22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы

 18 августа 2017 года исполнилось 70 лет члену-корреспонденту Российской академии наук, научному руководителю Омского научного центра Сибирского отделения РАН и Института проблем переработки углеводородов СО РАН, профессору Владимиру Александровичу Лихолобову.
18 августа 2017 года исполнилось 70 лет члену-корреспонденту Российской академии наук, научному руководителю Омского научного центра Сибирского отделения РАН и Института проблем переработки углеводородов СО РАН, профессору Владимиру Александровичу Лихолобову.
После окончания Новосибирского государственного университета в 1970 году Владимир Александрович работал в Институте катализа СО АН СССР (РАН), пройдя путь от стажера-исследователя до заведующего лабораторией и заместителя директора по научной работе. В 1973 г. получил ученую степень кандидата химических наук, в 1983 г. – степень доктора химических наук, в 2001 г. – звание члена-корреспондента РАН.
По инициативе Президиума СО РАН в 2000 году он переехал из Новосибирска в Омск, чтобы возобновить и развить деятельность Омского научного центра. В 2000 – 2015 гг. работал в должности председателя Президиума Омского научного центра СО РАН, в настоящее время является научным руководителем Центра. За время нахождения на посту председателя Президиума ОНЦ СО РАН Лихолобов В.А. существенно укрепил позиции Центра среди других региональных научных центров. Под его руководством сформирован квалифицированный научный коллектив, развиты интеграционные связи с вузами и промышленными предприятиями региона.
Под руководством В.А. Лихолобова в 2003 г. осуществлена реорганизация Омского филиала Института катализа СО РАН и Конструкторско-технологического института технического углерода СО РАН в Институт проблем переработки углеводородов СО РАН, директором которого Владимир Александрович был избран и утвержден в 2004 г. и проработал в этой должности до 2015 года. Институт к настоящему времени занял позиции одного из лидеров академической науки в области нефтепереработки, нефтехимии и синтеза углеродных материалов. В настоящее время В.А. Лихолобов в ИППУ СО РАН выполняет обязанности научного руководителя Института, заведующего лабораторией синтеза функциональных углеродных материалов, заведующего лабораторией зеленой химии ИППУ СО РАН (созданной в рамках проекта Российского научного фонда).
Владимир Александрович Лихолобов – специалист в области создания катализаторов и каталитических процессов нефтехимического и органического синтеза, целенаправленного синтеза функциональных углеродных материалов и каталитических композиций. Он известен отечественной и мировой научной общественности как один из инициаторов проведения исследований в области применения принципов гомогенного катализа для создания новых эффективных гетерогенных катализаторов органического синтеза, а также разработки методов каталитического матричного синтеза и их применения для создания практически важных материалов и каталитических композиций. Заметный вклад В.А. Лихолобов внес в разработку каталитических систем, имеющих перспективу значительного улучшения технико-экономических показателей продукции предприятий нефтехимического и органического синтеза.
С активным участием В.А. Лихолобова были разработаны научные основы и реализованы в опытно-промышленном масштабе технологии производства различных модификаций нового углеродного материала Сибунит и катализаторов на его основе для процессов органического синтеза; решены научно-технологические вопросы получения наноглобулярного углерода и его поверхностного и объемного модифицирования с целью создания новых функциональных материалов для решения вопросов экологии, защиты здоровья человека, обороноспособности государства.
В.А. Лихолобов является руководителем ведущей научной школы, поддержанной грантами Президента РФ, исследования которой направлены на развитие методов целенаправленного синтеза активных центров для создания нового поколения катализаторов нефтегазопереработки, нефтехимии, а также методов получения наноглобулярного углерода и продуктов его модифицирования с целью разработки материалов для природоохранных технологий, альтернативной энергетики, защиты здоровья, а также для специальных областей применения.
В.А. Лихолобов – автор и соавтор 542 научных публикаций, в том числе 137 охранных документов. За последние 5 лет (2012-2016) им опубликовано 117 научных работ, из них 24 патента. Общее количество публикаций, учтенных в базе данных Web of Science Core Collection, – 307, индекс Хирша – 28, индекс цитирования (Web of Science C.C.) – 3182, среднее количество цитирований на статью – 10,36.
Владимир Александрович имеет большой опыт научно-организационной работы, является членом редколлегий пяти научных журналов, ряда научных советов РАН и СО РАН, докторского диссертационного совета при ОмГТУ, Бюро Научного совета по катализу РАН, Совета по технической химии и новым материалам Военно-промышленной комиссии РФ, Координационного совета по промышленной и научно-технической политике Межрегиональной ассоциации «Сибирское соглашение», и.о. заместителя председателя СО РАН по вопросам импортозамещения, обороны и развития программ реиндустриализации.
В.А. Лихолобов активно занимается подготовкой молодых исследователей. С 1985 по 2003 гг. он возглавлял кафедру катализа и адсорбции факультета естественных наук Новосибирского государственного университета, читал основную часть лекций по курсу “Катализ”. С 2003 г. Владимир Александрович уделял серьезное внимание подготовке высококвалифицированных научных кадров в Омском государственном университете, возглавив кафедру химической технологии. В настоящее время заведует кафедрой созданного при его активном участии Нефтехимического института Омского государственного технического университета. Среди его учеников 9 докторов и 21 кандидат наук.
За свою многолетнюю активную научную и общественную деятельность В.А. Лихолобов награжден почетной грамотой Академии наук СССР (1974 г.), медалью Ордена “За заслуги перед отечеством” II степени (1999 г.), орденом Дружбы (2007 г.), Золотым Почетным знаком “Общественное признание” в номинации “Наука и образование” (2007). В 2007 г. получил государственную награду Омской области – медаль “За высокие достижения”, в 2015 году – почетный знак СО РАН «Золотая сигма».
Научный совет по катализу ОХНМ РАН и редакция Каталитического бюллетеня сердечно поздравляют Владимира Александровича с юбилеем, желают ему реализации намеченных планов и новых творческих достижений!
12 сентября 2017 года на заседании Президиума Российской академии наук было принято решение о присуждении Премии имени В.А. Коптюга академику РАН Валерию Васильевичу Лунину, доктору химических наук Екатерине Сергеевне Локтевой и кандидату химических наук Елене Владимировне Голубиной (Московский государственный университет имени М.В. Ломоносова) за серию работ «Разработка новых катализаторов процессов экологического катализа».
Представленная серия работ направлена на разработку катализаторов и способов осуществления реакций экологического катализа. Разработаны, запатентованы и проходят испытания в условиях реальных производств новые экологически чистые процессы и установки для утилизации токсичных и высоко-токсичных хлорсодержащих органических и неорганических отходов. Проведен большой объем исследований с целью создания новых эффективных катализаторов и научных основ технологии утилизации техногенных хлорорганических отходов с использованием восстановительных методов. Показана возможность экологически безопасной их переработки с получением полезных продуктов, предложены новые каталитические системы и реакции.
Помимо высоких научных достижений, авторы вносят огромный вклад в преподавательскую и просветительскую деятельность во многих университетах страны, пропагандируя через свои достижения основополагающие принципы «зеленой химии».
Поздравляем коллег с высокой наградой!
Премия имени В.А. Коптюга присуждается Российской академией наук с 2002 года за выдающиеся работы по химии в интересах сохранения окружающей среды и развития. Премия названа в честь российского химика, Вице-президента РАН, Председателя Сибирского отделения РАН В.А. Коптюга.
| 2002 | Г.А. Ягодин | За цикл работ по химии и химической технологии для устойчивого развития |
| 2005 | Г.В. Сакович А.Н. Загоруйко З.Р. Исмагилов | За работу “Разработка и внедрение новых каталитических технологий охраны окружающей среды и утилизации высокоэнергетических материалов” |
| 2008 | В.Т. Калинников В.Н. Макаров В.А. Чантурия | За цикл работ “Научные основы снижения экологической опасности горнопромышленных отходов и разработка эффективных технологий их утилизации” |
| 2011 | Н.З. Ляхов Н.П. Тарасова И.В. Коптюг | За цикл работ “Использование принципов зеленой химии в фундаментальных и прикладных исследованиях в интересах устойчивого развития” |
| 2014 | Ю.А. Золотов Г.И. Цизин Л.К. Шпигун | За цикл работ “Разработка общей методологии контроля химического состава объектов окружающей среды и создание комплекса высокочувствительных методов анализа воды” |

22-26 мая 2017 г., Нижний Новгород, Россия
Третий Российский конгресс по катализу Роскатализ-2017 прошёл 22-26 мая в Нижнем Новгороде в корпусах Национального исследовательского Нижегородского государственного университета им. Н.И. Лобачевского. Задачи Конгресса включали обсуждение состояния дел и перспектив развития всех областей катализа, поиск новых возможностей для импортозамещения в отношении стратегически важных катализаторов и химической продукции, стимулирование консолидации науки, бизнеса и высшего образования для решения фундаментальных и прикладных задач, обеспечивающих модернизацию и инновационное развитие химического комплекса России в соответствии с утверждённой Президентом России «Стратегией научно-технологического развития Российской Федерации на период до 2030 года».

Основным организатором Конгресса выступил Институт катализа им. Г.К. Борескова СО РАН (Новосибирск) при активной поддержке Национального исследовательского Нижегородского государственного университета им. Н.И. Лобачевского, Новосибирского национального исследовательского государственного университета, Московского государственного университета имени М.В. Ломоносова, а также Нижегородского технического университета им. Р.Е. Алексеева, Института нефтехимического синтеза им. А.В. Топчиева (Москва) и Института органической химии им. Н.Д Зелинского (Москва).
Финансовую поддержку Конгрессу оказали Российский фонд фундаментальных исследований и Федеральное агентство научных организаций. Генеральным спонсором мероприятия выступило ПАО «Газпром нефть». Спонсорская поддержка была получена от ПАО «СИБУР», ООО «Салаватский катализаторный завод», ООО «ФИЗЛАБПРИБОР», группы компаний «ГалаХим», ООО «Сервис центр «Хромосиб», ООО «Аналит Продактс», ООО «Новомичуринский катализаторный завод», фирмы SPECS Surface Nano Analysis GmbH (Германия), ЗАО «Нижегородские сорбенты», ООО «Центр технологий «Лантан», OOО «Брукер», Компания ООО «РИОС – Инжиниринг», ООО «СИ ЛАБ», АО «СКТБ «Катализатор», ООО «АБЦР ХЕМИ РУС». Партнёры конгресса: ООО «ЛУКОЙЛ-Нижегороднефтеоргсинтез» (г. Кстово), СИБУР-Нефтехим (г. Дзержинск) и ООО «Газпром трансгаз Нижний Новгород».
В Конгрессе приняли участие 358 представителей российских и зарубежных научных институтов и промышленных организаций из России, Азербайджана, Узбекистана, Белоруссии, Финляндии, Великобритании, Германии и Франции. На Конгрессе было представлено 6 пленарных лекций (40 мин.), 8 ключевых лекций (30 мин.), 4 приглашенных устных доклада (20 мин.), 105 устных докладов (20 мин.), 51 кратких устных докладов (10 мин.), 114 стендовых докладов. 36 молодых ученых, аспирантов и преподавателей ВУЗов представили широкой аудитории свои устные доклады. На двух Круглых столах с устными докладами выступили 14 участников. 35 человек присутствовали на Конгрессе в качестве слушателей: представители организаций Нижегородского региона и представители спонсоров Конгресса.
