
Премия имени А.А. Баландина 2001 года
Научный совет по катализу РАН -
Отчет о научно-организационной деятельности в 2001 году
За рубежом
Л.М. Кустов
Современные тенденции промышленного катализа
Дж. В. Ньемантсвердрит
Как готовить успешные устные и постерные презентации (часть 1)
Президиум Российской академии наук присудил премию им. А.А. Баландина 2001 года доктору химических наук Тамаре Витальевне Андрушкевич, кандидату химических наук Валентине Михайловне Бондаревой и кандидату химических наук Галине Яковлевне Поповой (Институт катализа им. Г.К.Борескова СО РАН) за серию работ "Гетерогенно-каталитическое окисление основных органических соединений в карбоновые кислоты: механизм, кинетика, дизайн катализаторов".
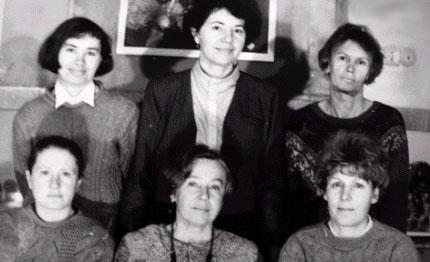
Лауреаты премии среди коллег:
1-й ряд, слева направо: Н.Е. Ванькова, Т.В. Андрушкевич, Л.А. Шадрина
2-й ряд: Г.Я. Попова, Е.М. Алькаева, В.М. Бондарева.
Редакция "Каталитического бюллетеня" сердечно поздравляет Тамару Витальевну, Валентину Михайловну и Галину Яковлевну с премией академика А.А. Баландина и желает доброго здоровья, удачи и успехов в новых исследованиях. Пусть в Вашем дружном коллективе царит взаимопонимание, творчество и хорошее настроение!
Предлагаем вниманию читателей интервью заведующей лабораторией гетерогенного селективного окисления, доктором химических наук Т. Андрушкевич корреспонденту газеты "Наука в Сибири" Л. Юдиной.
Новосибирские химики - лауреаты престижной академической премии
- Тамара Витальевна, из многих работ, выдвинутых на соискание премии им. А.А.Баландина, выбрана ваша. Удалось осуществить новые подходы в решении проблем?
- Представленный цикл - это разработка эффективных способов каталитического гетерофазного синтеза карбоновых кислот - акриловой, муравьиной и никотиновой из акролеина, формальдегида и 3-метилпиридина, соответственно. Эти кислоты - ценные химические продукты, они широко используются в производстве полимеров, химических волокон, душистых веществ, лекарственных препаратов, во многих других органических синтезах.
- А каковы же современные промышленные методы их синтеза?
- Дело в том, что в России промышленных производств этих кислот, кроме акриловой, сейчас нет. Способ получения акриловой кислоты, разработанный в Институте катализа много лет назад, является традиционным и отличается от зарубежных только химическим составом катализатора. Что касается муравьиной и никотиновой кислот, то это принципиально новые технологии. Существующие зарубежные производства - жидкофазные многостадийные процессы с большим количеством вредных стоков и отходов. Гетерофазные способы получения кислот, впервые предложенные нами, недефицитны по сырью, отличаются простотой технологии, низкой энергоемкостью, легкостью выделения продуктов. И что особенно важно - отсутствием жидких стоков, вредных газовых выбросов и твердых отходов. Достоинства способа оценили и за рубежом. Лицензию на получение никотиновой кислоты закупила у Института катализа одна германская фирма.
- Чем же определяется эффективность гетерофазных способов?
- В первую очередь - подбором катализаторов. Как теоретическую основу конструирования высокоселективных катализаторов мы использовали положение о промежуточном химическом взаимодействии реактантов и катализатора и об энергии связи промежуточных соединений, как основном факторе, определяющем скоростьи направление протекания реакции.
Впервые представление об оптимальной величине энергии промежуточного взаимодействия для решения задач предвидения каталитического действия в форме принципа энергетического соответствия мультиплетной теории катализа предложил именно А.А.Баландин. Далее этот подход последовательно и плодотворно развивался в работах Г.К.Борескова, главным образом, в окислительном катализе.
Мы исследовали механизм окисления альдегидов и 3-метилпиридина на ряде оксидных систем, определили структуру промежуточных соединений, их реакционную способность и кинетику превращения в конечные продукты и установили зависимость направления превращения промежуточных соединений от энергии связи их с активными центрами катализатора. Механизм образования кислот оказался аналогичным - через промежуточные соединения одного и того же типа и через одну и ту же последовательность их превращения.
Исходя из условия оптимальности энергии связи интермедиатов с активными центрами, мы сформулировали принципы подбора катализаторов для синтеза карбоновых кислот окислением органических соединений оснόвного типа, каковыми являются альдегиды и гетероциклы. Предложенные в соответствии с этими принципами химические композиции стали основой промышленных катализаторов для получения перечисленных кислот.
- Работу можно было считать завершенной?
- Определенный ее этап. Следующий, длительный и трудоемкий период - разработка технологичного способа приготовления катализаторов с заданными характеристиками: пористой структурой, поверхностью, прочностью, размером и формой гранул. Здесь большой вклад внесли другие подразделения института, в первую очередь - лаборатория приготовления катализаторов и опытно-химический цех.
Для всех реакций на катализаторах, удовлетворяющих промышленным требованиям по перечисленным характеристикам, в широком интервале условий были выполнены детальные кинетические исследования. На основании кинетических уравнений методом математического моделирования рассчитаны оптимальные режимы проведения реакции, проведены моделирование процессов и испытания опытных образцов в трубках, представляющих элементы промышленных реакторов.
- Что показали испытания?
- Хорошее совпадение расчетных и экспериментальных данных. Получены выходы акриловой кислоты - 95%, муравьиной - 83%, никотиновой - 82%.
