был признан одной из десяти прорывных идей в области энергетики по версии ассоциации «Глобальная энергия».
Как отметил Зинфер Ришатович, «Основные достижения, лежащие в основе конкурсной документации, которую подавали на премию «Глобальная энергия», были выполнены за последние пятьдесят лет в наших коллективах и были суммированы в 31 кандидатскую и семь докторских диссертаций. В конкурсную заявку в нашей номинации также вошли многие материалы, разработанные в наших международных проектах».
Ученые ФИЦ УУХ и ФИЦ ИК СО РАН под руководством З.Р. Исмагилова развили технологию низкотемпературного экологически чистого сжигания топлив в кипящем слое катализатора, разработали технологию очистки попутных нефтяных газов от сероводорода, освоили технологию создания сотовых блочных катализаторов, разработали различные варианты получения водорода, вели исследования в области синтеза углеродных наноматериалов, развили технологию утилизации отходов атомной промышленности, работали над решением экологических проблем угледобывающих и перерабатывающих регионов и т.д.
По словам Зинфера Ришатовича, он оценивает присуждение ему премии «Глобальная энергия» как величайшее достижение для любого ученого.
«Известно, что в число номинаций «Нобелевской премии» не входит энергетика, поэтому некоторые коллеги иронично называют премию «Глобальная энергия» – «энергетическим Нобелем», — рассказывает ученый. – Ее присуждение говорит о мировом признании наших научных достижений в области энергетики. Это очень большая честь не только для меня, но и для всей нашей исследовательской команды, которая работает как в новосибирском Институте катализа СО РАН, где были наработаны многие результаты исследований, так и в последнее десятилетие в Институте углехимии и химического материаловедения ФИЦ УУХ СО РАН в Кемерово. Оказалось, мое имя как победителя было обнародовано еще 3 сентября на пленарной сессии Восточного экономического форума с участием президентов России, Казахстана, Монголии, председателя КНР и премьер-министров Индии и Таиланда. На один из вопросов об энергетике от нашего президента, Владимира Владимировича Путина, модератор пленарного заседания, президент ассоциации «Глобальная энергия» Сергей Брилев, рассказал, что есть в стране ученые, занимающиеся получением водорода из угля и переработки углекислого газа каталитическими методами, и назвал мою фамилию. Потом 6 сентября на выездной сессии Ассоциации «Глобальная Энергия» в Казани состоялось торжественное объявление всех трех лауреатов по трем номинациям».
Вместе с З.Р. Исмагиловым премию получили заведующий лабораторией Института физиологии растений РАН Сулейман Аллахвердиев и директор Института энергетики Precourt (США) И Цуй.

«Остановив свой выбор на этих трех кандидатах, «Глобальная энергия», уверен, дала четкий сигнал, что научные и технологические новации как в традиционной, так и нетрадиционной энергетике могут привести к чистой энергетике будущего» – отметил председатель Международного комитета премии, нобелевский лауреат Рае Квон Чунг, передает ТАСС.
Это уже вторая премия «Глобальная энергия», которая была присуждена сотрудникам Института катализа СО РАН: в 2016 году лауреатом премии стал научный руководитель Института катализа СО РАН, академик РАН Валентин Николаевич Пармон за прорывную разработку новых катализаторов в области нефтепереработки и возобновляемых источников энергии, внесших вклад в развитие энергетики будущего.
Справка: Ассоциация «Глобальная энергия» – неправительственная организация, созданная в 2002 году для поддержки исследований и инноваций в области энергетики, а также содействия развитию энергетического сотрудничества. Членами Ассоциации являются ПАО «Газпром», ПАО «Сургутнефтегаз», «Россети ФСК ЕЭС». Ассоциация помогает формировать энергетику будущего, поддерживая передовые научно-технические разработки и стимулируя международное энергетическое сотрудничество в интересах всего человечества.

VI Семинар памяти профессора Ю.И. Ермакова
«Гомогенные и закрепленные металлокомплексные катализаторы
для процессов полимеризации и нефтехимии»
28 июня – 2 июля 2021 г.,
пос. Листвянка Иркутской обл., Россия
http://conf.ict.nsc.ru/ermak-VI/ru
VI Семинар памяти профессора Ю.И. Ермакова «Гомогенные и закрепленные металлокомплексные катализаторы для процессов полимеризации и нефтехимии», приуроченный к 85-летию со дня его рождения, состоялся с 28 июня по 2 июля 2021 года в поселке Листвянка Иркутской области. В рамках Семинара прошла Школа для молодых учёных и специалистов производственных компаний «Каталитическая полимеризация олефинов». Основу научных программ пяти предыдущих Семинаров (прошедших ранее, в 1995 и 2000 гг. в Новосибирске, в 2005 г. в Омске, в 2010 г. в пос. Листвянка и в 2015 г. в Республике Алтай) составляли работы учеников и последователей научной школы профессора, д.х.н. Ю.И. Ермакова, а также выступления молодых ученых.
Организаторами VI Семинара выступили ученики и последователи научной школы профессора Юрия Ивановича Ермакова, который в 70-е годы прошлого века внес крупный вклад в формирование и становление нового направления в синтезе активных центров гетерогенных катализаторов, связанного с применением принципов гомогенного катализа металлокомплексами, использования методологии целенаправленной сборки каталитически активных центров из металло-органических фрагментов и контроля такой сборки физико-химическими методами. Введённый впервые в работах Ю.И. Ермакова и его школы термин «точный активный центр» к настоящему времени трансформировался в определение «one-site catalysts», подход к его синтезу известен как «tailor-made catalysts», а методологические подходы для повышения информативности физических методов при исследовании таких «точных» систем внесли вклад в развитие и современные возможности науки о поверхности (surface science). В настоящее время с использованием такого типа «точных» катализаторов работают мощные установки риформинга на Рязанском, Куйбышевском, Новоуфимском нефтеперерабатывающих заводах.
Под руководством профессора Ю.И. Ермакова была разработана технология приготовления высокоэффективного катализатора полимеризации пропилена, который успешно использовался в течение многих лет на Томском нефтехимическом комбинате. В настоящее время в Институте катализа разработана новая технология приготовления современных нанесенных титанмагниевых катализаторов для производства полиэтилена и полипропилена.

Открытие Семинара, конф.-зал отеля Маяк
На церемонии открытия Семинара с приветственным словом выступили заместитель Президента РАН, член.-корр. РАН С.В. Люлин и руководители учреждений со-организаторов Семинара: В.И. Бухтияров, академик РАН, директор ФИЦ Института катализа им. Г.К. Борескова СО РАН; А.Б. Купчинский, к.б.н., директор Байкальского музея ИНЦ СО РАН; А.Ф. Шмидт, д.х.н., ректор Иркутского государственного университета. Во время церемонии открытия состоялась презентация изданной в Институте катализа книги «Воспоминания о Юрии Ивановиче Ермакове», приуроченной к 85-летию со дня его рождения и вышедшей в свет к началу работы Семинара.

Приветствие зам. Президента РАН, чл.-корр. РАН С.В. Люлина.