Конгресс Роскатализ-2017, как крупнейшее всероссийское научное мероприятие, проводился для обсуждения состояния дел во всех областях катализа, выявления актуальных задач для научных исследований и разработок, в том числе в таких приоритетных направлениях как модернизация и инновационное развитие химического комплекса России, глубокая переработка её углеводородных ресурсов. Конгресс придал новый импульс развитию науки и промышленному применению катализаторов и каталитических технологий в нефтепереработке, химии и нефтехимии.
Для поддержки участия молодых учёных и специалистов (студентов и аспирантов) Организационный комитет Конгресса провел конкурс среди аспирантов, студентов и молодых учёных, подавших заявки на участие в работе Молодёжной школы «Физико-химические методы исследования – ключ к пониманию принципов каталитического действия», по итогам которого 25 участников Конгресса, представивших лучшие устные и стендовые доклады, были освобождены от оплаты регистрационного взноса Мероприятия.
Тематика научных секций Конгресса охватила все наиболее важные области фундаментальной науки о катализе и промышленного применения катализаторов:
Секция 1. Физико-химические основы катализа
Секция 2. Научные основы производства катализаторов
Секция 3. Перспективные каталитические процессы
Секция 4. Промышленные катализаторы и каталитические процессы
В рамках Конгресса прошли сателлитные мероприятия – Круглые столы «Образование и катализ» и «Каталитические процессы в газохимии».
Программа Круглого стола «Образование и катализ» включала устные доклады по направлениям:
На Круглом столе «Каталитические процессы в газохимии» обсуждались следующие темы:
В рамках Конгресса была проведена Молодёжная школа «Физико-химические методы исследования – ключ к пониманию принципов каталитического действия». Научная программа школы для молодых учёных, студентов и аспирантов включала 4 пленарные лекции (40 мин.), 16 устных докладов (15 мин.) и 20 стендовых докладов по направлениям:
 В приветственной речи на церемонии открытия председатель программного
комитета конгресса академик РАН Валентин Николаевич Пармон отметил
успешное развитие каталитических технологий в России в последние
годы, а также растущий интерес к российским разработкам в области
катализа со стороны промышленности. «Мы нужны нашей
промышленности и нашему обществу, поэтому мы будем востребованы
всегда», – сказал Валентин Николаевич. Он также отметил
положительные сдвиги в области координации научных
исследований со стороны государства. «Общенациональная
программа по катализу находится на финише первого этапа: в рамках
ФАНО созданы комплексные планы научных исследований. Наш план
«Ресурсо- и энергоэффективные катализаторы и процессы»
охватывает девять институтов, и мы надеемся, что эта консолидация
вовлечет и университеты». Говоря о конгрессе, В.Н. Пармон
подчеркнул значимость выбора русского языка в качестве официального
для форума: «Конгресс делается на русском языке, чтобы усилить
связь между промышленностью и представителями науки, а также чтобы мы
не забывали, что мы можем консолидировать научную и не только научную
деятельность на всей территории бывшего Советского Союза».
В приветственной речи на церемонии открытия председатель программного
комитета конгресса академик РАН Валентин Николаевич Пармон отметил
успешное развитие каталитических технологий в России в последние
годы, а также растущий интерес к российским разработкам в области
катализа со стороны промышленности. «Мы нужны нашей
промышленности и нашему обществу, поэтому мы будем востребованы
всегда», – сказал Валентин Николаевич. Он также отметил
положительные сдвиги в области координации научных
исследований со стороны государства. «Общенациональная
программа по катализу находится на финише первого этапа: в рамках
ФАНО созданы комплексные планы научных исследований. Наш план
«Ресурсо- и энергоэффективные катализаторы и процессы»
охватывает девять институтов, и мы надеемся, что эта консолидация
вовлечет и университеты». Говоря о конгрессе, В.Н. Пармон
подчеркнул значимость выбора русского языка в качестве официального
для форума: «Конгресс делается на русском языке, чтобы усилить
связь между промышленностью и представителями науки, а также чтобы мы
не забывали, что мы можем консолидировать научную и не только научную
деятельность на всей территории бывшего Советского Союза».

 После
открытия Конгресса с презентационным докладом выступил
представитель генерального спонсора С.Ю.
Гурьевских (АО «Газпромнефть-ОМПЗ»). В своем докладе «Пилотная
установка FCC: преимущества, результаты испытаний» он рассказал о новых
возможностях и преимуществах, которые реализуются на современных
НПЗ при использовании пилотных установок для испытаний катализаторов.
Было подробно рассмотрено функционирование пилотной установки
гидрокрекинга, работающей на Омском нефтеперерабатывающем заводе.
После
открытия Конгресса с презентационным докладом выступил
представитель генерального спонсора С.Ю.
Гурьевских (АО «Газпромнефть-ОМПЗ»). В своем докладе «Пилотная
установка FCC: преимущества, результаты испытаний» он рассказал о новых
возможностях и преимуществах, которые реализуются на современных
НПЗ при использовании пилотных установок для испытаний катализаторов.
Было подробно рассмотрено функционирование пилотной установки
гидрокрекинга, работающей на Омском нефтеперерабатывающем заводе.
 Далее
были представлены научные лекции – пленарный доклад и три
ключевые лекции. В пленарном докладе «Применение
масс-спектрометрии ультра-высокого разрешения для анализа сложных
химических смесей», представленном чл.-корр. РАН, д.х.н.
Е.Н. Николаевым
(Сколковский институт науки и технологий, Москва), основное
внимание было уделено основам масс-спектрометрии ультра-высокого
разрешения и ее применению для анализа сложных органических
молекул. Были показаны первые результаты исследования состава
различных нефтей с помощью масс-спектрометрии ультра-высокого
разрешения.
Далее
были представлены научные лекции – пленарный доклад и три
ключевые лекции. В пленарном докладе «Применение
масс-спектрометрии ультра-высокого разрешения для анализа сложных
химических смесей», представленном чл.-корр. РАН, д.х.н.
Е.Н. Николаевым
(Сколковский институт науки и технологий, Москва), основное
внимание было уделено основам масс-спектрометрии ультра-высокого
разрешения и ее применению для анализа сложных органических
молекул. Были показаны первые результаты исследования состава
различных нефтей с помощью масс-спектрометрии ультра-высокого
разрешения.
 Ключевая лекция профессора РАН, д.х.н. О.Н. Мартьянова (Институт
катализа им. Г.К. Борескова СО РАН, Новосибирск) была посвящена
особенностям применения сверхкритических флюидов в
гетерогенном катализе. На ряде примеров была показана
перспективность проведения гетерогенных каталитических процессов при
повышенных температурах и давлениях. Обсуждались
сольватационные эффекты и эффекты локального концентрирования в
присутствии твердой поверхности, которые могут существенно
усложнять гетерогенные
каталитические процессы, стирая грань между гетерогенным и гомогенным
характером каталитического процесса.
Ключевая лекция профессора РАН, д.х.н. О.Н. Мартьянова (Институт
катализа им. Г.К. Борескова СО РАН, Новосибирск) была посвящена
особенностям применения сверхкритических флюидов в
гетерогенном катализе. На ряде примеров была показана
перспективность проведения гетерогенных каталитических процессов при
повышенных температурах и давлениях. Обсуждались
сольватационные эффекты и эффекты локального концентрирования в
присутствии твердой поверхности, которые могут существенно
усложнять гетерогенные
каталитические процессы, стирая грань между гетерогенным и гомогенным
характером каталитического процесса.
 Ключевая лекция к.т.н. В.П. Доронина (Институт проблем переработки углеводородов СО РАН, Омск) была посвящена
анализу мирового рынка и производству современных катализаторов
крекинга нефтяных фракций.
Ключевая лекция к.т.н. В.П. Доронина (Институт проблем переработки углеводородов СО РАН, Омск) была посвящена
анализу мирового рынка и производству современных катализаторов
крекинга нефтяных фракций.
 В ключевой лекции чл.-корр. РАН, д.х.н. С.Д.
Варфоломеева
(Институт биохимической физики им. Н.М. Эмануэля РАН, Москва) были
проанализированы возможности применения суперкомпьютерных
технологий в исследовании механизмов ферментативных
каталитических реакций. Было рассмотрено применение методов
молекулярной механики, молекулярной динамики, квантовой химии для
расшифровки элементарных процессов в каталитическом цикле белкового
катализа, а также применение суперкомпьютерных вычислений в
молекулярной медицине и конструировании лекарств.
В ключевой лекции чл.-корр. РАН, д.х.н. С.Д.
Варфоломеева
(Институт биохимической физики им. Н.М. Эмануэля РАН, Москва) были
проанализированы возможности применения суперкомпьютерных
технологий в исследовании механизмов ферментативных
каталитических реакций. Было рассмотрено применение методов
молекулярной механики, молекулярной динамики, квантовой химии для
расшифровки элементарных процессов в каталитическом цикле белкового
катализа, а также применение суперкомпьютерных вычислений в
молекулярной медицине и конструировании лекарств.
 Во второй день работы
Конгресса были представлены три лекции и ряд устных докладов. С
пленарной лекцией, посвященной катализу на наноструктурированных
и дисперсных системах в нефтехимии и нефтепереработке, выступил
д.х.н., профессор А.Л. Максимов (Институт
нефтехимического синтеза им. А.В. Топчиева РАН, Москва). В докладе
рассматривались особенности поведения наноразмерных каталитических
систем в реакциях гидрирования ароматических и непредельных
соединений, гидродеароматизации и гидрокрекинга газойлевых фракций и
гидродеоксигенации кислородсодержащих соединений. Обсуждались пути
синтеза дисперсных каталитических систем из различных типов
прекурсоров, использование различных вариантов формирования и стабилизации
дисперсий частиц активных компонентов (сульфидов, оксидов, цеолитов),
возможные подходы к повторному использованию диспергированных
катализаторов, возможности применения альтернативных сред в
указанных системах.
Во второй день работы
Конгресса были представлены три лекции и ряд устных докладов. С
пленарной лекцией, посвященной катализу на наноструктурированных
и дисперсных системах в нефтехимии и нефтепереработке, выступил
д.х.н., профессор А.Л. Максимов (Институт
нефтехимического синтеза им. А.В. Топчиева РАН, Москва). В докладе
рассматривались особенности поведения наноразмерных каталитических
систем в реакциях гидрирования ароматических и непредельных
соединений, гидродеароматизации и гидрокрекинга газойлевых фракций и
гидродеоксигенации кислородсодержащих соединений. Обсуждались пути
синтеза дисперсных каталитических систем из различных типов
прекурсоров, использование различных вариантов формирования и стабилизации
дисперсий частиц активных компонентов (сульфидов, оксидов, цеолитов),
возможные подходы к повторному использованию диспергированных
катализаторов, возможности применения альтернативных сред в
указанных системах.

 Академик РАН, д.х.н. А.М. Музафаров (Институт
элементоорганических соединений им. А.Н. Несмеянова РАН, Москва)
представил пленарную лекцию «Макромолекулы-частицы –
новая форма высокомолекулярных соединений».
Академик РАН, д.х.н. А.М. Музафаров (Институт
элементоорганических соединений им. А.Н. Несмеянова РАН, Москва)
представил пленарную лекцию «Макромолекулы-частицы –
новая форма высокомолекулярных соединений».