- Промышленная реализация разработанных технологий. Сделано уже немало. В частности, в Новосибирске, на АО "Химпласт" налаживается промышленное производство никотиновой кислоты мощностью 500 т в год. Готовится пуск первой очереди. Как
всегда - проблем множество. Хочется верить, что они не на очень длительный срок отодвинут полную реализацию нашей разработки в промышленном варианте.
Наука в Сибири, N 10, 2002
Под эгидой Научного совета по катализу РАН (НСК) и при активном участии членов НСК в подготовке и проведении мероприятий были проведены в 2001 году следующие важные для научной общественности конференции и совещания:
Научный совет по катализу способствовал формированию делегации российских ученых для участия в работе V Европейского конгресса по катализу "From Surfaces to Processes - Fundamentals to Applications" - EUROPACAT-V (September 2-7, 2001, Limerick, Ireland) и IV World Congress on Oxidation Catalysis (September 16-21, 2001, Potsdam, Germany).
НСК принимал активное участие в решении оргвопросов по созданию и выпуску в свет, начиная с 2001 года, нового полноформатного периодического научно-технического журнала "Катализ в промышленности".
Начиная с 1997 года, Научный совет по катализу продолжает ежеквартально выпускать и рассылать членам НСК "Каталитический бюллетень", в котором публикуется оперативная информация о важнейших результатах фундаментальных и прикладных исследований в области катализа в России и за рубежом, дается перечень приглашений на предстоящие конференции, публикуются приказы, документы РАН и источники дополнительного финансирования НИР (гранты, федеральные программы и т.п.), заметки об истории развития катализа в России и другие материалы.
Секретариат НСК продолжает сотрудничать с Академией наук, Министерствами, институтами разных ведомств и другими организациями России, дальнего и ближнего зарубежья по различным вопросам, касающимся научной, научно-организационной, учебно-преподавательской и общественной деятельности в области катализа.
Примеры важнейших результатов фундаментальных и прикладных исследований в области катализа, полученных в 2001 году членами Академии наук или возглавляемыми ими коллективами, работающими в отраслевых научных учреждениях, государственных научных центрах и ВУЗах
С применением in situ 13С ЯМР спектроскопии высокого разрешения в твердом теле и ex situ ГХ-МС анализа исследован механизм изомеризации н-бутана на сульфатированном оксиде циркония (SZ). Сделан вывод о протекании сложного процесса сопряженной полимеризации при превращении октена-1, возможного интермедиата изомеризации н-бутана, на SZ при 20-100 оС с одновременным образованием смеси алканов (в основном, изобутана) и устойчивых алкил-замещенных циклопентенильных катионов.
Показано, что при превращении н-бутана-1-13С на SZ при 20 ºС протекает два параллельных процесса - (1) перемешивание метки в н-бутане и (2) скелетная изомеризация н-бутана в изобутан при полном отсутствии продуктов диспропорцинирования бутана (пропана и пентанов) на начальной стадии реакции. На основе масс-спектрометрического анализа распределения меток 13С в бутанах после реакции установлено, что перемешивание метки в н-бутане протекает по внутри- (или моно-) молекулярному маршруту, тогда как изомеризация н-бутана в изобутан протекает по бимолекулярному механизму.
Наблюдаемые реакции открывают возможности использования сульфатированной окиси циркония в качестве твердого кислотного катализатора для получения различных циклоалканов.
(академик В.Н. Пармон,
Объединенный институт катализа СО РАН, Новосибирск)
Разработаны оригинальные блочные катализаторы сотовой структуры для процесса селективного окисления природного газа в синтез-газ при миллисекундных временах контакта и температурах 800-1000 оС. Использование нетрадиционных подходов к синтезу блочных носителей на основе корунда, а также выбор состава активного компонента - металлов платиновой группы со сложными оксидными системами со структурами перовскита и флюорита позволили обеспечить высокую активность и селективность данных катализаторов при содержании металлов платиновой группы существенно более низком по сравнению с известными зарубежными аналогами. Показано, что новый процесс по сравнению с ранее известными процессами позволяет уменьшить капитальные затраты в 5-10 раз, текущие затраты на 30-50 %.
В рамках подготовки к созданию первой промышленной установки на Норильском горно-металлургическом комбинате наработана опытно-промышленная партия катализаторов объемом 100 л. Разработан, изготовлен и поставлен заказчику вместе с катализатором промышленный реактор для процесса получения синтез-газа. Низкая себестоимость синтез-газа, полученного при селективном окислении природного газа, открывает большие перспективы для его использования в химической, металлургической и электронной промышленности.
(академик В.Н. Пармон,
Объединенный институт катализа СО РАН, Новосибирск)
На примере каталитической окислительной димеризации 2-меркаптобензтиазола впервые показана принципиальная возможность использования гетерогенных фталоцианиновых катализаторов для бесщелочной демеркаптанизации нефтяных углеводородов. Это позволяет приступить к разработке и оптимизации соответствующих нефтеперерабатывающих технологий.
(чл. - корр. РАН Г.Н. Ворожцов,
Государственный научный центр "НИОПИК", Москва)
Впервые получены количественные данные по кинетической неоднородности (полицентровости) активных центров при ионно-координационной полимеризации диенов, позволившие определить концентрацию и реакционную способность каждого типа активных центров. Предложенный метод основан на экспериментальных данных по ММР полимеров и решении интегральных уравнений, некорректность которых преодолевается с помощью метода регуляризации А.Н. Тихонова.
Показано, что процесс 1,4-цис- полимеризации диенов на лантанидных каталитических системах протекает при участии четырех, а 1,4-транс-полимеризации на ванадиевых катализаторах- трех типов активных центров. Это способствует построению адекватных моделей полимеризации и получению полимеров с заданными характеристиками.