В работе Семинара и Школы приняли участие 96 специалистов из 42 организаций, расположенных в 12 городах России. В течение четырех рабочих дней участники прослушали и обсудили 4 пленарные лекции (45 мин), 14 ключевых лекций (30 мин) Семинара, 5 ключевых лекций (45 мин) Школы и 48 устных (15 и 10 мин) докладов, включая два доклада спонсоров.
Ключевые лекции и устные доклады были представлены на двух тематических секциях:

Пленарная сессия, конференц-зал отеля Маяк.
Пленарные лекции были заслушаны на совместных заседаниях обеих секций.
В пленарной лекции академика РАН В.И. Бухтиярова «Катализаторы и процессы получения базовых мономеров С2-С4: сравнение маршрутов дегидрирования и окислительного дегидрирования» (ФИЦ Институт катализа СО РАН, г. Новосибирск) были рассмотрены новые катализаторы и процессы, разрабатываемые в Институте катализа СО РАН для получения С2-С4 олефинов с использованием реакций (неокислительного) дегидрирования и окислительного дегидрирования этана, пропана и бутена, и представлен сравнительный анализ результатов таких исследований с точки зрения выбора катализатора, технологического оформления процесса, обеспечивающего оптимальные показатели в получении этилена, пропилена, бутадиена для последующей практической реализации. Показано, что природа исходного углеводорода в значительной степени определяет выбор в пользу дегидрирования, окислительного дегидрирования или их совместного использования, на основании чего были обсуждены перспективные подходы к реализации каталитических процессов превращения этилена в пропилен, и получения бутенов.
Академик РАН В.П. Анаников (Институт органической химии РАН, г. Москва) в своей пленарной лекции «Динамические явления в катализе: взаимосвязь между гомогенными и закрепленными металлокомплексными катализаторами», сделанной в он-лайн режиме, отметил важность учёта динамической взаимосвязи между гомогенными и закреплёнными металлокомплексными катализаторами для понимания механизма их функционирования. Обсуждены вопросы интеграции передовых методов контроля типа каталитических систем и визуализации процессов с участием наноразмерных систем, а важность такого подхода продемонстрирована на примере сравнения катализаторов на основе никеля и палладия.
В пленарной лекции чл.-корр. РАН А.Л. Максимова «Гомогенные и закрепленные металлокомплексные катализаторы и каталитические композиции, образующиеся из нанесенных комплексов металлов, для процессов нефтехимии» (Институт нефтехимического синтеза РАН, г. Москва) рассмотрены и проанализированы подходы для создания каталитических композиций, которые бы обладали высокой регио- и субстратной селективностью за счет супрамолекулярных взаимодействий с субстратами. «Настройка» такого взаимодействия позволяет конструировать новые каталитические системы на основе комплексов и наночастиц переходных металлов, что представлено на примерах применения металл-содержащих композиций, полученных с использованием дендримерных «сеток», циклодекстринов, полиароматических пористых каркасов и других типов пористых полимеров для реакций селективного гидрирования, гидроформилирования, карбонилирования, окислительного обессеривания и др. Обсуждены возможности модификации такого рода материалов функциональными группами, различные варианты иммобилизации металлокомплексов и наночастиц металлов с применением таких материалов, достоинства и недостатки получаемых катализаторов и возможности их использования для процессов нефтехимии.
Д.х.н., проф. В.А. Захаров в своей пленарной лекции «Развитие представлений о процессах формирования, составе и функционировании активных центров нанесенных титан-магниевых катализаторов полимеризации олефинов» (ФИЦ Институт катализа СО РАН, г. Новосибирск) представил результаты исследований, выполненных в последние годы в лаборатории каталитической полимеризации Института катализа СО РАН по ряду направлений, касающихся процессов формирования и состава активных центров нанесенных титанмагниевых катализаторов для полимеризации олефинов, выявления роли алюминийорганического сокатализатора в обратимых процессах превращений активных центров в условиях полимеризации и установления связи между составом полицентровых титан-магниевых катализаторов и распределением активных центров по их стереоспецифичности.
Секция 1. «Гомогенные и закрепленные металлокомплексные катализаторы и каталитические композиции, образующиеся из нанесенных комплексов металлов, для процессов нефтехимии»
В ходе работы секции были заслушаны и обсуждены ключевые лекции: д.х.н. А.В. Лавренова (Центр новых химических технологий ИК СО РАН, г. Омск) о полифункциональном катализе в превращениях легких алкенов, в которой на примере реакций превращения этилена (олигомеризация, алкилирование, метатезис и т.п.) продемонстрирована перспективность применения полифункциональных композиций, сочетающих в себе мягкие льюисовские кислотные (ионы переходных металлов) или мягкие льюисовские основные (наночастицы металлов) центры с жёсткими бренстедовскими кислотными (кислотных неорганических носители: оксиды алюминия, циркония, цеолиты, аморфные алюмосиликаты) центрами; д.х.н. Б.Н. Кузнецова (Институт химии и химической технологии СО РАН, г. Красноярск) о металлокомплексном катализе в процессах получения ценных химических веществ из растительной биомассы, в которой были рассмотрены примеры использования растворенных металлокомплексных катализаторов в процессах окисления лигноцеллюлозной биомассы с получением целлюлозы и ароматических альдегидов, теломеризации лигнинов бутадиеном, а также использования закрепленных на оксидных подложках металлокомплексов и карбонильных кластеров переходных металлов в процессах гидрирования СО до легких олефинов и метатезиса олефинов; д.х.н. А.Ф. Шмидта (Иркутский государственный университет, г. Иркутск) о методах определения гомогенно-гетерогенных, скорость-определяющих, обратимых и квазиравновесных стадий в каталитических реакциях с демонстрацией действенности этих методов на примере реакций сочетания арилгалогенидов, катализируемых палладием, где были получены уникальные экспериментальные свидетельства, указывающие на протекание «классических» реакций кросс-сочетания Мицороки-Хека и Сузуки-Мияуры по нелинейному механизму так называемого «кооперативного» катализа с участием в образовании продуктов реакции двух различных палладийсодержащих интермедиатов; д.х.н. Е.С. Локтевой (Московский государственный университет, Химический факультет, г. Москва) о разработке и результатах исследований биметаллических катализаторов для восстановительной утилизации хлорированных микроэкотоксикантов в водных растворах, где на примере железо-палладиевых композиций, нанесённых на цирконо-кремнийоксидные и алюмооксидные носители продемонстрирована их высокая эффективность в реакции гидродехлорирования 4-хлорфенола и натриевой соли диклофенака.