С ключевой лекцией выступила д.х.н. З.П. Пай (Институт катализа СО РАН, Новосибирск). Ее доклад был посвящен каталитическим процессам малотоннажного производства органических соединений.
 Завершил
утреннюю сессию к.х.н. О.В.
Климов (Институт
катализа СО РАН, Новосибирск) устным приглашенным докладом
«Технология регенерации/реактивации
катализаторов гидроочистки дизельного топлива». Были
представлены полученные в Институте катализа СО РАН научные
результаты, положенные в основу технологии регенерации/реактивации
катализаторов гидроочистки дизельного топлива, приведены
данные, полученные в ходе наработки и эксплуатации промышленной
партии реактивированного катализатора, а также сделана экономическая
оценка разработанной технологии.
Завершил
утреннюю сессию к.х.н. О.В.
Климов (Институт
катализа СО РАН, Новосибирск) устным приглашенным докладом
«Технология регенерации/реактивации
катализаторов гидроочистки дизельного топлива». Были
представлены полученные в Институте катализа СО РАН научные
результаты, положенные в основу технологии регенерации/реактивации
катализаторов гидроочистки дизельного топлива, приведены
данные, полученные в ходе наработки и эксплуатации промышленной
партии реактивированного катализатора, а также сделана экономическая
оценка разработанной технологии.
Устные доклады проводились параллельно на трех секциях. В рамках секции «Физико-химические основы катализа» в этот день было сделано 17 устных докладов. Профессор Д.Ю. Мурзин (Университет Або Академи, Турку, Финляндия) представил доклад «Кинетический анализ влияния растворителя в сложных многостадийных каталитических реакциях» о результатах анализа математических моделей для описания кинетики каталитических реакций, учитывающих свойства растворителя (диэлектрическую проницаемость). Тему кинетических исследований продолжила к.х.н. Л.Б. Охлопкова (Институт катализа СО РАН, Новосибирск). Ее доклад был посвящен исследованию кинетических закономерностей реакции гидрирования диметилэтинилкарбинола в микрокапиллярном реакторе на Pd-Zn катализаторах. Далее д.х.н., профессор Л.Г. Брук (Московский технологический университет, Москва) рассказал о кинетике и механизме эпоксидирования аллилового спирта пероксидом водорода на титансиликалите TS-1. К.х.н. А.А. Барабанов (Институт катализа СО РАН, Новосибирск) представил результаты изучения кинетики полимеризации этилена и сополимеризации этилена с гексеном-1 на титанмагниевых катализаторах. Используя метод ингибирования полимеризации радиоактивным монооксидом углерода, автор исследовал влияние водорода и гексена-1 (в качестве сомономера) на число активных центров и константы скорости роста при полимеризации этилена на титан-магниевых катализаторах различного состава. К.х.н. Е.Е. Файнгольд (Институт проблем химической физики РАН, Черноголовка) также рассказал об исследованиях процессов полимеризации. Его доклад был посвящен использованию металлоценовых каталитических систем полимеризации олефинов и диенов, содержащих арилоксиды изобутилалюминия в качестве новых активаторов. К.х.н. Л.А. Ришина (Институт химической физики им. Н.Н. Семенова РАН, Москва) представила доклад о новой эффективной каталитической системе полимеризации олефинов на основе Ti(Oiso-C3H7)4, показав преимущества данной каталитической системы по сравнению с металлоценовыми и постметаллоценовыми системами. К.х.н. Д.А. Свинцицкий (Институт катализа СО РАН, Новосибирск) рассказал о каталитических свойствах частиц оксида меди в реакции окисления монооксида углерода. Завершил утреннюю сессию доклад к.х.н. Л.В. Синевой (Технологический институт сверхтвердых и новых углеродных материалов, Москва), в котором был рассмотрен вклад воды во вторичные превращения углеводородов в синтезе Фишера–Тропша.
Вечерняя сессия состояла из двух 20-минутных устных докладов и семи 10-минутных устных докладов. Первым выступил д.х.н., профессор В.Е. Гутерман (Южный федеральный университет, Ростов-на-Дону) с докладом «Стабильность и активность нанесенных Pt/C и Pt-M/C электрокатализаторов для низкотемпературных топливных элементов: роль морфологии и архитектуры наночастиц». Доклад был посвящен изучению характера корреляции между величинами активности и стабильности для различных, в том числе коммерческих, Pt/C и некоторых биметаллических Pt-M/C (M = Cu, Co) электрокатализаторов, полученных методами совместного или последовательного химического восстановления прекурсоров металлов на микрочастицах углеродного носителя Vulcan XC 72. Второй доклад также был посвящен электрокатализу – «Катализаторы электрохимического окисления водорода, содержащие палладий и золото: исследование влияния состава биметаллических частиц на каталитическую активность и толерантность к СО». Его представил П.А. Пыряев (Институт катализа СО РАН, Новосибирск). Далее шли короткие устные доклады: Н.Д. Евдокименко (Российский химико-технологический университет им. Д.И. Менделеева, Москва) «Каталитические и адсорбционные свойства наночастиц золота размером 4.6 нм и 19.4 нм, полученные цитратным методом», В.В. Сукулова (Институт катализа СО РАН, Новосибирск) «Исследование процесса формирования поверхностных титан-алкильных соединений – предшественников активных центров нанесенных титанмагниевых катализаторов полимеризации олефинов», П.М. Недорезова (Институт химической физики им. Н.Н. Семенова РАН, Москва) «Сополимеризация пропилена с метилвинилкетоном или его полимеризация в присутствии полилактида – перспективный путь к созданию полипропилена с контролируемой скоростью деструкции». В.В. Болтенков (Институт катализа СО РАН, Новосибирск) представил результаты сравнительного исследования пероксидного окисления метана, метанола и муравьиной кислоты в присутствии катализаторов Fe-ZSM-5. О.Э. Зиядуллаев (Ташкентский химико-технологический институт, Ташкент, Узбекистан) рассказал о гомогенном и гетерогенном каталитическом винилировании ацетиленовых спиртов. Н.М. Максимов (Самарский государственный технический университет, Самара) представил результаты исследования кинетических особенностей реакций гидродесульфуризации легкого газойля каталитического крекинга. В заключительном докладе к.х.н. Д.П. Иванов (Институт катализа СО РАН, Новосибирск) рассказал о влиянии морфологии цеолита MFI на его каталитические свойства в реакции гидроксилирования фенола закисью азота.
Вторая секция «Научные основы производства катализаторов» проходила под председательством д.х.н. Б.С. Бальжинимаева и д.х.н. А.А. Пимерзина. Секция была посвящена особенностям синтеза катализаторов для различных процессов – гидрирования, дегидрирования, кислородной и углекислотной конверсии углеводородов в синтез-газ, процесса Фишера-Тропша и других. Большой интерес вызвали доклады, посвященные новым подходам к синтезу катализаторов и перспективным процессам на их основе. Например, доклад д.х.н. О.П. Паренаго (Институт нефтехимического синтеза им. А.В. Топчиева РАН, Москва) был посвящен нанокатализаторам гидрирования, полученным в сверхкритических средах, которые обладают высокой активностью и селективностью. Интересный подход по выделению катализаторов из реакционной среды был предложен коллективом авторов из научной школы д.х.н., профессора Э.М. Сульман (Тверской государственный технический университет, Тверь). В ее докладе рассматривались перспективы использования магнитно-разделяемых палладиевых катализаторов в реакциях селективного гидрирования. Доклад д.х.н. С.Ф. Тихова (Институт катализа СО РАН, Новосибирск) был посвящен получению и применению в процессах окисления пористых керамометаллических микрооболочечных носителей и катализаторов на основе порошкообразных Me-Al (Me = Fe, Cu, Cr, Co) сплавов, полученных механохимической обработкой. Вечернюю секцию проводили д.х.н. Е.З. Голосман и д.х.н. А.В. Романенко, которые отметили ряд интересных докладов, представших, например, новые подходы к синтезу катализаторов и адсорбентов для переработки жидкого и газообразного углеводородного сырья (д.т.н. М.П. Юнусов, УзКФИТИ, Ташкент, Узбекистан), исследование кобальтовых катализаторов гидролиза боргидрида натрия, восстановленных in situ в реакционной среде гидрида (к.х.н. О.В. Нецкина, Институт катализа СО РАН, Новосибирск).
Третья секция была посвящена перспективным каталитическим процессам. Утреннюю секцию проводили д.х.н. А.М. Музафаров и д.х.н. М.Ю. Синев. Были представлены доклады, в которых в основном описывались процессы переработки растительного сырья, такие как гидрирование фурфурола, риформинг лигнина, гидрогенолиз целлюлозы, изомеризация эпоксида альфа-пинена в альдегиды. Следует отметить высокий уровень докладов д.х.н. Б.Н. Кузнецова (Институт химии и химической технологии СО РАН, Красноярск), который подробно рассмотрел возможности оптимизации интегрированных процессов каталитической переработки низкосортной древесины, и д.х.н. О.П. Таран (Институт катализа СО РАН, Новосибирск), чье выступление было посвящено жидкофазным каталитическим процессам переработки основных компонентов лигноцеллюлозной биомассы в ценные химические продукты. Хотелось бы также отметить доклад к.х.н. П. Мерчински (Лодзинский технический университет, Польша), выступление которого было посвящено изучению процесса Фишера-Тропша на катализаторах Fe-Ru/Al2O3-Cr2O3 в качестве эффективного способа получения топливных компонентов. Во второй половине дня заседание секции вели к.х.н. М.Д. Смоликов и д.х.н. О.П. Таран; были представлены как полновесные 20-минутные доклады, так и короткие 10-минутные презентации. Интересные устные доклады были сделаны к.х.н. Н.В. Смирновой (Южно-Российский политехнический университет, Новочеркасск) о каталитических процессах получения 5-гидроксиметилфурфурола и некоторых его производных из сырья растительного происхождения, а также К.А. Сашкиной (Институт катализа СО РАН, Новосибирск), которая рассказала о гетерогенных катализаторах Фентона на основе Fe-силикалита-1 для выделения радиокобальта из хелатов с ЭДТА. Из коротких докладов следует выделить сообщения, сделанные Д.Д. Уваркиной (Институт катализа СО РАН, Новосибирск) о влиянии соотношения Si/Al в исходном цеолите на свойства получаемого дизельного топлива в процессе гидроизодепарафинизации на конкретно выбранном катализаторе, и А.С. Коклюхиным (Самарский государственный технический университет, Самара) об использовании триметаллических NiCoMoS катализаторов в совместной гидроочистке растительного и нефтяного сырья.
В завершении второго дня Конгресса были проведены два параллельных круглых стола «Образование и катализ» и «Каталитические процессы в газохимии». В рамках круглого стола «Образование и катализ» был рассмотрен опыт преподавания курса «Термодинамика функционирующего катализатора» и мотивационного курса «Катализ, окружающая среда и устойчивое развитие» в Новосибирском государственном университете. Доклад сделал академик В.Н. Пармон (Институт катализа СО РАН, Новосибирск). Д.х.н. М.Ю. Синев (Институт химической физики РАН, Москва) рассказал о лекционном курсе по катализу в рамках аспирантской программы компании Хальдор Топсё, который проводится в Институте химической физики имени Н.Н. Семенова РАН, Москва. К.х.н. И.А. Касьянов (МГУ имени М.В. Ломоносова, Москва) рассказал об опыте преподавания катализа на кафедре физической химии МГУ имени М.В. Ломоносова. Д.х.н. Е.С. Локтева (МГУ имени М.В. Ломоносова, Москва) поделилась опытом участия в образовательной, научной и организационной деятельности Международного союза теоретической и прикладной химии (ИЮПАК). Интересное сообщение об использовании последних достижений науки в базовом курсе гетерогенного катализа для инженеров-химиков было сделано д.х.н. Д.Ю. Мурзиным (Университет Або Академи, Турку, Финляндия). В целом дискуссия получилась бурная и вышла за рамки отведенных полутора часов.