(академик Ю.Б. Монаков,
Институт органической химии АН РБ и УНЦ РАН)
Разработан микросферический оксидный алюмохромкалиевый катализатор дегидрирования парафинов, по своей механической прочности в 3-4 раза выше, чем катализаторы, используемые в настоящее время на установках дегидрирования парафинов с псевдоожиженным слоем. Способ приготовления таких катализаторов включает стадии термохимической активации тригидрата алюминия, гидратации и термообработки образующегося продукта, нанесения активных компонентов на полученный микросферический алюмооксидный носитель и термообработку при 600-650 оС в атмосфере воздуха.
Совместно с ЗАО "Каучук" (Стерлитамак) на оборудовании Ишимбайского специализированного химического завода катализаторов начаты работы по внедрению технологии приготовления микросферических катализаторов, что позволит уменьшить в 2-3 раза расход катализатора на установке дегидрирования изо-пентана в ЗАО "Каучук".
(чл-корр. РАН У.М. Джемилев,
Институт нефтехимии и катализа АН РБ и УНЦ РАН)
Впервые осуществлено гидрофосфинирование непредельных соединений (алкинов, стиролов, енаминов), катализируемое комплексами Pd или Ni. Разработаны условия регио- и стереоселективного синтеза алкенилдиарилфосфинов, арилэтил- и пиридилэтилдифенилфосфинов, а также 1,1-алкенилдифосфинов.
Все вновь полученные третичные фосфины представляют собой перспективные лиганды для гомогенного металлокомплексного катализа.
(академик И.П. Белецкая,)
Московский государственный университет им. М.В. Ломоносова, Москва)
The NATO Programme
The NATO Programme on Security-Related Civil Science and Technology announces three deadlines for receipt of applications - 1 February, 1 May and 1 October. Applications can be accepted for Advanced Research Workshops, Advanced Study Institutes, Collaborative Linkage Grants and Expert Visits. The Security-Related Civil Science and Technology area deals with (a) security of the environment in connection with all types of military activity; and (b) technologies for disarmament and conversion. It is aimed more at the application of the appropriate science and technology to the resolution of security-related problems, than at research per se. Topics include security-related biological science and technology (including biochemistry, biophysics, biotechnology, immunology, pharmacology, toxicology, epidemiology and related medical sciences); security-related nuclear science and technology; security-related chemical science and technology; security-related environmental science and technology; hazardous waste storage and disposal science and technology; conversion technologies; risk assessment; detection science and technology; weapons dismantlement technologies; and other security-related science and technology matters affecting both individuals and society.
Further information (and downloadable applications) on the support mechanisms described above can be found on the Internet at http://www.nato.int/science. Alternatively, application forms can be requested by: Tel: +32.2.707.4231; Fax: +32.2.707.4232; E-mail: science.fell@hq.nato.int
SUPRAMOLECULAR CLUSTER CATALYSIS
Conventional wisdom holds that in organometallic catalysis, substr
H-BOND DONORS AS ACID CATALYSTS
Hydrogen-bond donors such as thioureas that form multiple hydrogen bonds with carbonyl oxygen atoms in di-enophiles have been shown to make complexes that function in a fashion similar to traditional metal-containing Lewis acid catalyst adducts in Diels-Alder reactions [Org.Lett., published Jan. 4 ASAP, http://pubs.acs.org/journals/orlef7]. The results point to the potential use of H-bond additives as environmentally friendly replacements for Lewis acids in industrial processes, notes chemistry professor Peter R.Schreiner of the University of Georgia. Using NMR and IR spectroscopy and ab initio calculations, Schreiner and graduate student Alexander Wittkopp determined that complexation of thioureas with N-acyloxazolidinones is "well in line" with that of Lewis acids. In Diels-Alder additions of N-acyloxazolidinone to cyclopentadiene, the thioureas achieve near 75 % yield of the addition product at room temperature in 48 hours, in contrast to uncatalyzed reactions that require high temperature and give lower yields. Reactions with Lewis acids AlCl3 and TiCl4 achieve better than 90% yield in one hour at low temperature with slightly better diastereoselectivity.
C&EN/JANUARY 14, 2002
http://pubs.acs.org/CEN/
HYDROGEL OPALS
What is iridescent like a precious opal, yet soft, elastic, and water-swollen like gelatin? It's a hydrogel opal, a new type of bulk hydrogel that sparkles in many colors because of the way its crystalline structure diffracts light [Adv. Mater., 13, 1708 (2001)]. Polymer physicist Zhibing Hu and coworkers Xihua Lu and Jun Gao at the University of North Texas make the opals by first preparing nanoparticles consisting of N-isopropylacrylamide co-polymerized with acrylic acid or 2-hydroxyethyl acrylate. The nanoparticles are allowed to self-assemble in solutions, after which they are cross-linked to form a stable 3-D network. The resulting gels are as much as 97 % by weight water. The color and volume of the gels respond to changes in temperature and electric field. Temperature changes can make the gels become cloudy, obscuring their iridescence quickly and reversibly. The gel's fast response rate could be a major advantage over conventional colloidal crystal arrays for developing sensor or display technologies.
C&EN/November 19, 2001
http://pubs.acs.org/CEN/
TOMATOES WITH A TASTE FOR SALT
You'd hardly look to tomatoes for cutting-edge research news, but advances in genetic engineering have just turned the spotlight on that juicy red salad ingredient. Eduardo Blumwald, pomology professor at the University of California, Davis, and Hong-Xia Zhang, a postdoc in the botany department at the University of Toronto, report that genetically modified tomato plants flourish on a diet of 200-mM salt water and produce fruit that's healthy looking and tasty [Nat. Biotechnol., 19, 765 (2001)]. Extending this salt tolerance to other crops would be a boon to agriculture in vast regions of the world where farming is compromised by the adverse effects of salty irrigation water or salt-damaged soil. Typically, plants exposed to high salt concentrations dehydrate and die. But the genetically modified tomato plants manage to thrive on salt-water because the researchers engineered the plants to produce high levels of Na+/H+ antiport, a transport protein that provides plants with an ion-shuttling service. The protein directs sodium ions away from regions of plant cells where salt can interfere with metabolic functions and holds them in cellular storage compartments known as vacuoles.