Серия устных докладов касалась актуальных вопросов: синтеза нанесённых катализаторов с использованием сверхкритических сред (д.х.н. О.О. Паренаго с соавт.); получения и свойств гомогенных, гетерогенных и закреплённых гетерополикислотных катализаторов для процессов переработки целлюлозы (д.х.н. О.П. Таран с соавт.); механизма формирования (закрепления и трансформации) хлоридных комплексов палладия в структуре слоистых двойных алюмо-магниевых гидроксидов (к.х.н. О.Б. Бельская с соавт.); процессов теломеризации бутадиена с метанолом, катализируемых ацетилацетонатными комплексами палладия (к.х.н. М.В. Быков с соавт.); синтеза и свойств металлокомплексных катализаторов окисления высших тиолов кислородом в углеводородных средах; свойств медь-содержащих церий-кремниевых и цирконий-кремниевых оксидных катализаторов селективного окисления окиси углерода в избытке водорода (к.х.н. И.Ю. Каплин с соавт.); активации арилхлоридов в условиях «безлигандного» катализа палладием реакций кросс-сочетания (к.х.н. А.А. Курохтина с соавт.); свойств иммобилизованных ионных жидкостей в окислении пероксидом водорода серусодержащих органических соединений (д.х.н. И.Г. Тарханова с соавт.); синтеза каталитических композиций на основе наночастиц платины и модифицированного кобальтом цеолита для процессов окисления окиси углерода и метана (д.х.н. Т.Н. Ростовщикова с соавт.); модифицирования платины в составе катализаторов на основе магний-алюминиевых слоистых гидроксидов (к.х.н. Л.Н. Степанова с соавт.); синтеза катализаторов для превращения этилена в пропилен (к.х.н. Т.Р. Карпова с соавт.); улучшения физико-механических свойств резин, полученных с применением механически активированного технического углерода (к.х.н. О.А. Княжева с соавт.); строения нанесённых платиновых катализаторов дегидрирования пропана (к.х.н. Н.Н. Леонтьева с соавт.); синтеза и свойств оксокатионных комплексов кобальта и церия в каталитическом окислении окиси углерода на цеолитах (д.х.н. М.И. Шилина с соавт.); определения дифференциальной селективности в реакциях с участием арилиодида и диарилацетилена в условиях «безлигандного» катализа соединениями палладия (к.х.н. Е.В. Ларина с соавт.); синтеза специальных марок силикагеля как сорбента и носителя катализаторов нефтехимии и полимеризации (к.х.н. Г.В. Мамонтов с соавт.); природы промотирующего действия фосфора на свойства палладиевых катализаторов получения пероксида водорода антрахиноновым способом (к.х.н. Т.П. Стеренчук с соавт.); механизма каталитической дегидратации этанола в этилен на анион-модифицированном оксиде алюминия (В.А. Ковеза с соавт.); природы кислородных центров на поверхности оксида лантана в окислительной конденсации метана (к.х.н. Е.И. Вовк с соавт.).
Секция 2. «Каталитическая полимеризация олефинов с использованием гомогенных и нанесенных металлоценовых и постметаллоценовых катализаторов и нанесенных катализаторов циглеровского типа»
В ходе работы секции 2 были заслушаны и обсуждены 11 ключевых лекций (30 мин), 24 устных доклада (15 мин) и 5 устных сообщений (10 мин). Представленные ключевые лекции и доклады включают результаты по различным важнейшим направлениям исследований в области каталитической полимеризации олефинов, в частности:
В ключевых лекциях д.х.н. Т.Б. Микенас (Институт катализа СО РАН) и к.х.н. С.А. Сергеева (Институт катализа СО РАН) представлены данные о процессах формирования высокоэффективных нанесенных титанмагниевых катализаторов, полученных с использованием в качестве исходного компонента оригинальных магнийорганических соединений, данные о химических процессах, протекающих на отдельных стадиях формирования этих катализаторов, составе и структуре образующихся соединений и возможностях регулирования каталитических свойств получаемых катализаторов и морфологии образующихся частиц полимеров для процессов полимеризации этилена и сополимеризации этилена с α-олефинами (лекция Т.Б. Микенас) и процессов стереоспецифической полимеризации пропилена (лекция С.А. Сергеева).
В ключевых лекциях д.х.н. И.Э. Нифантьева (Институт нефтехимического синтеза РАН) и д.х.н. А.З. Воскобойникова (Московский государственный университет) представлены данные о результатах, полученных при разработке новых методов синтеза и новых структур гомогенных цирконоценовых комплексов в качестве катализаторов полимеризации олефинов. В частности, в лекции И.Э. Нифантьева представлены результаты, полученные при синтезе гомогенных цирконоценовых комплексов, содержащих донорные группы в циклопентадиенильном лиганде, и информация о свойствах этих комплексов в полимеризации пропилена. В лекции А.З. Воскобойникова представлены данные, полученные при реализации нового подхода к рациональному дизайну заданных структур анса-цирконоценовых комплексов в качестве катализаторов с повышенной стереоспецифичностью при полимеризации пропилена.
В четырех ключевых лекциях представлены результаты, полученные при разработке и исследовании гомогенных и нанесенных постметаллоценовых катализаторов различного состава. В лекции к.х.н. Д.В. Уборского (Московский государственный университет) представлены данные о разработке новой группы постметаллоценовых комплексов циркония и гафния, содержащих структурно-жесткие бис(фенолатные) лиганды. Эти комплексы обладают высокой стереоспецифичностью при полимеризации пропилена; в частности, катализатор на основе комплекса гафния позволяет получать полипропилен с высокой изотактичностью (Tm = 100°C) при температурах полимеризации 70 – 100°C. В лекции д.х.н. Е.П. Талзи (Институт катализа СО РАН) представлены данные спектральных исследований каталитических систем на основе бис(иминовых) комплексов Ni(II). Определено строение комплекса Ni(II), образующегося при взаимодействии с активаторами МАО и ММАО. Установлено образование комплексов Ni(I) при низких температурах с последующим их превращением в комплексы Ni(II) при повышении температуры и появлении активности в полимеризации этилена. В ключевой лекции д.х.н. Ю.Ю. Титовой (Иркутский институт химии СО РАН и Иркутский государственный университет) представлены результаты исследования формирования и функционирования каталитической системы на основе диацетата Ni(II) с алюминийорганическим сокатализатором в процессе олигомеризации олефинов. На основе полученных результатов предполагается, что в процессе формирования каталитической системы образуются никельсодержащие наночастицы, на которых закреплены активные в олигомеризации комплексы Ni(II).
В ключевой лекции к.х.н. М.А. Мацько (Институт катализа СО РАН) представлены данные о получении разветвленного полиэтилена (РПЭ) на нанесенных катализаторах, содержащих комплексы Ni(II) с бис(иминовыми) лигандами на носителе SiO2(Al). Показано, что на этих катализаторах образуется РПЭ (17 – 18 CH3/1000 C) при гомополимеризации этилена (в отсутствие сомономера), по своим свойствам близкого к промышленным образцам ЛПЭНП, получаемым путем сополимеризации этилена с α-олефинами.
В ключевой лекции д.х.н. Л.Н. Новокшоновой (Институт химической физики РАН) представлены результаты о возможности модификации реологических свойств сверхвысокомолекулярного полиэтилена (СВМПЭ) путем введения в СВМПЭ низкомолекулярной полиэтиленовой компоненты при двухстадийной полимеризации этилена, а также возможности получения композиционных материалов на основе СВМПЭ путем введения в СВМПЭ различных микро- и нанонаполнителей в процессе полимеризации этилена in situ на катализаторе, закрепленном на поверхности наполнителя.