На Круглом столе «Каталитические процессы в газохимии» обсуждались каталитические процессы переработки природного газа, окислительное дегидрирование легких углеводородов и перспективы развития катализаторов и процессов газохимии в России. Наиболее интересный доклад был сделан д.х.н. А.Л. Максимовым (Институт нефтехимического синтеза им. А.В. Топчиева РАН, Москва), который рассказал о перспективных путях наращивания углеводородного скелета с использованием синтез-газ в качестве исходного сырья. Отмечалась перспектива получения легких оксигенатов как строительный блок для дальнейшего получения топлив.
 Третий день работы Конгресса
также был открыт пленарной лекцией, двумя ключевыми докладами и
приглашенным устным докладом. Пленарную лекцию «Сульфиды
переходных металлов в гидропереработке, электрокатализе и
фотокатализе: общие черты и различия; модели и реальность»
представил профессор П.В.
Афанасьев из
Лионского университета (Université
Claude
Bernard
Lyon,
Лион, Франция). Был дан критический обзор современного состояния
исследований активных фаз сульфидов переходных металлов, с упором на
работы нескольких последних лет, выполненных с участием докладчика.
Третий день работы Конгресса
также был открыт пленарной лекцией, двумя ключевыми докладами и
приглашенным устным докладом. Пленарную лекцию «Сульфиды
переходных металлов в гидропереработке, электрокатализе и
фотокатализе: общие черты и различия; модели и реальность»
представил профессор П.В.
Афанасьев из
Лионского университета (Université
Claude
Bernard
Lyon,
Лион, Франция). Был дан критический обзор современного состояния
исследований активных фаз сульфидов переходных металлов, с упором на
работы нескольких последних лет, выполненных с участием докладчика.
 Ключевую лекцию «Катализаторы
процессов получения алкилароматичеких соединений» прочитал
д.х.н. Н.Ю. Адонин
(Институт катализа СО РАН, Новосибирск). Основной акцент был
сделан на обзоре алкилароматических продуктов, представляющих
практический интерес, и методах их получения. Автор провел
сравнительный анализ применяемых кислотных катализаторов и условий
осуществления реакций алкилирования в их присутствии, а также
рассмотрел основные тенденции в развитии процессов алкилирования.
Ключевую лекцию «Катализаторы
процессов получения алкилароматичеких соединений» прочитал
д.х.н. Н.Ю. Адонин
(Институт катализа СО РАН, Новосибирск). Основной акцент был
сделан на обзоре алкилароматических продуктов, представляющих
практический интерес, и методах их получения. Автор провел
сравнительный анализ применяемых кислотных катализаторов и условий
осуществления реакций алкилирования в их присутствии, а также
рассмотрел основные тенденции в развитии процессов алкилирования.
 Вторая
ключевая лекция была посвящена применению in
situ
методов в гетерогенном катализе. Ее представил к.ф.-м.н. В.В.
Каичев (Институт
катализа СО РАН, Новосибирск). Был приведен ряд примеров
использования методов РФЭС, РФА и колебательной спектроскопии для in
situ исследований окисления спиртов на поверхности ванадий-титановых
катализаторов, а также окисления метана и пропана на поверхности
никеля.
Вторая
ключевая лекция была посвящена применению in
situ
методов в гетерогенном катализе. Ее представил к.ф.-м.н. В.В.
Каичев (Институт
катализа СО РАН, Новосибирск). Был приведен ряд примеров
использования методов РФЭС, РФА и колебательной спектроскопии для in
situ исследований окисления спиртов на поверхности ванадий-титановых
катализаторов, а также окисления метана и пропана на поверхности
никеля.
 В заключение утренней сессии профессор
К. Нейман
(Барселонский университет, Барселона, Испания) сделал доклад на тему
«Компьютерное конструирование биметаллических
катализаторов для ускорения их целенаправленного приготовления»,
рассказав об оригинальном методе для определения
химического порядка в биметаллических наночастицах на основе
расчётов методом функционала плотности.
В заключение утренней сессии профессор
К. Нейман
(Барселонский университет, Барселона, Испания) сделал доклад на тему
«Компьютерное конструирование биметаллических
катализаторов для ускорения их целенаправленного приготовления»,
рассказав об оригинальном методе для определения
химического порядка в биметаллических наночастицах на основе
расчётов методом функционала плотности.
Устные доклады проводились параллельно на трех секциях. На заседании первой секции, «Физико-химические основы катализа», в этот день было представлено 16 устных докладов. Первый доклад сделал д.х.н., профессор В.А. Садыков (Институт катализа СО РАН, Новосибирск). Тема его доклада – «Механизм трансформации этанола в синтез-газ на шпинели MnCr2O4, промотированной Pt, Ru, Ni+Ru: исследования in situ и квантово-химический анализ». Далее выступил к.х.н. Ю.А. Чесалов (Институт катализа СО РАН, Новосибирск) с докладом «In situ исследование механизма образования никотиновой и изоникотиновой кислот при газофазном окислении β- и γ-пиколина на ванадий-титановых оксидных катализаторах». Автор показал, что окисление β-пиколина протекает согласно модифицированному механизму Марса – ван Кревелена. Реокисление катализатора и выделение продукта происходят в совмещенной стадии. Никотинат, координированный к восстановленному ванадиевому центру, является прочносвязанным и не превращается в никотиновую кислоту. Никотинат, связанный с реокисленным ванадиевым центром, становится слабосвязанным и легко превращается в кислоту. Следующие два докладчика представляли Московский государственный университет. К.х.н. М.И. Шилина рассказала об исследованиях полиядерных катионных комплексов кобальта в каталитическом окислении монооксида углерода. Д.х.н. Д.А. Пичугина представила результаты квантовохимических расчетов структурных и электронных эффектов диссоциации кислорода на кластерах золота и серебра. Доклад к.х.н. Н.В. Дохликовой (Институт химической физики им. Н.Н. Семенова РАН, Москва) также был посвящен квантовохимическим исследованиям – в приближении функционала электронной плотности исследовано влияние адсорбции атомарного водорода на электронное и атомное строение нанокластеров золота Aun с размерами 0.6, 0.8, 1, 1.4 и 1.5 нм (n = 13, 20, 55, 100 и 147). Установлено, что возмущение, обусловленное адсорбцией водорода, локализовано в области порядка длины связи Au-Au, и процесс последовательной адсорбции атомов водорода слабо коррелирован. Влияние ранее адсорбированных атомов связано, в основном, с перестройкой делокализованных s-состояний золота, дающих относительно небольшой вклад в энергию взаимодействия Au-H. При этом тенденция некоторого уменьшения энергии связи Au-H с ростом числа атомов водорода обусловлена сдвигом d-зоны в область меньших энергий. Продолжил тему теоретических исследований д.х.н. Р.С. Шамсиев (Московский технологический университет, Москва). В его докладе были представлены результаты квантовохимического исследования механизма гидрирования фенилацетилена на поверхности палладиевых частиц. К.х.н. С.С. Лалетина (Институт химии и химической технологии СО РАН, Красноярск) рассказала о результатах теоретического исследования дегидрирования метанола на кластерах платины. Закрыл утреннюю сессию к.х.н. А.Г. Шамов (Казанский национальный исследовательский технологический университет, Казань) докладом «Квантовохимическое исследование акватермолиза органических сульфидов и тиофена».
Вечерняя сессия состояла из трех 20-минутных устных докладов и пяти 10-минутных устных докладов. Первые два докладчика были из Тверского государственного технического университета: к.х.н. В.Ю. Долуда с докладом «Изучение Zn/Cu смешенных оксидных катализаторов на основе сверхсшитого полистирола методом XAS» и А.Е. Филатова, рассказавшая о результатах кинетического исследования гидрирования нитробензола. Доклад к.т.н. В.В. Скудина (Российский химико-технологический университет им. Д.И. Менделеева, Москва) был посвящен Кнудсеновскому режиму транспорта в мембранных катализаторах. Д.х.н. А.Ф. Шмидт (Иркутский государственный университет, Иркутск) сделал доклад «Операндо исследование процессов превращения катализатора за пределами каталитического цикла реакции арилирования алкенов ангидридами ароматических кислот». К.х.н. Л.С. Кибис (Институт катализа СО РАН, Новосибирск) представила результаты исследования окислительно-восстановительных свойств поверхности катализаторов на основе Rh/CeOx. О.А. Кунгурова (Институт катализа СО РАН, Новосибирск) рассказала о синтезе и каталитических свойствах Ru-промотированных кобальталюминиевых катализаторов синтеза Фишера-Тропша. А.А. Мерк (Томский государственный университет, Томск) представила результаты исследования влияния соединений Cu и Zn на структуру и свойства алюмохромовых катализаторов дегидрирования легких парафинов. Заключительный доклад на тему «Синтез цирконий содержащих молибдат- и ванадат-фосфатов, их каталитические свойства в превращениях метанола» сделал А.С. Шипилов из Нижегородского государственного университета им. Н.И. Лобачевского (Нижний Новгород).
В рамках утренней секции № 2
«Научные основы производства катализаторов», которую вели
д.х.н. О.П. Паренаго и
д.т.н. В.М.
Воротынцев, были
представлены разнообразные доклады, освещающие традиционные области
приготовления катализаторов, такие как синтез и свойства
нанесенных сульфидных вольфрамсодержащих катализаторов
гидропереработки нефтяных фракций (В.Ю. Перейма, Институт катализа СО
РАН), так и новые перспективные подходы синтеза, например, подходы
для синтеза полифункциональных материалов с использованием
сверхкритических флюидов. Был представлен доклад, где описывается
применение углеродных материалов как носителей катализаторов и
обсуждается влияние свойств поверхности наноалмаза на формирование
нанесенных частиц металла и их каталитическую активность (к.х.н. Е.В.
Голубина, МГУ имени
М.В. Ломоносова).
 Интересный доклад был сделан к.х.н. В.Ю.
Бычковым (Институт
химической физики РАН, Москва) об эффекте ускоренного развития
поверхности Ni и Pd и увеличения их каталитической активности под
действием автоколебаний. После обеда секцию вели д.х.н. Л.А.
Исупова
и д.х.н. Э.М. Сульман,
которые выделили целый ряд интересных докладов, такие как доклад
к.х.н. А.В. Нартовой
(Институт катализа СО
РАН) о влиянии условий приготовления на характеристики
алюмоплатиновых катализаторов, к.х.н. О.В.
Комовой (Институт
катализа СО РАН) о влиянии режимов горения биметаллических
глицин-нитратных предшественников на фазовый состав образующихся
продуктов, О.В. Джикия
(Институт проблем
переработки углеводородов СО РАН, Омск) об исследовании влияния
содержания палладия и условий активации катализаторов на показатели в
реакции изомеризации н-гексана.
Интересный доклад был сделан к.х.н. В.Ю.