C&EN/AUGUST 13, 2001
http://pubs.acs.org/CEN/
CRANKING UP THE TURNOVER NUMBER
High turnover numbers (TONs) have been observed during selective epoxidation of alkenes with molecular oxygen at one atmosphere catalyzed by a polyoxometalate [Angew. Chem. Int. Ed., 40, 3639 (2001)]. Using a di-iron-substituted silicotungstate, chemists Yoshiyuki Nishiyama, Yoshinao Nakagawa, and Noritaka Mizuno at the University of Tokyo find a TON of 10,000 for conversion of cyclooctene to cyclooctene oxide with 98 % selectivity over the alcohol and ketone co-products. That TON is 100 times higher that has previously been achieved with the same substrate and oxidant, the researchers note. Conversion of cyclododecene, 1-octene, 2-octene, 2-heptene, and 2-hexene proceed with TON's ranging from 1,300 to 9,600 with selectivity for the epoxide over the alcohol or ketone co-products. These encouraging results raise "the prospect of using this type of inorganic catalyst for industrial epoxidation processes", the researchers write. But for the reaction to be of practical use, the reaction times and the selectivities must be improved.
LOW-ENERGY ELECTRONS INDUCE SURFACE REACTIONS
Low-energy electrons can be used as selective probes to investigate structure, chemical bonding, and reactivity in thin films of hydrocarbons, according to a new study at the University of Southern California. Chemistry professor Bruce E. Koel and graduate student Denis Syomin find that beams of 30 eV electrons directed at metal surfaces covered with hydrocarbon layers induce C-H bond cleavage in a highly selective manner [Surf. Sci. Lett., 492, L693 (2001)]. The electron-induced dissociation process can be used to examine adsorbed layers of molecules or to modify the films chemically - for example, by covering the molecules to reactive intermediates or to stable products. Using a surface-sensitive IR spectroscopy technique and a mass spectrometry method that analyzes molecules desorbing from a specimen's surface, the researcher observes that under the influence of an electron beam, benzene that is weakly bonded to gold is converted to phenyl fragments. These species subsequently react with one another to form biphenyl. No other products are detected. Similarly, cyclohexane undergoes electron-induced bond cleavage, leaving a gold surface coated with reactive cyclohexyl fragments.
ACIDS, BASES AVOID MUTUAL DESTRUCTION
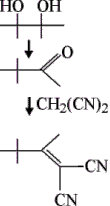
Chemists at Hebrew University of Jerusalem have managed to carry out sequences of acid- and base-catalyzed reactions in one pot by sequestering the acids and bases from one another [Angew. Chem. Int. Ed., 40, 3647 (2001)]. Chemistry professors David Avnir and Jochann Blum and graduate student Faina Gelman keep the acid and base from neutralizing one another by entrapping them in silica gel matrices. Acids are molybdic acid or a sulfonated perfluoro resin. Bases are N-2-aminoethylamino-propylated silica or 1,5,7-triazabicyclo[4.4.0]dec-5-ene. The chemists demonstrate their technique by heating benzene solutions of pinacol, malononitrile, and entrapped sulfonated resin and aminopropylated silica. In the sequence shown, the acid catalyzes rearrangement of pinacol to pinacolone, which undergoes base-catalyzed condensation with malononitrile to yield 83% overall of a 1,1-dicyano-1-butene.
C&EN/OCTOBER, 1 2001
http://pubs.acs.org/CEN/
FLUOROUS BIPHASIC TRANSESTERIFICATION
Catalyst-solvent system mediates quantitative 1:1 ester-alcohol reactions
A new fluorinated catalyst-solvent system that overcomes some of the traditional limitations of transesterification reactions - thus boosting its potential as a green industrial process - has been developed by a research team at Okayama University of Science in Japan.
Professor Junzo Otera, assistant professor Akihiro Orita, and coworkers in the department of applied chemistry report the 1:1 reaction of esters with alcohols using a fluorinated distannoxane catalyst in perfluorohexanes. The method leads to essentially 100 % conversion and yield of the desired esters [Angew. Chem. Int. Ed., 40, 3670 (2001)].
Generally, transesterification involved the acid- or base-catalyzed reaction of an ester and an alcohol that leads to double displacement of substituent groups. They are equilibrium-dependent reactions, meaning a large excess of the ester or alcohol is needed to drive the reaction, with one of the reactants usually acting as the solvent.
Otera and coworkers were looking for the "ultimate practical transesterification" that could satisfy some of the tenets of green chemistry. The goals included having a 1:1 ratio of ester and alcohol reactants (atom economy), a neutral catalyst that is readily recoverable, and 100% conversion and yield.
Earlier work of Otera's group on tetraalkyldistannoxanes led to the idea that such a catalyst with polyfluoroalkyl groups, when dissolved in a perfluorocarbon solvent, could lead to a useful fluorous biphasic reaction system. In this system, the catalyst and solvent form one layer, while the ester and alcohol reactants form a separate organic layer. On heating, the two layers are sufficiently miscible for the reaction to occur; on cooling, the organic and fluorinated layers separate.
The system was tested by carrying out a series of millimolar-scale reactions of methyl and ethyl esters with various high-boiling alcohols, including some acid-sensitive alcohols and protected alcohols. One representative reaction was ethyl-3-phenyl-propionate with 1-octanol at 150 oC for 16 h that gave greater than 99% yield of octyl-3-phenylpropionate. The pure ester was recovered by evaporation of the ethanol coproduct.