Различные аспекты каталитической полимеризации олефинов на катализаторах различного состава отражены также в устных докладах и сообщениях, которые можно классифицировать по тем же направлениям, что и ключевые лекции:
В программу Семинара впервые была включена
Важной особенностью Семинара явилось участие в его работе представителей крупнейших российских компаний – производителей полиолефинов (ООО «СИБУР», ООО «Нижнекамскнефтехим», ООО «Газпромнефть Салават», ООО «Казаньоргсинтез»). От этих компаний было представлено 9 устных докладов по результатам освоения ряда каталитических систем, закупаемых по импорту, ряду совместных работ, выполненных с российскими научно-исследовательскими организациями, а также общей информацией о перспективах развития этих производств и организации научно-технологической базы по развитию технологий и новых каталитических систем.
В рамках Семинара состоялся Круглый стол «Взаимодействие науки и бизнеса в России», кураторами которого выступили ПАО «СИБУР Холдинг» и Институт катализа СО РАН. Темами Круглого стола являлись следующие вопросы и задачи:

Круглый стол «Взаимодействие науки и бизнеса в России»,
конф.-зал Байкальского музея ИНЦ СО РАН
В заседании Круглого стола приняли участие представители академической и вузовской науки, крупного бизнеса (ПАО «Сибур») и руководства Российской академии наук. С сообщениями за Круглым столом выступили: член-корр. РАН С.В. Люлин (заместитель Президента РАН), академик РАН В.И. Бухтияров (директор ИК СО РАН); чл.-корр. РАН А.Л. Максимов (директор ИНХС); чл.-корр. РАН В.А. Лихолобов (ИК СО РАН), д.х.н., профессор А.Ф. Шмидт (ректор ИрГУ); д.т.н., профессор А.С. Носков (зам. директора ИК СО РАН); Ив Рамжуа (ООО СИБУР), В.Д. Альперн (ООО СИБУР), А.С. Алябьев (ООО «Газпромнефтехим Салават»).
В ходе заседания было отмечено сокращение механизмов участия государства в интеграции научных исследований с потребностями бизнеса. Так, деятельность Федеральной целевой программы «Исследования и разработки по приоритетным направлениям развития научно-технологического комплекса России» завершилась, а предложенный механизм в виде комплексных научно-технических программ (проектов) так и не стартовал. Высказано мнение, что на данном этапе инновационного развития технологических направлений роль государства в структуре взаимодействий «образование – наука – производство (бизнес) – государство (власть)» приобретает особую актуальность. На примере технологического направления, связанного с производством полимеров и композиционных материалов на их основе, со стороны крупного бизнеса было предложено разработать гражданско-правовую форму и механизм функционирования Российского института полимеров, который бы объединил возможности бизнеса и различные исследовательские подразделения академических институтов и университетов.
В качестве совершенствования взаимодействия науки и бизнеса было предложено расширить масштабы проводимых конференций путем привлечения к участию в них представителей зарубежных компаний и международного круга ученых.
Во время работы Семинара был проведен Конкурс устных докладов молодых ученых. По результатам конкурса были награждены Дипломами за наиболее содержательные работы и хорошее представление материала: Костомарова Оксана Дмитриевна (ООО «НИОСТ», г. Томск), Антонов Артём Артёмович (ИК СО РАН, г. Новосибирск), Степанова Людмила Николаевна (ЦНХТ ИК СО РАН, г. Омск), Каплин Игорь Юрьевич (МГУ, Химический факультет, г. Москва).

В.А. Захаров вручает Диплом победителю конкурса молодых ученых
Костомаровой Оксане Дмитриевне (ООО «НИОСТ», г. Томск),
конференц-зал Байкальского музея ИНЦ СО РАН
Основные итоги проведённого Семинара
В области синтеза новых катализаторов были продемонстрированы интересные и важные результаты по дизайну нанокомпозитных и наноструктурированных катализаторов, в том числе на основе закреплённых комплексов и наночастиц металлов на неорганических оксидных и «неоксидных», углеродных и полимерных носителях, обладающих высокой эффективностью и стабильностью в широком круге промышленно важных реакций, включая реакции трансформации как традиционных углеводородов, так и биомассы в ценные химические продукты (что можно отнести к отличию прошедшего Семинара от более ранних, IV-го и V-го). Наметилось повышение интереса исследователей к использованию в синтезе катализаторов сверхкритических флюидов, ионных жидкостей, а также к изучению роли динамических равновесий «комплекс металла в растворе/кластер металла в растворе/наночастица металла на поверхности носителя» в функционировании каталитических композиций; в последнем случае можно отметить, что успех многих работ, доложенных на Семинаре, был связан с применением современных физических методов и соответствующих расчётно-теоретических подходов для исследования состояния катализаторов в условиях протекания каталитических реакций.
Продемонстрированы достигнутые в последнее десятилетие успехи в разработке современных высокоэффективных катализаторов полимеризации, отмечена высокая готовность академических институтов к решению проблемы импортозамещения по катализаторам полимеризации этилена и пропилена, отмечена заинтересованность представителей крупных промышленных предприятий в этих катализаторах (ПАО Сибур, ПАО Нижнекамскнефтехим, ПАО Казаньоргсинтез, ОАО Газпром нефтехим Салават и др.). Отмечено, что основным вектором развития научного направления получения новых полимерных материалов является дизайн молекулярной структуры полимеров и получение нанокомпозитов, в том числе наполнение при полимеризации in situ для формирования новых свойств известных материалов (полиэтилена, в том числе сверхвысокомолекулярного, полипропилена и т.д.), позволяющих существенно расширить области применения известных полимеров. Традиционно актуальным здесь является дизайн новых каталитических систем для получения полимеров с уникальными свойствами, которые невозможно получать с использованием традиционных катализаторов.
Прошедший VI Семинар позволил провести анализ полученных результатов и наметить перспективные направления исследований как по синтезу катализаторов металлокомплексной природы, изучению природы их активных центров, механизма их функционирования, так и по прикладным аспектам применения таких катализаторов в процессах полимеризации и нефтехимии – основной области научных интересов исследований, оригинальных идей, заложенных в свое время профессором Ю.И. Ермаковым, к которым и сегодня специалисты продолжают проявлять интерес, разрабатывают новые проекты по металлокомплексному катализу.
Курс лекций Школы «Каталитическая полимеризация олефинов» способствовал повышению квалификации и привлечению молодых ученых к исследованиям новых полимерных наносистем, как в плане разработки методов их синтеза, так и с точки зрения разработки методов изучения их состава и структуры.
Следуя традиции семинаров Ермакова, в 2021 году для участников и сопровождающих лиц были организованы интересные экскурсии по Байкальскому музею, посещение архитектурно-этнографического музея «Тальцы», буддистского храмового комплекса – Иркутского дацана и сказочного Минералогического музея предприятия «Байкалкварцсамоцветы». Особо запомнился однодневный пост-тур по озеру Байкал на теплоходе «Бабушкин» и прогулка по заповедной тропе мыса Кадильный.