Бычковым (Институт
химической физики РАН, Москва) об эффекте ускоренного развития
поверхности Ni и Pd и увеличения их каталитической активности под
действием автоколебаний. После обеда секцию вели д.х.н. Л.А.
Исупова
и д.х.н. Э.М. Сульман,
которые выделили целый ряд интересных докладов, такие как доклад
к.х.н. А.В. Нартовой
(Институт катализа СО
РАН) о влиянии условий приготовления на характеристики
алюмоплатиновых катализаторов, к.х.н. О.В.
Комовой (Институт
катализа СО РАН) о влиянии режимов горения биметаллических
глицин-нитратных предшественников на фазовый состав образующихся
продуктов, О.В. Джикия
(Институт проблем
переработки углеводородов СО РАН, Омск) об исследовании влияния
содержания палладия и условий активации катализаторов на показатели в
реакции изомеризации н-гексана.
Утреннюю секцию «Перспективные каталитические процессы» вели к.х.н. О.В. Климов и к.х.н. А.В. Лавренов. Из представленных докладов следует выделить презентацию А.С. Лядова (Институт нефтехимического синтеза им. А.В. Топчиева РАН, Москва), посвященную получению органических карбонатов из многоатомных спиртов и карбамида, и доклад к.х.н. И.Л. Симаковой (Институт катализа СО РАН) о новом перспективном направлении получения жирных спиртов через конденсацию пентанола по реакции Гербе-Марковникова на металлах VIII группы. Промышленное внедрение могут получить результаты работы д.х.н. Н.И. Кузнецовой (Институт катализа СО РАН), которая представила доклад о стратегии в производстве терефталевой кислоты с низким содержанием п-карбокси бензальдегида. При этом обсуждалось применение ацетата аммония и ионных жидкостей в синтезе и окислительной очистке. Фундаментальный интерес представляет работа М.В. Грабченко (Томский государственный университет), которая представила доклад «Исследование роли взаимодействия Ag-CeO2 в Ag/CeO2 и Ag/CeO2/SiO2 катализаторах в реакции окислительного дегидрирования этанола». Во второй половине дня работу секции организовывали д.х.н. И.Л. Новокшонова и к.х.н. И.Л. Симакова. В рамках этой секции О.Б. Бельская (Институт проблем переработки углеводородов СО РАН, Омск) рассказала о роли начальных стадий синтеза катализаторов в формировании свойств нанесенного металла Pt(Pd) на носителях различной природы. Другой представитель омской каталитической школы, к.х.н. Р.М. Мироненко, обсудил результаты селективного каталитического гидрирования фурфурола до циклопентанола. О практически перспективном процессе получения из угля синтетических реактивных топлив, взаимозаменяемых с нефтяными топливами типа Джет А-1 и Т-8В, рассказал к.х.н. А.Б. Куликов из Института нефтехимического синтеза им. А.В. Топчиева РАН, Москва.
 Четвертый
день работы Конгресса
был открыт пленарной лекцией профессора И.В.
Кожевникова из
Ливерпульского Университета (Ливерпуль,
Великобритания), за которой последовали ключевой доклад профессора
Е.С. Локтевой из Московского государственного университета имени М.В.
Ломоносова и приглашенный устный доклад профессора Е.В. Кондратенко
из Института катализа при университете Ростока (Германия). Пленарная
лекция была посвящена применению гетерополикислот в
катализе. Профессор И.В. Кожевников рассмотрел научные основы и
промышленное использование гетерополикислот.
Четвертый
день работы Конгресса
был открыт пленарной лекцией профессора И.В.
Кожевникова из
Ливерпульского Университета (Ливерпуль,
Великобритания), за которой последовали ключевой доклад профессора
Е.С. Локтевой из Московского государственного университета имени М.В.
Ломоносова и приглашенный устный доклад профессора Е.В. Кондратенко
из Института катализа при университете Ростока (Германия). Пленарная
лекция была посвящена применению гетерополикислот в
катализе. Профессор И.В. Кожевников рассмотрел научные основы и
промышленное использование гетерополикислот.
 Профессор
Е.С. Локтева
представила доклад на тему «Взаимодействия металлов с
углеродной подложкой в катализе». В докладе на базе
литературных данных и собственных результатов был проведен анализ
взаимодействий между переходными металлами и углеродными материалами
в составе носителей/углеродных отложений, и выявлены направления
воздействия на каталитические свойства.
Профессор
Е.С. Локтева
представила доклад на тему «Взаимодействия металлов с
углеродной подложкой в катализе». В докладе на базе
литературных данных и собственных результатов был проведен анализ
взаимодействий между переходными металлами и углеродными материалами
в составе носителей/углеродных отложений, и выявлены направления
воздействия на каталитические свойства.
 Профессор Е.В.
Кондратенко в своем
докладе рассмотрел альтернативные технологии и катализаторы
для целенаправленного производства пропилена.
Профессор Е.В.
Кондратенко в своем
докладе рассмотрел альтернативные технологии и катализаторы
для целенаправленного производства пропилена.
Устные доклады проводились параллельно на трех секциях. В этот день было представлено 10 устных докладов. Д.х.н., профессор В.А. Собянин из Института катализа СО РАН (Новосибирск) представил доклад на тему «Газофазное карбонилирование диметоксиметана в метилметоксиацетат на твердых кислотах: влияние кислотности на каталитическую активность». М.C. Никульшина из Самарского государственного технического университета (Самара) рассказала о синергетическом эффекте Mo-W в полиметаллических (Ni)WMo/Al2O3 катализаторах гидроочистки. П.С. Солманов из Самарского государственного технического университета (Самара) представил результаты исследования влияния содержания фосфора в носителе P-Ni-Mo-W катализаторов на морфологию активной фазы и каталитическую активность в реакции гидрогенолиза дибензотиофена. Доклад Е.Д. Сущенко из Томского государственного университета (Томск) был посвящен исследования влияния соотношения Mg:V в нанесенных MgO-V2O5/γ-Al2O3 катализаторах на состав и строение поверхностных ванадатов магния и их каталитические свойства в реакции окислительного дегидрирования пропана. И.Г. Тарханова из Московского государственного университета им. М.В. Ломоносова (Москва) представила доклад на тему «Селективное галогенирование и окисление органических соединений, катализируемое иммобилизованными ионными жидкостями». А.Б. Ильин из Института нефтехимического синтеза им. А.В. Топчиева РАН (Москва) рассказал о применении каркасных катализаторов со структурой NASICON для каталитического превращения спиртов. Д.х.н. В.Р. Флид из Московского технологического университета (Москва) рассмотрел в своем докладе ключевые интермедиаты в катализируемых никелем и палладием реакциях с участием норборнадиена. С.Е. Шуляка из Российского химико-технологического университета им. Д.И. Менделеева (Москва) представила результаты исследования окисления смеси ксилолов в присутствии солей переходных металлов. Д.Ю. Эберт из Томского государственного университета (Томск) рассказал о своем исследовании нанесенных Mo-Fe-O/SiO2 катализаторов окисления пропиленгликоля. Заключительный доклад сделал А.С. Чикунов из Института катализа СО РАН (Новосибирск) на тему «Стабилизированные крахмалом микрогетерогенные коллоидные Mn, Fe, Co и Co катализаторы реакции окисления воды в присутствии комплекса трехвалентного трисбипиридил рутения как функциональная модель кислородвыделяющего комплекса фотосистемы II».
Утренняя секция № 3 «Перспективные каталитические процессы» под председательством д.х.н. Н.Ю. Адонина и д.т.н. А.А. Ламберова была представлена радом интересных докладов, посвященных, например, риформеру дизельного топлива для энергоустановок на основе ТОТЭ (к.х.н. П.В. Снытников, Институт катализа СО РАН), высокоактивным сульфидным катализаторам для гидрогенизационных процессов нефтепереработки (д.х.н. А.А. Пимерзин, Самарский государственный технический университет). Д.х.н. А.В. Восмериков (Институт химии нефти СО РАН, Томск) представил обстоятельный доклад о применении псевдобемита и гидраргиллита в качестве компонентов цеолитсодержащих катализаторов облагораживания прямогонных бензиновых фракций нефти. Интересным с практической точки зрения был доклад к.т.н. А.И. Грудановой (ОАО «ВНИИ НП» Москва) о пилотных испытаниях катализатора изодепарафинизации среднедистиллятных фракций для получения дизельного топлива для арктических условий и авиационного керосина.

 B этот же день была проведена секция «Промышленные катализаторы и
каталитические процессы», которая включила цикл докладов о
практическом применении каталитических наработок в промышленности
РФ. В рамках данной секции представители ряда крупнейших предприятий
России сделали доклады о практике применения новых катализаторов в
своем производстве. Так, например, к.х.н. И.Н.
Воропаев рассказал об
особенностях выбора катализаторов в компании СИБУР: критерии,
подходы, результаты. Директор Салаватского катализаторного
завода Д.А. Медведев
поделился опытом разработки, внедрения и опытно-промышленных
испытаний новых адсорбентов для подготовки газа.
К.т.н. В.Б. Сиднев
из ОАО НИИ «Ярсинтез» представил опыт промышленной
эксплуатации Российского катализатора дегидрирования
этилбензола. Тема прямого
гетерогенно-каталитического окисления сероводорода для очистки
попутных нефтяных газов была освещена к.х.н. С.Р.
Хайрулиным как опыт
внедрения разработки Института катализа АО «СМП-НЕФТЕГАЗ»,
Альметьевск. Д.И.
Кирьянов (Институт
проблем переработки углеводородов СО РАН, Омск) представил новый
процесс переработки бензиновых фракций для производства автобензинов
Евро-5, 6 и перспективных классов.
B этот же день была проведена секция «Промышленные катализаторы и
каталитические процессы», которая включила цикл докладов о
практическом применении каталитических наработок в промышленности
РФ. В рамках данной секции представители ряда крупнейших предприятий
России сделали доклады о практике применения новых катализаторов в
своем производстве. Так, например, к.х.н. И.Н.
Воропаев рассказал об
особенностях выбора катализаторов в компании СИБУР: критерии,
подходы, результаты. Директор Салаватского катализаторного
завода Д.А. Медведев
поделился опытом разработки, внедрения и опытно-промышленных
испытаний новых адсорбентов для подготовки газа.
К.т.н. В.Б. Сиднев
из ОАО НИИ «Ярсинтез» представил опыт промышленной
эксплуатации Российского катализатора дегидрирования
этилбензола. Тема прямого
гетерогенно-каталитического окисления сероводорода для очистки
попутных нефтяных газов была освещена к.х.н. С.Р.
Хайрулиным как опыт
внедрения разработки Института катализа АО «СМП-НЕФТЕГАЗ»,
Альметьевск. Д.И.
Кирьянов (Институт
проблем переработки углеводородов СО РАН, Омск) представил новый
процесс переработки бензиновых фракций для производства автобензинов
Евро-5, 6 и перспективных классов.
 Заключительный, пятый день
Конгресса был открыт
пленарной лекцией д.х.н., профессора А.В.
Романенко из
Института катализа СО РАН (Новосибирск), ключевым докладом О.В.
Гиязова из ООО «РРТ»
(Санкт-Петербург) и приглашенным устным докладом А.Н.