The catalyst can be recovered from the perfluorocarbon solvent, the researchers note, but it is more practical to reuse the solution directly in subsequent reactions. The viability of the fluorous phase was thus demonstrated by running a set of 14 different esterifications consecutively with the same catalyst-solvent sample, each of which proceeded in near 100 % conversion and yield. In a future paper, the Okayama group plans to explore how the 1:1 reactions proceed so efficiently. - Steve Ritter
C&EN/OCTOBER, 1 2001
http://pubs.acs.org/CEN/
Trimerization of acetylenes yields aromatic
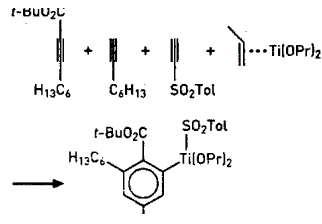
t-Bu=tert-butyl, Pr=isipropil, Tol=p-tolyl
A trimerization of acetylene derivatives devised by biomolecular researchers at Tokyo Institute of Technology in Kanagawa yields a phenyltitanium reagent that reacts to give a variety of substituted benzenes (J. Am. Chem. Soc., 123, 7925 (2001)]. Their reaction could lead to synthesis of "druglike" combinatorial libraries based on four-component reactions. In one example, biomolecular engineering professor Fumie Sato, graduate student Daisuke Suzuki, and biological information professor Hirokazu Urabe mix tert-butyl 2-nonynoate,1-octyne, and η2-propylene-titanium(II) isopropoxide to get a titanofuran intermediate, which reacts with p-toluenesul-fonylacetylene to give a 3,5-dihexyl-2-carbo-tert-butoxy-phenyltitanium reagent. The phenyltitanium reacts with protons, iodine, or benzaldehyde to replace the titanium. Total overall yields are 46 to 88 %.
FTIR screens catalyst library
Fourier transform IR imaging can be used to analyze the reaction products from an entire array of catalysts at once, according to researchers at Purdue University's School of Chemical Engineering [Angew. Chem. Int. Ed., 46, 3028 (2001)]. They claim that the technique is the first truly parallel high-throughput screening method for monitoring gas-phase reaction products from combinatorial catalysts libraries. Associate professor Jochen Lauterbach, research fellow Chris M. Snively, and graduate student Gudbjorg Oskarsdottir have developed a gas-phase array attached to a multiple sample reactor that currently can investigate 16 supported catalysts simultaneously. They demonstrated the technique by analyzing the oxidation of propene over commercial and custom-synthesized samples of platinum-group metal catalysts, recording FTIR absorbance images every 30 seconds as they increased the temperature. The experiment showed seven catalysts oxidizing propene to CO2 at 440 K, while 12 catalysts were active at 650 K. The technique is capable of screening hundreds to thousands of catalyst samples on a timescale of seconds, they conclude.
(по материалам V Европейского конгресса по катализу)
1. Общая информация
В начале сентября 2001 г. в г. Лимерик (Ирландия) проходил очередной V Европейский конгресс по катализу (EUROPACAT-V), который продолжил серию предыдущих конгрессов (Монпелье, 1993; Маастрихт, 1995; Краков, 1997; Римини, 1999). Как и на предшествующих форумах, этот конгресс собрал более 1000 ученых из большинства стран Европы, а также США, Японии, Китая, Индии и др., которые по традиции постоянно участвуют в европейских конгрессах по катализу. В целом, несмотря на некоторые организационные недоработки и, в основном, неприветливую, типично ирландскую погоду, конгресс, по общей единодушной итоговой оценке, можно считать успешным.
Выдающимися учеными из разных стран были прочитаны пять пленарных лекций, посвященных принципиально различным направлениям катализа. В лекции Д. Кинга (Кембриджский университет, Великобритания) на примере нескольких модельных реакций были продемонстрированы потенциальные возможности различных методов исследования поверхности для целенаправленного дизайна активных фаз. Ф. Фажюла (Национальная высшая химическая школа, Монпелье, Франция) представил убедительные свидетельства того, как можно управлять каталитическими свойствами микропористых, главным образом цеолитных, и мезопористых, в частности силикатных систем типа МСМ-41, в реакциях парциального окисления и кислотно-основного типа. Особенно подробно автор остановился на перспективах исследований сложных, высокоорганизованных систем на основе цеолитов и мезопористых материалов в тонком органическом синтезе.
Академиком А.Е. Шиловым (Институт биохимической физики РАН, Россия) была прочитана пленарная лекция, посвященная проблемам биокатализа (биомиметический катализ). Основное внимание докладчик уделил применению биомиметических систем для активации (окисления) молекул парафинов.
Г. Вайнберг (фирма "Symyx", США) в своей пленарной лекции впервые на подобном форуме изложил основы комбинаторной химии (методы скоростного и высокопроизводительного скрининга - high throughput experimentation/screening) применительно к синтезу и тестированию катализаторов различных классов. Комбикат в течение последних 2-3 лет стремительно ворвался в практику каталитического эксперимента. Разумное сочетание обоснованного научного подхода (планирование эксперимента, генерация идей, обоснованный выбор составов катализаторов и соответствие катализаторов реакциям, в которых они испытываются) и методологии высокоскоростного химического эксперимента (разработка надежных систем для приготовления и испытания "библиотек" катализаторов, аналитическая часть, алгоритмы обработки массивных результатов) определяет практическое использование комбинаторной химии в будущем.
Дж. Армор (фирма "Air Products and Chemicals", США) в заключительной пленарной лекции изложил перспективы и приоритетные направления в области катализа в новом тысячелетии, а также дал оценку основным достижениям катализа за последние 10 - 20 лет, в первую очередь успехам промышленного катализа. Среди приоритетных направлений следует отметить экологическую направленность катализа, использование возобновляемых энергетических и сырьевых ресурсов, смещение от задач базовой химии к проблемам тонкой химии и биохимии, возрастающую роль высокоорганизованных каталитических систем (наносистем), комплексных подходов в катализе.
Кроме пленарных лекций на соответствующих симпозиумах были также представлены 42 ключевые (keynote) лекции, посвященные анализу основных принципиальных направлений в катализе, которые в неменьшей степени, чем пленарные лекции, безусловно были полезны для специалистов, работающих в разных областях катализа.