Общее фото участников Семинара. Отель Маяк.
Материал подготовили:
В.А. Лихолобов, В.А. Захаров, М.А. Мацько, Л.Я. Старцева, М.А. Клюса
(Институт катализа СО РАН, Новосибирск)
21 сентября 2021 года, Казань, Россия
http://conf.nsc.ru/RusCat-2021/ru/ruscat-2021_sci-prog;jsessionid=7C0EF1EEE91B5A056EF3F5C0244B79E1
21 сентября 2021 года в рамках IV Российского конгресса “Роскатализ” проведено заседание Некоммерческого партнерства “Национальное каталитическое общество”.
1. Заслушан отчет о делах Партнерства;
2. Обсуждены вопросы взаимодействия Партнерства с Научным советом ОХНМ РАН по катализу и вопросы приема новых членов в Партнерство;
3. Избраны в соответствии с Уставом директор, президент, вице-президент и сформировано правление Партнерства:
Президентом Некоммерческого партнерства “Национальное каталитическое общество” избран
Вице-президентом Некоммерческого партнерства “Национальное каталитическое общество” избран
Исполнительным директором Некоммерческого партнерства “Национальное каталитическое общество” избран А.Б. Куликов, кандидат химических наук, заместитель директора ИНХС РАН.
4. Избраны национальные российские представители
в советы IACS, EFCATS:
В.Н. Пармон, академик РАН, доктор химических наук, профессор, научный руководитель ИК СО РАН, вице-президент РАН, председатель СО РАН;
А.Л. Максимов, член-корреспондент РАН, доктор химических наук, профессор, директор ИНХС РАН;
в совет APACS:
О.Н. Мартьянов, доктор химических наук, профессор РАН, заместитель директора ИК СО РАН;
О.П. Таран, доктор химических наук, профессор РАН, директор ИХХТ СО РАН.
22 сентября 2021 года, Казань, Россия
http://conf.nsc.ru/RusCat-2021/ru/ruscat-2021_sci-prog;jsessionid=7C0EF1EEE91B5A056EF3F5C0244B79E1
На I, II и III Российских конгрессах по катализу по инициативе академика В.В. Лунина (МГУ имени М.В. Ломоносова) и руководства Института катализа СО РАН успешно прошли круглые столы, посвященные образованию в области катализа. Эта необычная для подобных мероприятий тематика вызвала оживленный интерес среди участников конгрессов. В результате аналогичный международный круглый стол В.В. Лунин и проф. Д.Ю. Мурзин (Турку, Финляндия) организовали на 12-м Европейском конгрессе по катализу «ЕВРОПАКАТ» (2015, Казань, Россия). Анализ результатов этого круглого стола проведен в статье [1].
«Наследником» этих мероприятий стал новый Круглый стол «Исследовательская карьера: от молодого ученого до лидера проекта», который состоялся 22 сентября 2021 года в рамках IV Российского конгресса «Роскатализ» (Казань, 2025 сентября 2021 г.) в качестве сателлитного мероприятия. Модераторы Круглого стола – д.х.н. Козлов Денис Владимирович из Института катализа им. Г.К. Борескова и д.х.н., проф. Локтева Екатерина Сергеевна, представитель химического факультета МГУ имени М.В. Ломоносова, предложили для обсуждения следующую тематику:
Модераторы пригласили к участию в Круглом столе как исследователей, так и представителей промышленных предприятий и фирм. Круглый стол вызвал значительный интерес и собрал около ста участников конгресса. Открыл заседание Д.В. Козлов с проблематизирующим докладом, в котором очертил круг вопросов, которые возникают при обсуждении вариантов карьеры молодых исследователей, и призвал участников к обмену мнениями.
За две недели до Круглого стола на сайтах конгресса «Роскатализ» и химического факультета МГУ имени М.В. Ломоносова организаторы провели анонимный опрос, посвященный карьерным перспективам и их реализации для ученых разных возрастов.
Итоги опроса подвела в своем выступлении Е.С. Локтева. Они хорошо согласуются с результатами опроса [2], в число респондентов которого входило более 7000 участников, из них примерно треть из области естественных наук.
Выяснилось, что участники опроса на сайте конгресса «Роскатализ» (88 человек, половина из них женщины) высоко оценивают полученное образование, однако до 10% опрошенных чувствуют недостаток определенных компетенций, связанных с практическими и социальными навыками, умением находить финансирование и коммерциализировать результаты исследований. Важно, что менее половины опрошенных проходили практику на промышленных предприятиях, и даже из них значительная часть не получила никаких выгод от таких практик в отношении развития практических навыков, научного и практического сотрудничества. Также менее половины участников опроса проходили зарубежные стажировки, как длительные, так и кратковременные, причем осуществить оба типа стажировок смогли лишь два человека. Предполагается, что стажировки должны способствовать не только повышению квалификации, но и завязыванию долговременного научного и технического сотрудничества, однако о налаживании таких связей сообщила лишь малая часть опрошенных, для остальных стажировки не послужили основой для последующей коллаборации.
В опросе звучали вопросы о том, какие позиции в исследовательской сфере занимают участники. Подавляющее большинство занимает позиции исследователей, и меньшее число являются лидерами групп или проектов. Ответы на вопрос о желаемой позиции показали, что среди исследователей, которые пока не занимают таких постов, лишь малое количество хочет стать лидером проекта или группы, а в основном люди видят продолжение своей карьеры на пути исследователя. Практически все участники опроса имеют опыт руководства проектами, диссертациями, дипломными или курсовыми работами. Следовательно, даже студенты старших курсов нарабатывают необходимый опыт при руководстве исследовательскими проектами младшекурсников (упоминались также проекты школьников).
В качестве важных компетенций для успешной карьеры участники опроса в первую очередь назвали умение общаться с людьми, коммерциализировать результаты исследований, работать с литературой. Параметры, наиболее способствующие успеху в карьере – это качества характера, и только на втором месте образование, а далее с большим отрывом идут удача/везение, связи, общественная работа и др. То есть участники опроса хорошо видят, что не все они способны повести за собой людей, собрать коллектив, наметить цели и исполнить намеченное, а также обеспечить финансирование разработок.
Среди причин, препятствующих успешному построению карьеры, 44% участников назвали низкую престижность профессии ученого, 41% – отсутствие специалистов по коммерциализации исследований, которые способны помочь в практической реализации результатов, 35% – низкую мотивацию самих исследователей к такой коммерциализации, и несколько меньше – низкий уровень подготовки управленцев и самих исследователей.
Два взаимосвязанных вопроса касались перспектив работы в исследовательской сфере в России или за рубежом: для себя лично или для ближайшего родственника (брат, сестра). Интересно, что для себя лишь малая часть опрошенных планирует для себя работу за рубежом, и особенно на постоянной основе. А вот младшему брату/сестре карьеру исследователя если бы и посоветовали, то преимущественно за рубежом.