Озерина из Института
синтетических полимерных материалов им. Н.С. Ениколопова РАН
(Москва). Пленарная лекция была посвящена вопросам применения
углеродных материалов в катализе. О.В. Гиязов в своей ключевой лекции
рассказал о новом реакционно-ректификационном отечественном
процессе изомеризации бензиновых фракций. А.Н. Озерин нацелил
свой доклад на ознакомление профессиональной аудитории с современным
состоянием дел, связанных с разработкой новой технологии переработки
СВМПЭ в высокопрочные и высокомодульные пленки и пленочные нити и
представил требования к структуре и морфологии РП СВМПЭ,
обеспечивающие достижение наиболее высоких упруго-прочностных
характеристик получаемых по этой технологии ориентированных
материалов.
Заключительный, пятый день
Конгресса был открыт
пленарной лекцией д.х.н., профессора А.В.
Романенко из
Института катализа СО РАН (Новосибирск), ключевым докладом О.В.
Гиязова из ООО «РРТ»
(Санкт-Петербург) и приглашенным устным докладом А.Н.
Озерина из Института
синтетических полимерных материалов им. Н.С. Ениколопова РАН
(Москва). Пленарная лекция была посвящена вопросам применения
углеродных материалов в катализе. О.В. Гиязов в своей ключевой лекции
рассказал о новом реакционно-ректификационном отечественном
процессе изомеризации бензиновых фракций. А.Н. Озерин нацелил
свой доклад на ознакомление профессиональной аудитории с современным
состоянием дел, связанных с разработкой новой технологии переработки
СВМПЭ в высокопрочные и высокомодульные пленки и пленочные нити и
представил требования к структуре и морфологии РП СВМПЭ,
обеспечивающие достижение наиболее высоких упруго-прочностных
характеристик получаемых по этой технологии ориентированных
материалов.
Устные доклады проводились параллельно на трех секциях. На заседании секции «Физико-химические основы катализа» в этот день было представлено 9 устных докладов. Первый доклад сделала д.х.н. Е.Г. Жижина из Института катализа СО РАН (Новосибирск) на тему «Высокоэффективные катализаторы на основе гетерополикислот для окисления органических соединений в жидкой фазе». Следующим докладчиком был А.В. Чистяков из Института нефтехимического синтеза им. А.В. Топчиева РАН (Москва). Тема его доклада – «Превращение сверхкритических этанола и изопропанола в бутанол-1 и пентанол-2 в присутствии биметаллических золотосодержащих катализаторов». Далее выступила Т.Н. Ростовщикова из Московского государственного университета имени М.В. Ломоносова с докладом «Влияние соотношения СО:О2 и степени заполнения поверхности наночастицами Pt, осажденными лазерным электродиспергированием, на свойства Pt/Al2O3 катализаторов в окислении СО». М.В. Гришин из Института химической физики им. Н.Н. Семенова РАН (Москва) представил доклад «Управление адсорбционными свойствами нанесенных наночастиц: влияние электрического потенциала». С.К. Игнатов из Нижегородского государственного университета им. Н.И. Лобачевского (Нижний Новгород) рассказал об изучении реакции и динамики водорода на химически модифицированных наночастицах платины и их агрегатах в электрическом поле. Следующим выступал д.х.н. Х.Э. Харлампиди из Казанского национального исследовательского технологического университета (Казань) с докладом «Непереходные металлы – катализаторы окисления алкилароматических углеводородов». Продолжил выступления к.х.н. А.В. Бухтияров из Института катализа СО РАН (Новосибирск) с докладом «Биметаллические Pd-Pt/Al2O3 катализаторы полного окисления метана: in situ РФЭС исследование». В заключение работы сессии прозвучали два коротких устных доклада. С.Ю. Братская из Института химии ДВО РАН (Владивосток) представила доклад «Влияние характеристик полимерной матрицы на активность палладий- и никельсодержащих катализаторов гидрирования и гидродехлорирования». М.В. Магомедова из Института нефтехимического синтеза им. А.В. Топчиева РАН (Москва) представила доклад «Синтез этилена и пропилена из ДМЭ в различных реакционных средах: вопросы химии и технологии».
 Секция
«Промышленные катализаторы и каталитические процессы»
продолжила свою работу в формате устных докладов, из которых хотелось
бы выделить доклад д.т.н. А.А.
Ламберова (ФГАОУ ВО
«Казанский (Приволжский) федеральный университет») о
катализаторах нефтехимии с рассмотрением некоторых аспектов
соединения теории и практики. К.х.н. М.Д.
Смоликов (Институт
проблем переработки углеводородов СО РАН, Омск) рассказал об опыте
промышленной эксплуатации катализаторов риформинга серии ПР. Аспирант
А.В. Федоров
из Института катализа СО РАН поделился новыми разработками в области
исследования сферических катализаторов глубокого окисления для
процесса сжигания бурого угля в кипящем слое, которые являются
продолжением многолетних исследований каталитических генераторов
тепла, проводимых в ИК СО РАН.
Секция
«Промышленные катализаторы и каталитические процессы»
продолжила свою работу в формате устных докладов, из которых хотелось
бы выделить доклад д.т.н. А.А.
Ламберова (ФГАОУ ВО
«Казанский (Приволжский) федеральный университет») о
катализаторах нефтехимии с рассмотрением некоторых аспектов
соединения теории и практики. К.х.н. М.Д.
Смоликов (Институт
проблем переработки углеводородов СО РАН, Омск) рассказал об опыте
промышленной эксплуатации катализаторов риформинга серии ПР. Аспирант
А.В. Федоров
из Института катализа СО РАН поделился новыми разработками в области
исследования сферических катализаторов глубокого окисления для
процесса сжигания бурого угля в кипящем слое, которые являются
продолжением многолетних исследований каталитических генераторов
тепла, проводимых в ИК СО РАН.
26 мая секция «Перспективные каталитические процессы» проводилась под председательством д.х.н. М.П. Юнусова и к.х.н. С.Р. Хайрулина. Интересный доклад представила д.х.н. Л.А. Исупова (Институт катализа СО РАН, Новосибирск) об удалении закиси азота в производстве азотной кислоты. Представитель Нижнего Новгорода к.х.н. А.В. Воротынцев из НГТУ сделал доклад о новых аспектах низкотемпературного каталитического гидрирования, диспропорционирования и восстановления хлорсиланов для производства поликремния. О новых наноразмерных металл-полимерных катализаторах трехфазного синтеза Фишера-Тропша рассказала к.х.н. М.В. Куликова из Института нефтехимического синтеза им А.В. Топчиева РАН, Москва. Последним докладом в этой секции был доклад аспиранта Г.В. Соснина (Институт катализа СО РАН, Новосибирск), который представил результаты применения наноразмерных катализаторов в перспективном процессе парового крекинга тяжелого нефтяного сырья для повышения глубины переработки тяжелых нефтей, битумов и облегчения их транспортировки.
На церемонии закрытия Конгресса были отмечены молодые участники, сделавшие наиболее интересные доклады.
Лучшие устные доклады Конгресса
Лучшие стендовые доклады Конгресса
Лучшие устные доклады Молодёжной школы по катализу
Устный доклад иностранного участника Молодёжной школы по катализу
По результатам проведения III Российского конгресса по катализу «Роскатализ-2017» были приняты следующие решения:
1. Конгресс отмечает, что за время после проведения II Российского Конгресса по катализу «Роскатализ-2014» произошло формирование устойчивых консорциумов разработчиков из разных организаций, которые работают в согласованных направлениях, обмениваются опытом и объединяют усилия при решении общих задач, формулируемых отечественной промышленностью и министерствами. Это обусловлено, в первую очередь, существенно возросшим интересом промышленности к отечественным катализаторам и каталитическим технологиям в рамках импортозамещения. Очевидно постепенное восстановление широкого фронта отечественных исследований во многих областях катализа, которые закрывают большинство самых «горячих» направлений фундаментальных, поисковых и прикладных исследований в области катализа и сдерживаются в основном отсутствием целевых средств на исследования и соответствующей координирующей программы по катализу федерального уровня.
2. Конгресс с удовлетворением отмечает сохранение и дальнейшее упрочнение связей российских специалистов–каталитиков со своими коллегами из республик СНГ и стран дальнего зарубежья: Узбекистана, Азербайджана, Республики Беларусь, Германии, Франции, Финляндии, Польши, Великобритании, Испании.
3. Учитывая ключевую роль катализа в развитии экономики страны, Конгресс настоятельно рекомендует разработать в рамках РАН, ФАНО России и других ведомств специализированную комплексную программу фундаментальных и поисковых исследований по катализу, так как за прошедшие три года такая программа так и не была разработана.
4. Конгресс отмечает наличие очевидных приоритетов общегосударственной значимости, на которые в настоящее время должно быть направлено особое внимание отечественного сообщества специалистов–каталитиков. Этими приоритетами являются:
5. Конгресс подтверждает следующие основные отечественные приоритеты в области фундаментальных исследований:
5. Конгресс подтверждает следующие основные отечественные приоритеты в области прикладных исследований:
7. Конгресс отмечает возросшую необходимость проведения регулярных рабочих совещаний и симпозиумов отечественных специалистов на русском языке по более узким тематикам, некоторые из которых могут быть организованы в виде сателлитных мероприятий к Конгрессу.
8. Конгресс подтверждает целесообразность проведения IV Российского конгресса по катализу «Роскатализ-IV», в городе Санкт-Петербурге в 2020 году и обращается в связи с этим к руководству Санкт-Петербургского государственного технологического института и к другим российским организациям и промышленным предприятиям с просьбой оказать всемерную поддержку в подготовке и проведении Российского конгресса по катализу «Роскатализ-IV».
9. Конгресс обращается к Федеральному государственному бюджетному учреждению науки Институту катализа им. Г.К. Борескова Сибирского отделения Российской академии наук с просьбой выступить в роли основного организатора IV Российского конгресса по катализу «Роскатализ-2020» и взять на себя ответственность за обеспечение решения оперативных финансовых вопросов, связанных с проведением Конгресса.
Материал подготовили:
В.А. Яковлев, В.В. Каичев, М.А. Клюса
(ИК СО РАН, Новосибирск)
A promising drug-targeting approach is made easier thanks to a new rhodium-based catalyst
Antibody-drug conjugates are promising next-generation cancer therapies that can target and selectively kill malignant cells while sparing healthy ones. These conjugates—in which a drug is bound to an antibody through a small chemical linker—harness the antibody’s ability to recognize markers specific to cancer cells and bring the potent drugs to their intended site of action. But one conundrum that has plagued drug developers and scientists is how to consistently tether a drug onto a predictable site on an antibody.
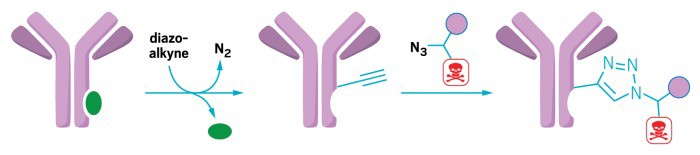
A rhodium-peptide catalyst binds to an antibody (left) and facilitates the addition of an alkyne-functionalized diazo group (center). The group serves as a chemical linker for attaching drugs, such as the cancer drug doxorubicin. The drug is modified with an azide group for the attachment reaction and desthiobiotin (purple circle) to make purification with chromatography easier.