Работа конгресса проходила в рамках 21 специального симпозиума, что почти вдвое превышало их количество на предыдущем конгрессе в г. Римини. Были организованы следующие симпозиумы:
Помимо пленарных и ключевых лекций на симпозиумах конгресса, проходящих на четырех параллельных секциях, было заслушано 333 устных доклада. В течение четырех дней также проходили постерные секции (724 стендовых доклада), на которых были представлены собственные результаты исследований и проходили обсуждения и встречи с учеными из разных стран.
Можно отметить некоторые существенные отличия последнего конгресса от предыдущих форумов.
1. Конгресс проходил "под знаменем" экологической химии ("green chemistry"): было выделено два специальных симпозиума, посвященных решению экологических проблем с помощью катализа ("Environmental Catalysis: End-of-Pipe Technology" и "Environmental Catalysis: Catalysis for Green Chemistry").
2. Более четко обозначились ценностные ориентиры в переработке углеводородного сырья (симпозиум "Hydrocarbon Valorization", на котором, кстати, было прочитано 7 ключевых докладов, тогда как на многих других - лишь по одному).
3. Во многих работах была представлена вся цепочка в разработке катализаторов и процессов: от лабораторных исследований до пилотных и полупромышленных испытаний, причем круг авторов не ограничивался учеными из университетов, но и включал сотрудников промышленных фирм.
4. Впервые был проведен симпозиум по комбинаторным методам в катализе; о возрастающей роли комбинаторной химии свидетельствует и приглашение в качестве пленарного докладчика Г. Вайнберга, директора фирмы "Symyx", одной из недавно (в 1995 г.) появившихся компаний, специализирующейся в этой области, а также в разработке оборудования для высокоэффективного скрининга катализаторов.
5. Появилось несколько новых симпозиумов, названия которых также свидетельствуют о том, что Европейский конгресс по катализу явно "позеленел" и ориентирован на практические цели: "Reactions in Unusual Solvents", "Industrial Catalysis", "Catalytic Reaction and Reactor Engineering", "Catalysis of Renewables", "Biocatalysis".
6. В симпозиуме по электрокатализу, который на предыдущих конгрессах обычно совмещали с фотокатализом, вторым направлением был катализ для топливных элементов (Fuel Cell Catalysis) - одна из стремительно развивающихся областей катализа. В свою очередь, фотокатализ был выделен как отдельный симпозиум ("Excited State Catalysis").
7. Можно отметить также появление значительного числа работ, в которых каталитические процессы проводились в новых средах: суперкритическом СО2, суперкритических углеводородах или в ионных жидкостях - низкотемпературных расплавах солей, содержащих органический катион (типа алкилимидазолия или алкилпиридиния) и неорганический анион (например, BF4-, PF6-, Al2Cl7-).
Что касается тематики докладов, то наибольшее их количество приходилось на разработку катализаторов для удаления оксидов азота, гидропроцессов в нефтепереработке с учетом новых требований к моторным топливам (изомеризация парафинов, гидрирование ароматики, гидродесульфуризация), окислительного дегидрирования низших парафинов, а также разработку и исследование новых каталитических материалов различной природы, отличающихся пористыми свойствами и другими ценными характеристиками. Самый крупный симпозиум конгресса, который фактически состоял из 4 подсимпозиумов, был посвящен фундаментальным проблемам катализа и исследованиям поверхности ("Fundamental Catalysis and Surface Science"). На нем было представлено рекордное количество ключевых докладов - 8.
2. Промышленный катализ
Проблемы промышленного катализа обсуждались хотя бы в одном докладе на каждом симпозиуме, кроме "Fundamental Catalysis and Surface Science", а наиболее подробно - на специальных симпозиумах "Industrial Catalysis", "Catalytic Reaction and Reactor Engineering", а также (в большей степени, чем на других) "Environmental Catalysis: End-of-Pipe Technology", "Hydrocarbon Valorization" и "Environmental Catalysis: Catalysis for Green Chemistry".
На конгрессе было представлено несколько докладов представителей таких компаний, разрабатывающих или использующих катализаторы, как "Shell", "Jonson-Metyu", "Haldor Topsoe", "ABB Lummus", "Institute Francais du Petrole", "Syntetics", "Solutia", "Sasol", "General Motors" и др. Остановимся лишь на некоторых, наиболее интересных докладах, посвященных разработке промышленных катализаторов и процессов.
На симпозиуме "Fisher-Tropsch Catalysis" в докладе от фирмы "Sasol" (П. Гибсон) был представлен последний вариант процесса Синтол (высокотемпературный процесс Фишера - Тропша) преимущественно для получения олефинов из синтез-газа, в котором селективность по олефинам достигает 70 %. Процесс реализован в промышленном масштабе на заводе в г. Секунда (ЮАР).
Дж. Шен (Департамент энергетики США) представил анализ процессов получения различных ценных продуктов из синтез-газа, разработанных по проектам Департамента энергетики США: метанола (процесс LPMeOHTM реализован в масштабе демонстрационной установки компанией "Eastman Chemical",
г. Кингспорт, США), диметилового эфира (процесс находится на заключительной стадии пилотных испытаний фирмой "La Porte", которая недавно была приобретена компанией "Degussa-Huels Corp.") и углеводородов (синтез Фишера -
Тропша на железосодержащих катализаторах).
Интересные данные по синтезу Фишера - Тропша в суперкритических условиях приведены в докладе K. Фуджимото. Такая технология суперкритики позволяет увеличить селективность по олефинам за счет снижения вклада процесса метанирования.
На различных симпозиумах были заслушаны доклады (Ф. Каптайн, К. Рамшев и др.) по новым реакторным технологиям, типам реакторов или структурированным катализаторам (монолиты, тарельчатые системы, сетки, ячеистые материалы и др.), которые во многих случаях продемонстрировали преимущества в сравнении с традиционными системами, в частности в экзотермических реакциях, например, парциальном окислении углеводородов, гидрировании различных соединений, получении продуктов из синтез-газа и др. Эти системы, очевидно, перспективны и для процессов, протекающих при ультрамалых временах контакта (short contact time processes), т.е. при скоростях газовых потоков ~ 0,5¸ 1,0 млн ч1.