На круглом столе остро стояли вопросы оплаты труда ученых. Опрос показал не слишком уверенное материальное положение, лишь 30.7% опрошенных имеют доходы выше 100 тыс. руб. При ответе на вопрос о желаемом доходе участники опроса проявляют определенную скромность, 38.8% хотели бы иметь доходы в интервале 100-200 тыс. руб., что примерно соответствует средней зарплате ученого за рубежом; около 30% согласны на меньшие зарплаты. Вопрос о материальном обеспечении и о роли этого фактора при выборе научной карьеры постоянно звучал в выступлениях участников круглого стола. Так, А. Бугаев (Ростов-на-Дону) отметил, что главный драйвер работы в науке – это интерес, счастье заниматься любимым делом, и при условии, что человек получает удовольствие от этой деятельности, он найдет возможности с большим или меньшим успехом обеспечивать материально себя и своих сотрудников. Об этом свидетельствует личный опыт А. Бугаева, который временами имел одновременно четыре исследовательских проекта и возможность содержать значительную научную группу, а в иные периоды был вынужден увольнять членов коллектива или переводить на низкие ставки в связи с отсутствием средств.
Звучали также вопросы о том, решит ли предоставление квартиры от предприятия вопрос о закреплении кадров в науке. А. Глотов (РГУНГ им. Губкина) прокомментировал, что ученый должен зарабатывать достаточно для самостоятельного решения квартирного вопроса, а не надеяться на помощь предприятия, что дает большую свободу в выборе жизненной траектории.
Анна Вутолкина, представляющая химический факультет МГУ имени М.В. Ломоносова, исходя из собственного опыта молодого ученого, разделила свою карьеру на три этапа – обучение (до 20), начало работы над научными проектами (20-25 лет), и после 25 лет, когда произошел переход к роли лидера научного проекта. Она воспринимает переходы на каждый следующий этап как вызовы, требующие существенного изменения подходов. Двигателем этого развития является научный интерес, удовольствие от исследований, реализация идей, но возрастающая ответственность несколько давит. И очень хотелось бы видеть адекватной материальную составляющую работы ученого, не только в плане зарплаты, но и в отношении обеспеченности необходимым оборудованием и материалами. Пока Анне сложно представить, «есть ли жизнь после 35», но ей хотелось бы, чтобы исследовательская деятельность обеспечивала ей и ее семье приемлемый уровень жизни в дополнение к обеспечению потребностей в творчестве.
Андрей Чистяков (ИНХС РАН) также подчеркнул, что интерес к работе – определяющее качество исследователя. Финансирование приходит и уходит, а образование остается с человеком. В науке остаются те, кто получают удовольствие от самой научной деятельности и постоянного саморазвития, а развитие влечет за собой лидерство.
Модераторы в самом начале круглого стола предложили участникам подумать о том, что они понимают под карьерой. Понятно, что для исследователя переход на позицию руководителя группы, проекта, лаборатории или более высокие административные посты, безусловно, является карьерным ростом. Но остается вопрос, можно ли считать карьерой повышение экспертной ценности ученого в роли исследователя (повышение индекса Хирша, публикация в более престижных журналах, вхождение в экспертные группы). Многие выступающие отметили, что этот второй вариант они тоже рассматривают как карьеру, и некоторые даже выделили его как предпочтительный вариант карьеры. Такая позиция нашла подтверждение в выступлении представителя промышленности И.Н. Воропаева (Сибур), который подчеркнул, что экспертученый высокого уровня в рамках фирмы может рассчитывать даже на более высокую оплату труда по сравнению с менеджерами, при условии, что квалификация менеджера в своей сфере не так высока.
Людмила Степанова (Омский филиал ИК СО РАН) наиболее привлекательным аспектом работы в науке назвала отсутствие границ – расширение возможностей познания и удовлетворение собственного научного любопытства, возможность работать долгие годы, даже и на пенсии, возможность перемещения из одного научного центра в другой в рамках конференций, стажировок и пр. Она особо отметила, что для нее «горизонтальное» развитие, связанное с развитием экспертных навыков, также является привлекательной карьерой.
На круглом столе выступали представители предприятий разного масштаба, от небольших до крупных. Сотрудник фирмы «Экоальянс», занимающейся разработкой и внедрением катализаторов для автомобильных каталитических нейтрализаторов, Е. Аликин посетовал на то, что не слишком высокие зарплаты на предприятии вызывают текучку кадров, уходящих на соседний Новоуральский трубный завод. Однако работа с молодежью, школьниками, студентами дает возможность сработать на интересе, привлечь новые кадры постановкой и решением перспективных задач. Как отметил Е. Аликин, классик катализаторной науки и практики Хальдор Топсе, основатель одноименной датской фирмы, писал, что хорошее предприятие должно иметь более 50 работников и оборудования более чем на 20 млн долларов. Эти задачи не так легко решить небольшому предприятию. Выход он видит на пути широкого сотрудничества с научными и образовательными учреждениями, располагающими современным оборудованием для исследования катализаторов и расширяющими возможности для такого исследования.
Представитель значительно более крупной фирмы ООО «Газпромнефтькаталитические системы» А.А. Зайцев рассказал о том, что в Омской области эта фирма строит современный высокопроизводительный катализаторный завод, который позволит обеспечить импортозамещение в этой важной сфере. Вскоре откроется более 120 вакансий для исследователей не только в научной, но и в организационной и коммерческой сфере. А.А. Зайцев призвал участников Круглого стола сохранить его контакты и обращаться в отдел кадров предприятия, где будут созданы отличные условия для развития карьеры во всех возможных направлениях. Фирма предлагает «точки карьерного входа» на самых разных уровнях для ученых, находящихся на разных стадиях карьеры, обладающих как небольшим, так и значительным опытом работы.
Работающий в «Сибуре» с 2000 года бывший научный сотрудник Института катализа им. Г.К. Борескова СО РАН И.Н. Воропаев подчеркнул, что развитие карьеры может выстраиваться по трем маршрутам: эксперт – позиция подходит для тех, кто получает удовольствие от работы в науке; менеджер или управленец, которому больше приходится работать с людьми, и который лишь отчасти развивает проектную деятельность; и проектный маршрут, подразумевающий наличие успешных навыков коммуникации, ответственности, планирования и других «мягких» навыков. Выступающий предложил искать, где лежит собственный интерес, и повышать квалификацию и нарабатывать навыки в выбранной области для повышения собственной ценности на рынке труда. Он призвал молодых ученых оценивать не только свою зарплату, но и ценность собственной деятельности, и повышать эту ценность путем наработки «жестких» и «мягких» навыков. Его посыл подразумевал, что нужно не только повышать квалификацию, но и искать возможности применения сил во всех отраслях, не обязательно в области фундаментальной науки.
Ему возразила Г. Шайдуллина из фирмы «Leco Corporation». Она подчеркнула, что с 2000х годов, когда предыдущий выступающий оставил научную карьеру, ситуация в России для молодых исследователей радикально изменилась, и теперь они обладают существенно более перспективными возможностями для развития на высоком научном уровне. Она призвала участников круглого стола оставаться работать в науке и искать там драйв и возможности применения своих сил.