Now, chemists have reported a new metallopeptide catalyst that can reliably link drug molecules to a variety of antibodies, at the same location on the antibody every time (J. Am. Chem. Soc.2017, DOI: 10.1021/jacs.7b06428 ). Inconsistent linkage can make it difficult for researchers to study the biological effects of these new compounds, or interfere with the antibody’s ability to bind to its target.
Previously, scientists used genetic engineering to create antibodies with chemical groups to which drugs could be attached. But this new approach bypasses the need for genetic engineering, a time-intensive process. The catalyst consistently binds to a particular location on an antibody’s so-called “constant chain,” an amino acid sequence that is found in all human antibodies and is conserved among many species. The catalyst produces reproducible results on human, pig, rabbit, and dog antibodies, the researchers found.
“Antibodies are pretty complicated molecules,” says Dennis G. Gillingham, a chemical biologist at the University of Basel not involved in this study. “This new technique is truly unique because it doesn’t require any engineering of any amino acids; the catalyst controls the reaction.”
Zachary T. Ball of Rice University and colleagues made the catalyst by taking a segment of a protein isolated from the bacterium Staphylococcus aureus that binds to the asparagine-79 residue on an antibody’s constant chain and then coordinated the peptide with three rhodium (II) ions. The rhodium complexes catalyze the addition of a linker group to the antibody—an alkyne-functionalized diazo that can react with many other small molecules, including drugs or fluorescent dyes, in a simple click-chemistry reaction.
To prove that antibody-drug conjugates made with the catalyst can target cells, the authors attached fluorescent dyes and the chemotherapy drug doxorubicin to Herceptin, an antibody drug that recognizes the HER2 protein on the surface of mammalian breast cancer cells, and then visualized the localization using confocal microscopy. The dye appeared at the cell surface, confirming that the antibody still recognized the cell surface markers.
“We’re building next-generation drug conjugates using this method and testing those in cell and tumor model studies,” Ball says. He adds that it would be relatively easy to produce the catalyst in bulk for future drug development applications.
New catalyst converts carbon dioxide to two- and three-carbon compounds

Copper nanoparticles on carbon paper fuse to form cubes, which provide an interface for catalyzing carbon dioxide reduction.
Through photosynthesis, plants and some bacteria use energy from sunlight to turn carbon dioxide and water into useful organic chemicals. Chemists have serious enzyme envy because designing inorganic catalysts that can do the same is difficult. Now researchers have developed a catalyst that can turn CO2 into ethanol and propanol when operating at voltages that solar cells could provide (Proc. Natl. Acad. Sci. USA 2017, DOI: 10.1073/pnas.1711493114).
This process of powering chemical synthesis via solar power, called artificial photosynthesis, could enable carbon-neutral fuels. In such a system, every molecule of CO2 emitted when a fuel is burned could be captured to make another fuel molecule. But developing catalysts that can recycle CO2 is challenging, says Peidong Yang, a chemist at the University of California, Berkeley.
Using catalysts based on iron, copper, indium, and other metals, researchers have successfully transformed CO2 into single-carbon compounds, including carbon monoxide, formate, and methanol. Making multicarbon compounds directly has proved more difficult.
Because biology’s metabolic capabilities are hard to beat, Yang’s group and others have built systems that pair catalysts and microorganisms to build molecules with more than one carbon. Other labs have made two- and three-carbon molecules using nanostructured copper and copper oxides, but these catalysts work only at voltages too high to be supplied by solar cells. And these high voltages waste electricity—only a small fraction of the energy gets stored in chemical bonds.
Yang’s group found that the right amount of copper nanoparticles loaded on carbon paper can catalyze the reduction of CO2 to two- and three-carbon compounds, including ethylene, ethanol, and n-propanol, with 60% selectivity at 600 millivolts, a voltage Yang believes is low enough to be supplied by solar cells. Previous catalysts performed similar reactions but required 900 millivolts, Yang says.
The team discovered this better catalyst by systematically studying the performance of different densities of copper nanoparticles on support structures made of various materials. Graduate student Dohyung Kim found the best catalytic performance when he covered carbon paper with about 45 µg of the copper particles per square centimeter of the paper.
Despite these fastidious efforts, Yang says the researchers are not yet sure why the catalyst works so well. The researchers observed that after seven minutes under reaction conditions, the spherical nanoparticles fuse into larger cubic ones, with an interface between copper, copper oxide, and carbon from the paper. The group is still investigating the mechanism, but they believe this interface is key to the structure’s catalytic activity. “We’ve finally identified a key active interface to produce two- and three-carbon compounds,” Yang says.
The new work “helps move the bar in investigating the applications of copper-based catalysts for electrochemical transformation of CO2 to useful products,” says Ellen Williams, a nanoscale physicist at the University of Maryland, College Park, who serves on the board of the Global CO2 Initiative. However, she notes, it remains to be proven whether this catalyst works on large, industrial scales.
Yang says that in addition to exploring the catalyst’s mechanism, his group is now testing the catalyst in a full system that’s coupled to photovoltaics.
Chemists would like to be able to modify compounds containing multiple hydroxyl groups, such as the toxin ouabain and the antiparasitic drug ivermectin, to generate new molecules with diverse uses in medicine, molecular biology, and agroscience. One way to do this would be to selectively oxidize individual hydroxyls to ketones, which are synthetically versatile groups in that they can be readily converted to nitrogen-based groups, such as oximes and amines, or can be leveraged to add adjacent groups in a molecule’s carbon framework.
A team of researchers at the University of California, Berkeley, has now designed a catalyst capable of such a feat.
Dehydrogenating selected hydroxyls to ketones in multi-hydroxylated compounds known as polyols has been difficult. A common strategy is to add protecting groups to the hydroxyls you don’t want to react and then deprotect them later. But this is laborious, requiring multiple reactions instead of just one. Although a few synthetic procedures can oxidize specific hydroxyls in polyols to ketones, they generally cause undesirable side reactions or are poorly selective for specific hydroxyls, producing complex mixtures instead of relatively pure ketone products.
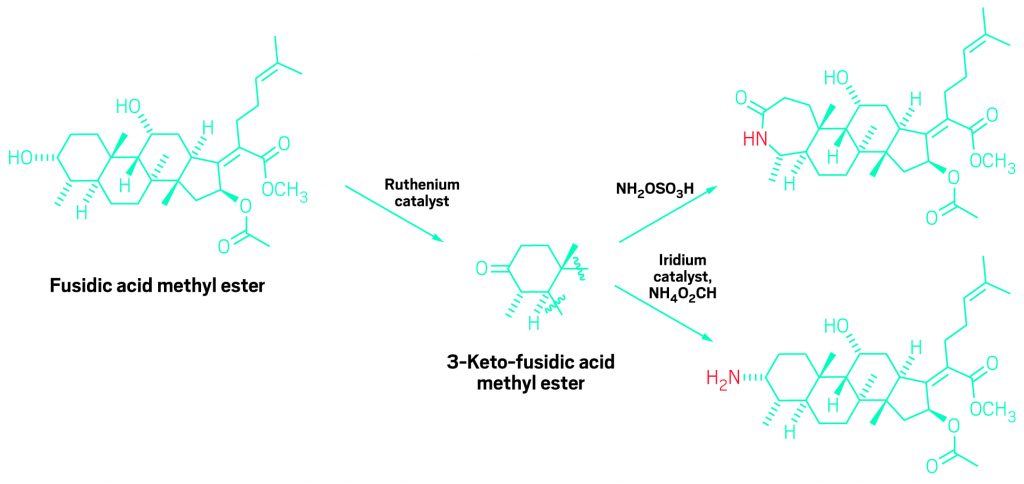
In this example of the new reaction, the secondary hydroxyl in the antibiotic natural product fusidic acid (left) is selectively oxidized to a ketone. The resulting ketone can then be converted readily to an amine (bottom right) or the ketone’s ring can be expanded by insertion of a nitrogen group (top right).
John F. Hartwig and Christopher K. Hill of UC Berkeley speculated that one way to improve selectivity would be to develop a catalyst that oxidizes polyol secondary alcohol groups—hydroxyls on carbon atoms that are bonded to two other carbon centers—much more readily than primary alcohol groups—hydroxyls on carbons connected to only a single carbon center.
The catalyst they designed has strongly electron-donating phosphine ligands that make its ruthenium center electron-rich, weakening its oxidizing powers. That makes the catalyst selective for secondary hydroxyls, which are more electron-rich than primary hydroxyls and thus better substrates for a weakly oxidizing catalyst.
Hartwig and Hill demonstrated the catalyst’s power and versatility by using it to selectively oxidize a single hydroxyl group in over a dozen polyol natural products and using subsequent catalytic reactions to convert the resulting ketones into nitrogen-modified products. They recently reported the work in Nature Chemistry (2017, DOI: 10.1038/nchem.2835) and presented it during the ACS national meeting in Washington, D.C., during a session sponsored by the Catalysis Science & Technology Division.
The researchers developed a set of rules that predict which hydroxyls in a polyol the catalyst will oxidize selectively. In general, the catalyst is selective for electron-rich, sterically hindered secondary hydroxyls. The reaction does not produce dione products by oxidizing multiple hydroxyls in the same compound, owing to the catalyst’s high sensitivity for electronic and steric differences in the properties of different hydroxyls.
The new selective alcohol dehydrogenation reaction “has many advantages over traditional methods, including lower catalyst loading, milder conditions, higher yields, and improved selectivity,” says transition-metal catalysis specialist Guangbin Dong of the University of Chicago. “I am most impressed by the reaction’s unusual chemoselectivity in the dehydrogenation of secondary hydroxyls. Without doubt, this technology is going to be highly useful for medicinal chemistry.”
The technique represents “a very important step forward in the ability to do catalytic, site-selective alterations of complex molecules,” says Scott Miller of Yale University, an expert on site-selective catalysts. It will be useful for achieving selectivity in the conversion of complex starting materials to bioactive analogs, he says.
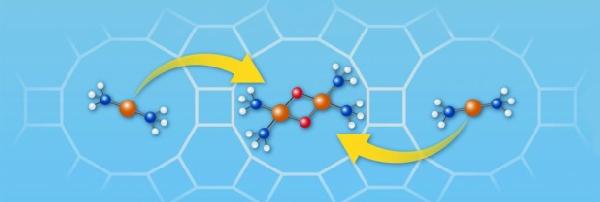
Single copper ions in a zeolite catalyst (white geometric shapes) bind two ammonia molecules, migrate through the zeolite, and form oxygen-bridged dimer complexes, which catalyze exhaust cleanup.
High schoolers aren’t the only ones who pair-up and break-up frequently. Catalytic species in the systems that clean up engine exhaust do it too, according to a study presented at the American Chemical Society national meeting in Washington, D.C., on Tuesday.
The investigation uncovers an unusual mechanism in the catalytic process that rids diesel engine exhaust of smog-causing nitrogen oxides (NOx). The findings may eventually lead to more effective catalysts.
Diesel-powered engines score high marks for fuel efficiency, which is why nearly all long-haul trucks use them. But they emit various pollutants including hydrocarbons, CO, and NOx.
Selective catalytic reduction (SCR) systems—part of the overall exhaust cleanup equipment on diesel vehicles—reduce NOx to nitrogen and water through a reaction with ammonia. The ammonia comes from an aqueous urea solution, which is carried on-board like windshield cleaner. SCR systems catalyze NOx reduction with the help of chabazite zeolite that has been treated to incorporate copper in its lattice.
These systems come standard on many diesel vehicles, yet details of how they work remain a subject of debate.