Одним из перспективных направлений в катализе безусловно следует считать и каталитическую дистилляцию - процесс, сочетающий катализ и разделение продуктов и исходных веществ. Для технологии каталитической дистилляции характерны такие преимущества, как снижение капитальных и эксплуатационных расходов, увеличение выхода продукта для реакций, ограниченных термодинамическим равновесием, повышение энергетической эффективности благодаря использованию теплового эффекта реакции в процессе дистилляции, увеличение селективности по целевому продукту в результате разделения непосредственно в каталитическом реакторе, снижение количества отходов и побочных продуктов, а также увеличение срока эксплуатации катализатора. Эта технология подробно обсуждалась в докладах Ф. Даутценберга и Ф. Нг на примере гидрирования ароматических соединений, олигомеризации олефинов и альдольной конденсации ацетона.
В докладе Дж.-П. Ланге были обсуждены пути повышения эффективности каталитических процессов (выбор сырья, снижение потерь на теплообмене, повышение селективности и т.д.), рассмотрены экономические критерии внедрения новых каталитических процессов, в частности были определены пороговые величины активности (производительности), ниже которых промышленное использование процесса представляется невыгодным (0,1 т продукта / (т× ч) катализатора), и пороговый показатель стабильности (расход катализатора не должен превышать 1 кг/т продукта). Были указаны наименее экологически чистые каталитические процессы и пути повышения эффективности таких производств, как получение капролактама, метилметакрилата, нитросоединений и др.
К.В. Гент в своем докладе рассмотрел возможность применения циклов Кондратьева для описания экономических тенденций и выработки долгосрочных прогнозов в промышленной химии, которая, как известно, на ~90 % базируется на каталитических процессах. Однако автор явно недоучел роль политических, энергетических и других факторов в формировании такого рода прогнозов. Большой интерес аудитории вызвал также анализ перемен, происходящих в сфере кооперации промышленной и университетской (академической) науки.
В докладе П. Ноттe (фирма "Solutia") в очередной раз были показаны преимущества процесса окисления бензола в фенол с использованием мягкого окислителя -
закиси азота, разработанного совместно с Институтом катализа СО РАН (г. Новосибирск) (процесс AlphOxв). В этой связи интересен также доклад
A. Райтцмана, в котором были представлены новые системы для получения N2O восстановлением NO под действием водорода или синтез-газа с использованием алюмородиевых или алюмопалладиевых катализаторов. Выход закиси азота при восстановлении водородом или синтез-газом достигает 70 и 95 %, соответственно. Катализаторы показывают высокую стабильность в отличие от процесса с использованием СО как восстановителя.
В связи с проблемой ограничения использования метил-трет-бутилового эфира как высокооктановой добавки к бензинам, обсуждающейся в последнее время на всех уровнях, значительный интерес представляет работа M. Сильвекоски, посвященная димеризации изобутена в изооктан с использованием ионообменных смол в качестве катализаторов.
Еще один каталитический процесс на кислотных катализаторах -
перегруппировка Бекмана на борсодержащих цеолитах ZSM-5 с целью получения капролактама -
обсуждался в докладе
В. Холдрич.
Участники конгресса продемонстрировали много примеров сокращения числа стадий и повышения селективности для различных процессов основного и тонкого органического синтеза.
Около 20 участников из России представили в общей сложности около 60 устных и стендовых докладов.
В.А. Садыков (с соавторами) выступил с пятью устными (не считая стендовых) докладами на 3 разных симпозиумах, чем, безусловно, произвел впечатление на участников конгресса, в первую очередь разноплановостью исследований и широким применением различных физико-химических методов.
Большое внимание привлек доклад А. Белого, В.К. Дуплякина, В.А. Лихолобова и В.Н. Пармона, посвященный разработке методов и катализаторов для процесса получения жидкого углеводородного топлива с целью вовлечения легких алканов в целевые продукты.
В докладе К.Г. Ионе обсуждались цеолитные катализаторы (типа пентасила) и процесс получения моторных топлив из прямогонного бензина, попутных газов и газов, содержащих олефины (процесс Zeoforming). Процесс внедрен в России (Нижневартовский газоперерабатывающий завод) и Польше (завод в г. Горлице).
В ограниченном объеме настоящего сообщения, к сожалению, невозможно дать полный и обстоятельный анализ тенденций, прослеживающихся в последние годы в промышленном катализе, равно как и представить все интересные работы, обсуждавшиеся на конгрессе. Возможно, это станет предметом отдельной публикации на страницах журнала "Катализ в промышленности".
Л.М. Кустов
ИОХ им Н.Д. Зелинского РАН, г. Москва
Перепечатано с разрешения журнала
"Катализ в промышленности", N1, 2002 г
Глубокоуважаемые читатели!
Мы все прекрасно понимаем, как сильно впечатление и эффект от результатов Вашей научной работы зависят от удачно найденного стиля изложения опубликованной статьи и, тем более, от удачно сделанного Вами доклада на научных конференциях.
Существуют несложные, но, к сожалению, практически неформализированные и поэтому малоизвестные алгоритмы несомненного повышения вероятности успеха от презентации результатов Вашей работы. Поэтому было очень приятно видеть на прошедшем недавно конгрессе "Европакат-5" небольшую брошюру с соответствующими рекомендациями, подготовленную Президентом Европейской федерации каталитических обществ (EFCATS) 1999-2001 годов профессором Дж. В. Ньемантсвердритом. Мы с удовольствием предлагаем нашим читателям перевод этой брошюры, любезно подготовленный для Каталитического бюллетеня Н.С. Крыловой.
EFCATS
Европейская Федерация каталитических обществ
Дж. В. Ньемантсвердрит
Институт катализа, Технологический Университет, Эйндховен, Нидерланды
Часть 1. Как подготовить успешное устное выступление
Создайте собственный стиль выступлений...