Е. Андрусенко, работающая на организационных должностях в нескольких фирмах и университетах, отметила боязнь многих молодых ученых брать ответственность за выполнение проекта, особенно если он подразумевает значительное финансирование или выход за границы привычной деятельности.
Из аудитории постоянно звучали вопросы о том, удается ли выступающим совмещать исследовательскую карьеру со строительством семьи и рождением детей, и ответ в целом был положительным.
Подводя итоги Круглого стола «Исследовательская карьера: от молодого ученого до лидера проекта», можно отметить живой и активный интерес участников конгресса «Роскатализ» к этой тематике. Времени для обсуждения всех поднятых вопросов не хватило, поэтому мероприятия подобного рода необходимо проводить и в дальнейшем.
Молодые ученые, участники круглого стола, думают над вопросами карьеры постоянно. Не все они нацелены на постоянное лидерство, да это и не требуется, поскольку для этой роли необходимы определенные качества характера: определенная смелость, решительность, а получение навыков лидера, как правило, происходит постепенно. Но большинство видят в науке интересную и перспективную область применения своих сил, а также возможность построения «горизонтальной» карьеры с углублением и расширением экспертных компетенций.
Для увеличения числа возможных лидеров необходимо в ходе обучения обеспечивать такие компетенции, как умение работать с людьми, а также привлекать финансирование и коммерциализировать исследования. Кроме того, больше внимания нужно уделять качественной организации практик и стажировок для того, чтобы эти важные этапы помогали в приобретении необходимых навыков и расширении круга людей, способных и желающих участвовать в совместных разработках.
Основные разочарования в ходе построения научной карьеры связаны с низкой престижностью профессии и недостаточной оплатой, причем завышенных ожиданий у молодых ученых нет. Эти вопросы необходимо решать на самом высоком уровне.
Литература:
Материал подготовили:
Е.С. Локтева
(Химический факультет МГУ имени М.В.Ломоносова, e.lokteva@rambler.ru),
Д.В. Козлов
(Институт катализа им. Г.К.Борескова СО РАН,
kdv@catalysis.ru)
Glass catalysis shatters material’s inert reputation
The surface of glass can act as a strong base to catalyze reactions and degrade certain reagents
Beakers, flasks, petri dishes—glassware is a hallmark of chemistry labs. Though commonly thought of as inert, glass containers are known to affect certain chemical reactions, but researchers have not understood exactly how or to what extent. Now, a team reports that glass beads can catalyze many base-catalyzed reactions, suggesting that glass could be used as a green catalyst in place of toxic or expensive chemicals (Chem. Science, 2021, DOI: 10.1039/d1sc02708e)
Late last year, Yangjie Li of Graham Cooks’s lab at Purdue University published work based on an accidental discovery that an amine transfer reaction proceeded faster in glassware than in plastic, and that adding glass beads accelerated reactions even further (Angew. Chem., Int. Ed. 2020, DOI: 10.1002/anie.202014613). Li wanted to find out more.
Li and colleagues tested thousands of reaction conditions and dozens of reactions to see how the presence of glass microspheres affected various reaction types. “We found that there are so many base-catalyzed reactions that can be catalyzed by just adding these glass particles in the solution”—as opposed to the expensive, caustic basic chemicals typically used for these kinds of reactions, she says. Glass beads accelerated elim-ination, solvolysis, condensation, and oxidation reactions 30- to 2,000-fold.
The surface of glass is covered with dissociable silanol groups, giving it a negative charge when in contact with solution. The researchers determined that this allows glass to act as a strong base while producing nucleophilic solvent anions that catalyze reactions.
“The second very important part of [the study] is that we found that glass can degrade biomolecules such as lipids,” Li says. “So it could be a huge impact in bioanalytical work and in clinical settings.”
Cooks says that for standard concentrated solutions stored in a glass container, “there’s no problem at all” because “99.9% of the material will not make it to the surface.” But he adds that for specialty low-concentration ingredients or analytical tests at low concentrations, “we found significant loss of the material.”
“I think it is an outstanding new direction,” says Richard Zare, a physical and analytical chemist at Stanford University who was not part of the study. Zare is interested in how this can be scaled up as a practical means of making new chemicals.
Core-shell catalysts made of two metals combine high activity with high selectivity
Careful preparation avoids catalytic trade-offs
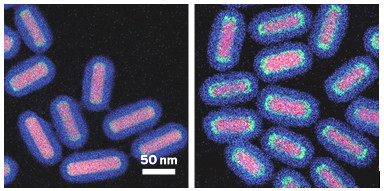
These catalysts consist of a gold core, a palladium shell ranging in thickness from
one atom (left) to six (right), and a protective silica layer.
By carefully controlling the structure and composition of bimetallic nanoparticles, researchers have come up with a high-performance model catalyst that blends the best features of each metal. The advance could lead to commercial catalysts that cut manufacturing costs and reduce waste. Heterogeneous catalysts, which generally contain one type of metal, drive most industrial-scale chemical processes. Blending two metals can increase catalysts’ capabilities, for example, by mediating separate steps of a reaction. But most methods for preparing mixed-metal catalysts lead to particles with variations in composition, size, and shape. The lack of control often causes a trade-off between catalytic activity and chemical selectivity. A team of researchers led by Jessi E. S. van der Hoeven, Alfons van Blaaderen, and Petra E. de Jongh of Utrecht University has now shown how to combine metals to make catalysts that are active and selective. The team made a series of uniform nanorods, each with a gold core and a palladium shell of predetermined thickness. The researchers capped the rods with porous silica and tested them catalytically in the selective hydrogenation of 1,3-butadiene, a process used for purifying propene feedstocks. The core-shell catalysts were all highly selective, a trademark of gold, yet they were up to 50 times as active as catalysts made of a single metal or random mixtures of the metals (Nat. Mater. 2021, DOI: 10.1038/s41563-021-00996-3).
Copper catalysts team up for chiral amide synthesis
Blue light powers a radical route to ubiquitous functional group

A pair of copper catalysts can create chiral amides in a new, light-driven process, offering a useful alternative to the standard method for making these staples of medicinal chemistry (Nature 2021, DOI: 10.1038/s41586-021-03730-w).
“Amides are ubiquitous functional groups in bioactive molecules, such as peptides,” says Gregory C. Fu at the California Institute of Technology, who coled the work.
The conventional route to amides involves reacting an amine with a carboxylic acid derivative. So if chemists want to selectively create just one of the mirror-image enantiomers of a chiral amide, they generally start with a single enantiomer of the amine. That sequence typically involves at least two steps—one to control the stereochemistry of the amine precursor and a second to form the amide.
In contrast, the Caltech team’s method achieves this in a single step without using single-enantiomer precursors by controlling the stereochemistry of the amide as it forms. “We’re hoping this leads to a more efficient process,” Fu says.
The reaction relies on two different copper catalysts and combines an amide with an alkyl bromide to form a chiral secondary amide—where the nitrogen atom is bonded to a hydrogen and two carbons, one of which is chiral.