A team led by Rajamani Gounder of Purdue University and William F. Schneider of the University of Notre Dame studied some of the details in copper-chabazite SCRs. Using X-ray spectroscopy, kinetics measurements, and quantum calculations, the researchers tracked unusual behavior of the zeolite’s copper ions.
Speaking at a symposium sponsored by the Division of Catalysis Science & Technology, Gounder reported that in the presence of ammonia, copper ions form Cu(NH3)2 complexes. The NH3 moities make the species mobile, allowing them to migrate through openings that interconnect hollow cages in the porous zeolite framework. As the coppers species move about, they briefly and reversibly form dimers that are bridged by a pair of oxygen atoms. The dimers facilitate an O2-mediated Cu(I) to Cu(II) redox step that’s central to reducing NOx to nitrogen and water (Science 2017, DOI: 10.1126/science.aan5630).
Gounder explained that, in effect, individual copper ions come together and work in tandem to carry out the difficult step of breaking apart oxygen molecules. The copper ions then go back to being isolated after the reaction is complete. He noted that this step might be accelerated by fine-tuning the spatial distribution of copper ions in the zeolite, leading to lower NOx emissions at cooler operating temperatures than is possible with current SCR systems.
“Exquisite” is how Robert J. Davis described the team’s techniques for exploring this SCR system. Davis, a catalysis specialist at the University of Virginia, remarked that this exciting finding regarding the mobility of copper ions and the dynamic formation of paired copper species may also be relevant to the high selectivity of Cu-treated zeolites used for oxidizing methane to methanol.
Gallium-palladium droplets drive alkane dehydrogenation with high selectivity
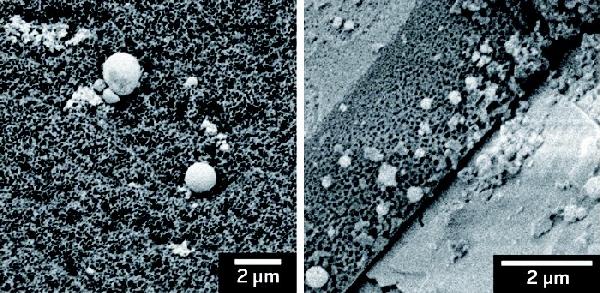
A Ga-Pd alloy forms microscopic droplets on glass (left) and serves as an active dehydrogenation catalyst, remaining liquid even after 20 hours of reaction (right).
Gallium’s quirky liquid-state properties have pushed that element into the scientific spotlight recently, as researchers have tapped the liquid metal for applications in stretchable electronics and three-dimensional printing. Now gallium is back in the news, this time as a catalyst.
Researchers in Germany report that liquid droplets of Ga-Pd alloys function as active and durable catalysts for alkane dehydrogenation. That industrial-scale reaction converts low-value alkanes to higher-value olefins, compounds with C=C bonds that are used to make polymers and chemicals (Nat. Chem. 2017, DOI: 10.1038/nchem.2822).
Gallium and some of its alloys exhibit a handful of unique properties, such as a tendency to remain liquid over an enormous temperature range—about 2,000 °C. The metal also has a knack for spontaneously forming an ultrathin oxide skin that stabilizes liquid droplets but easily breaks, allowing the metal to flow momentarily until the skin re-forms around the liquid.
A team including Nicola Taccardi and Peter Wasserscheid of Friedrich-Alexander University, Erlangen-Nьrnberg, took advantage of those properties of gallium and its ability to dissolve numerous metals, generating alloys with various concentrations of palladium, a catalytically active metal. Then they deposited the liquid metals onto porous glass, forming supported liquid metal catalysts, and used them in a test reaction: butane dehydrogenation.
Homogeneous, solution-phase catalysts have the advantage of possessing clearly defined active sites and mechanisms. The aim of the new work was to create a hybrid catalyst with these advantages that can also be easily separated from reaction products and reused, a task that’s currently difficult to carry out with homogeneous versions. Various researchers have attempted this feat previously. But the stability of their liquid-phase catalysts typically limited reactions to roughly 200 °C and below, far lower than temperatures required in many industrial catalytic processes.
The Friedrich-Alexander team ran test reactions at roughly 450 °C and found that gallium-rich catalysts, for example, ones with a Ga-to-Pd ratio of 10:1, had high activity for butane dehydrogenation, produced butene with high selectivity (85%), and remained in the liquid state even after 20 hours of reaction. In addition, they did not accumulate the layer of carbon (coke) that gunks up and deactivates commercial Pt-Al2O3 and Cr2O3-Al2O3 dehydrogenation catalysts.
“Supported liquid metal catalysis is an interesting concept,” says Arizona State University’s Jingyue (Jimmy) Liu, a catalysis specialist. He is particularly intrigued by the researchers’ atomic-level description of their catalyst as individual isolated Pd atoms supported on the surface of a Ga-Pd liquid metal. He adds, “Systematic investigations are needed to better understand reaction processes in such a system and to provide deeper insights into the nature of the newly synthesized liquid metal.”
Chemical & Engineering News
|
30th International Microprocesses and Nanotechnology Conference (MNC 2017) Jeju, Korea |
http://mnc2017.org/ |
|
VII Международная конференция “Деформация и разрушение материалов и наноматериалов” Москва, Россия |
http://dfmn.imetran.ru |
|
7th Annual European Biomass to Power Conference Aarhus, Denmark |
http://www.wplgroup.com/aci/event/ european-biomass-to-power/ |
|
Школа молодых ученых “Новые каталитические процессы глубокой переработки углеводородного сырья и биомассы” Новосибирск, Россия |
http://www.catalysis.ru/resources/science/ conferences/school_2017 |
|
Sultan Qaboos University Chemistry Conference: Green and Sustainable Chemistry Muscat, Oman |
http://www.rsc.org/events/detail/25659 |
|
Всероссийская конференция по квантовой и математической химии Уфа, Республика Башкортостан, Россия |
http://www.qmchem2017.ru |
|
II Международная научно- практическая конференция “Графен и родственные структуры: синтез, производство и применение” Тамбов, Россия |
http://graphene.tstu.ru/ |
|
II Всероссийская молодежная конференция “Проблемы и достижения химии кислород- и азотсодержащих биологически активных соединений” Уфа, Республика Башкортостан, Россия |
http://www.bashedu.ru/ru/novosti- khimicheskogo-fakulteta |
|
Ежегодная конференция молодых ученых “Элементоорганические соединения, полимеры, органическая химия, теоретические и физико- химические методы исследования строения вещества” Москва, Россия |
https://ineos.ac.ru/competitions/young |
|
Научно-практическая конференция “Актуальные задачи нефтеперерабатывающего и нефтехимического комплекса” Москва, Россия |
conference@vnipineft.ru |
|
2nd International Symposium on Recent Progress of Energy and Environmental Photocatalysis Tokyo, Japan |
https://photocatalysis2.wixsite.com/pirc |
|
|
|
|
Conference on Advances in Catalysis for Energy and Environment (CASEE 2018) Mumbai, India |
http://www.tifr.res.in/~cacee |
|
4th International Symposium on Chemistry for Energy Conversion and Storage (CHEMENER 2018) Berlin, Germany |
www.chemener2018.org |
|
Lignofuels 2018 Conference (Advanced Biofuels & Materials) Amsterdam, The Netherland |
http://www.wplgroup.com/aci/event/ lignocellulosic-fuel-conference-europe |
|
5th European Biopolymer Summit Dusseldorf, Germany |
http://www.wplgroup.com/aci/event/ biopolymer-conference-europe |
|
2nd International Conference on Catalysis and Chemical Engineering (CCE 2018) Paris, France |
http://unitedscientificgroup.com/ conferences/catalysis |
|
International Conference of Computational Methods in Sciences and Engineering 2018 (ICCMSE 2018) Thessaloniki, Greece |
http://www.iccmse.org |
|
3rd SynGas Convention “Fuels and Chemicals from Synthesis Gas: State of the Art” Cape Town, South Africa |
www.syngasconvention.com |
|
15th International School-Conference on Magnetic Resonance and its Applications (Spinus 2018) St. Petersburg, Russia |
http://spinus.spb.ru |
|
27th Biennial Conference of Organic Reactions Catalysis Society San Diego, CA, USA |
http://orcs.org/27th-meeting |
|
III Всероссийская конференция с международным участием “Исследования и разработки в области химии и технологии функциональных материалов” Апатиты, Россия |
http://chemi-ksc.ru/m-osnovnoe/konferentsii/ 577-konferentsii-2018 |
|
III Scientific-Technological Symposium “Catalytic Hydroprocessing in Oil Refining” (STS HydroCat-2018) Lyon, France |
http://conf.nsc.ru/STS_3 |
|
Designing Nanoparticle Systems for Catalysis. Faraday Discussion London, UK |
http://www.rsc.org/events/detail/25362 |
|
5th International School- Conference on Catalysis for Young Scientists “Catalyst Design: From Molecular to Industrial Level” Moscow, Russia |
http://conf.nsc.ru/catdesign2018 |
|
13th International Symposium on the Synthesis and Applications of Isotopes and Isotopically Labelled Compounds Prague, Czech Republic |
http://www.iis-prague2018.cz |
|
1st International Conference on Reaction Kinetics, Mechanisms and Catalysis Budapest, Hungary |
https://rkmc.akcongress.com |
|
2nd International Symposium on Clean Energy from Ethanol (ISCEE 2018) Krakow, Poland |
http://iscee.com.pl/conference/conference-site |
|
EFCATS School on Catalysis Liblice Castle, Czech Republic |
http://www.jh-inst.cas.cz/efcats.school |
|
The World Conference on Carbon (Carbon 2018) Madrid, Spain |
www.carbon2018.org |
|
Post-graduate Summer School on Green Chemistry Venice, Italy |
|
|
12th International Symposium on Scientific Bases for the Preparation of Heterogeneous Catalysts (PREPA12) Louvain-La-Neuve, Belgium |
http://www.prepa12.org |
|
27th IUPAC International Symposium on Photochemistry Dublin, Ireland |
https://iupac.org/event/27th-iupac-international -symposium-photochemistry |
|
World Congress & Expo on Chemical Engineering & Catalysis (WCECEC-2018) Osaka, Japan |
http://scientificfederation.com/catalysis-2018 |
|
International Symposium on Zeolites and Microporous Crystals (ZMPC 2018) Yokohama, Japan |
http://www.jaz-online.org/ZMPC2018 |
|
8th Tokyo Conference on Advanced Catalytic Science and Technology (TOCAT8) Yokohama, Japan |
http://www.shokubai.org/tocat8zmpc2018 |
|
7th EuCheMS Chemistry Congress Liverpool, UK |
https://www.euchems2018.org |
|
XII Международная конференция молодых ученых по нефтехимии Звенигород, Россия |
http://conference.forenewchemistry.ras.ru |
|
15th International Conference on Micro Reaction Technology (IMPET 2018) Karlsruhe, Germany |
www.dechema.de/IMRET2018 |
|
|
|
|
6th European Conference on Environmental Applications of Advanced Oxidation Processes (EAAOP-6) Portorose, Slovenia |
http://eaaop6.ki.si |
|
50th General Assembly & 47th IUPAC World Chemistry Congress Paris, France |
https://www.iupac2019.org |
|
14th EuropaCat – European Congress on Catalysis “Catalysis without Borders” Aachen, Germany |
www.europacat2019.eu |