The first catalyst is a copper (I) bisphosphine phenoxide complex. Once activated by blue light, it removes bromine from the alkyl bromide to release an alkyl radical. Meanwhile, the second catalyst, a chiral copper (II) diamine complex, binds to the primary amide and unites it with the alkyl radical. This stereoselectively forms a C–N bond, replacing one of the amide’s hydrogen atoms with the alkyl group to produce a secondary amide. As these two catalytic cycles keep turning, the copper complexes alternate between +1 and +2 oxidation states.
The reaction is tolerant to air or moisture and works for a diverse array of primary amides. It is also compatible with various functional groups in the alkyl bromide precursor. Amide yields range from 53%–95%, with a single enantiomer making up at least 95% of the product. “I find it quite remarkable that Caiyou Chen, the postdoc who’s done this work, has been able to showcase efficacy over such a broad range of directing groups, with such high yields and enantioselectivity,” says Caltech’s Jonas C. Peters, who coled the work with Fu.
“I think this paper is really amazing,” says Nicolas Blanchard, a French National Center for Scientific Research research director at the University of Strasbourg, who was not involved in the research. “With three simple ligands, they’ve been able to solve something that was really unattainable, in my view.”
Blanchard’s own research involves copper-catalyzed reactions for organic synthesis, and he says the new amide-forming reaction highlights growing interest in the metal. “Copper is used more and more, especially in photocatalyzed reactions,” he says. “These authors were pioneers in that regard.”
Chemical & Engineering News
|
| |
|
November
26-27, 2021 9th International Conference on Nanomaterials and Materials Engineering (ICNME 2021) Chengdu, China |
http://www.icnme.org |
|
2-3
декабря 2021 г. XII международная конференции «Газохимия. Нефтехимия. Возможности зеленого будущего» Омск, Россия |
http://ntk2021.onhp.ru/ |
|
December
16-21, 2021 International Chemical Congress of Pacific Basin Societies (Pacifichem 2021) Honolulu, Hawaii, USA |
http://www.chemistry.or.jp/en/events/pacifichem.html |
|
2022
6th International Conference “Catalysis for Renewable Sources: Fuel, Energy, Chemicals” (CRS-6) |
http://conf.nsc.ru/CRS6/en |
|
2022
г. XIV Конференция «Металлургия цветных, редких и благородных металлов» в рамках XII Международного конгресса и выставки «Цветные металлы и минералы» Красноярск, Россия |
https://nfmsib.ru/ |
|
January
8-10, 2022 5th International Conference on Smart Materials Applications (ICSMA 2022) Bangkok, Thailand |
http://www.icsma.org |
|
February
13-17, 2022 Central European Conference on Photochemistry (CECP 2022) Bad Hofgastein, Austria |
http://www.cecp.at |
|
February
28 – March 4, 2022 13th Winter Symposium on Chemometrics (WSC-13) Moscow, Russia |
https://wsc.chemometrics.ru/ |
|
March
2-3, 2022 7th International Conference on Ecological & Environmental Chemistry (EEC-2022) Chisinau, Moldova |
http://eec-2022.mrda.md/ |
|
March
6-10, 2022 Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy (Pittcon 2022) Atlanta, GA, United States |
https://pittcon.org/
|
|
March
14-18, 2022 2022 Winter School “A roadmap for catalysis to support a society powered by renewable energies” CNRS CAES Paul-Langevin Center, Aussois, France |
https://catenerchem.cpe.fr/ |
|
March
16-18, 2022 German Catalysis Meeting Weimar, Germany |
https://dechema.de/en/katalytiker2022.html |
|
March
28-30, 2022 10th Conference on Catalysis, Chemical Engineering and Technology (CCET 2022) Singapore |
https://catalysis.magnusconferences.com/ |
|
April
20-22, 2022 Faraday Discussion – Photoelectron Spectroscopy and the Future of Surface Analysis London, United Kingdom |
https://www.rsc.org/events/detail/ 45900/photoelectron-spectroscopy-and-the-future- of-surface-analysis-faraday-discussion |
|
May
16-20, 2022 International Symposium on Green Chemistry (ISGC 2022) La Rochelle, France |
https://www.isgc-symposium.com/ |
|
May
23-27, 2022 10th International Symposium “Molecular Order and Mobility in Polymer Systems” (MOMPS-X) Saint Petersburg, Russia |
http://momps2020.macro.ru/ |
|
May
25-27, 2022 New Trends in Polymer Science: Health of the Planet, Health of the People Turin, Italy |
https://polymers2022.sciforum.net |
|
June,
2022 International BioEPR School-Сonference 2022 Novosibirsk, Russia |
http://www.bioepr2020.ru |
|
June
6-8, 2022 19th Nordic Symposium on Catalysis Espoo, Finland |
https://19nsc.fi/ |
|
June
6-10, 2022 11th European Conference on Solar Chemistry and Photocatalysis: Environmental Applications (SPEA11) Turin, Italy |
http://www.spea11.unito.it/home |
|
June
26-29, 2022 19th International Symposium on Relations between Homogeneous and Heterogeneous Catalysis (ISHHC 19) University of Oslo, Blindern |
https://www.mn.uio.no/kjemi/english /research/groups/catalysis/ishhc19/ |
|
July
3-8, 2022 12th Triennial Congress of the World Association of Theoretical and Computational Chemists (WATOC 2020) Vancouver, Canada |
http://watoc2020.ca |
|
July
4-7, 2022 International Conference of the International Association for Spectral Imaging (IASIM 2022) Esbjerg, Denmark |
https://2020.iasim.net/ |
|
July
17-22, 2022 28th IUPAC Symposium on Photochemistry Amsterdam, Netherlands |
https://photoiupac2022.amsterdam |
|
July
18-22, 2022 2nd International Conference on NonCovalent Interactions (ICNI 2021-2022) Strasbourg, France |
http://icni2021.unistra.fr/ |
|
July
18-22, 2022 26th IUPAC International Conference on Chemistry Education (ICCE 2020) Cape Town, South Africa |
https://iupac.org/event/26th-iupac-international-conference- on-chemistry-education/ |
|
July
24-28, 2022 84th Prague Meeting on Macromolecules – Frontiers of Polymer Colloids Prague, Czech Republic |
https://www.imc.cas.cz/sympo/84pmm/ |
|
July
25-27, 2022 2nd International Conference “Materials and Nanomaterials” (MNs-22) Rome, Italy |
https://mns-20.com/
|
|
August
28 – September 2, 2022 44th International Conference on Coordination Chemistry Rimini, Italy |
https://www.iccc2020.com/ |
|
September,
2022 7th International Conference on Metal-Organic Frameworks and Open Framework Compounds Dresden, Germany |
https://dechema.de/en/MOF2020.html |
|
September
– October, 2022 9th IUPAC International Conference on Green Chemistry (ICGC-9) Athens, Greece |
http://www.greeniupac2020.org |
|
August
27 – September 1, 2023 15th European Congress on Catalysis (EuropaCat 2023) Prague, Czech Republic |
https://www.europacat2023.cz/ |