... но старайтесь не делать типичных ошибок
Введение
Часто ли, слушая сообщение по интересному научному вопросу, вам с трудом удавалось удержать внимание до конца доклада? Часто ли вы теряли интерес, когда докладчик не рассказал и половины? Что было причиной: сам предмет доклада или способ представления материала?
Во многих выступлениях предлагается интересный материал, но тем не менее, сообщение воспринимается с трудом, поскольку докладчик неосознанно совершает множество ошибок, связанных с формой изложения. Наверное, самое серьезное упущение состоит в том, что выступающий не понимает, как аудитория слушает. Если вы четко представляете, каких ошибок следует избегать, высока вероятность, что вам удастся значительно повысить эффективность своего выступления.
Кривая уровня внимания
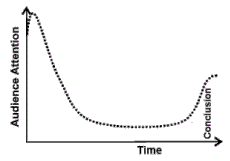
Как правило, участник конференции готов вас выслушать, но при этом его нетрудно отвлечь. Вы должны понимать, что лишь немногие пришли специально на ваше выступление. Остальные могут оказаться на вашем докладе по самым разным причинам: чтобы дождаться следующего сообщения, составить общее представление об области исследований, или зачем-то еще.
Рассмотрим, как меняется внимание усредненной аудитории во время типичного выступления, рассчитанного, к примеру, на 30 минут. Сначала слушают все, но где-то на половине доклада уровень внимания может снизиться до 10-20% от начального. Ближе к завершению многие опять начинают слушать, особенно если вы переходите к выводам, поскольку надеются что-то извлечь из вашего выступления.
Что стоит предпринять для удержания внимания слушателей в течение всего доклада? Несколько рецептов можно извлечь непосредственно из кривой уровня внимания:
Почему слушатели отвлекаются?
Для этого может быть немало причин, некоторые из них от вас не зависят, например, плохой микрофон, некачественный проектор, шумный конференц-центр с картонными перегородками между комнатами, в которых параллельно идут две сессии. Все, что в ваших силах, - это стараться избегать ошибок, которые могут побудить аудиторию прекратить слушать. Такие ошибки делятся на два класса: ошибки докладчика и ошибки презентации. Ниже приводятся наиболее типичные ошибки, большинство из них не нуждаются в комментариях.

Как организовать ваше выступление
Следует иметь в виду принципиальное отличие устного выступления от письменного сообщения. На лекции слушатель поневоле следует тому порядку, в котором докладчик излагает материал. При чтении статьи можно перескочить от одной части к другой, вернуться к разделу о материалах, знакомясь с результатами, заглянуть в выводы, и т.п. Именно поэтому все научные статьи имеют примерно одинаковую структуру: резюме, введение, экспериментальные методы, результаты, обсуждение, выводы, ссылки. Однако такая структура совершенно неуместна в устном выступлении. Тем не менее, большинство докладов на конференциях следуют именно этому порядку.
Почему эта общепринятая структура не подходит для лекций? Потому что слушателю приходится держать в голове детали экспериментальных методов до изложения результатов и припоминать различные результаты, когда докладчик переходит к обсуждению. Другими словами, детали, которые следует объединять
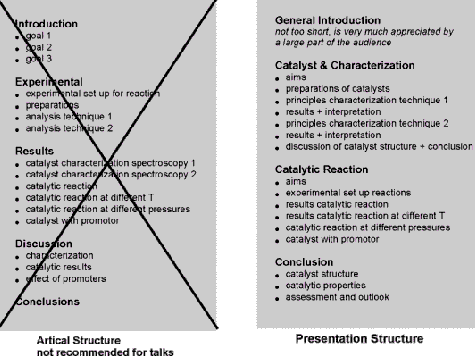
(почему, как, что, и что все это значит в отдельном эксперименте), рассматриваются по отдельности. Вы слишком многого требуете от слушателей, рассчитывая, что они будут помнить все эти факты и рисунки до заключительного момента, когда вы объясните, как части складываются в целостную картину.
Гораздо лучший способ организации выступления состоит в группировке сведений, связанных друг с другом. Так, если вы обсуждаете характеризацию, например, методом XPS, вы начинаете эту часть выступления несколькими замечаниями о том, что вы хотите узнать о рассматриваемом катализаторе, как метод XPS поможет получить эту информацию, затем показываете несколько результатов и обсуждаете их значение. Завершаете выводом. Затем переходите к следующему вопросу, например, к определению размера частицы методом TEM. Покончив с этим вопросом, можете сделать общее заключение о состоянии вашего катализатора, прежде чем перейти к обсуждению поведения катализатора.
Десять шагов в подготовке успешного выступления
Вы должны четко осознавать два ключевых момента в подготовке доклада:Послание: что должно остаться в головах слушателей, когда я закончу выступление.Аудитория: как построить выступление, чтобы слушатели поняли и запомнили мое сообщение.
1) Начните вовремя
Как только вы подали заявку на участие в конференции, пришло время задуматься, как организовать материал при выступлении, если ваша заявка будет принята. Читайте материалы по истории вопроса, знакомьтесь с работами по теме, регулярно обращайтесь к собственным результатам и обдумывайте наиболее уместные выводы. Постарайтесь представить, с какими слушателями придется иметь дело, и подумайте, какие сведения общего характера стоит включить в доклад

2) Послание
Постарайтесь выразить основную мысль вашего доклада в одном предложении. Это сложно. Вам удастся это сделать, только если вы действительно владеете своим предметом (что является основным условием, позволяющим ясно изложить материал слушателям).
3) Проведите отбор и упорядочение результатов
Используйте предложение, обозначенное в пункте (2), как критерий определения того, какие результаты включить в доклад, в каком порядке, какая общая информация нужна для восприятия этих результатов, какие детали эксперимента необходимы, а какие нет. Отбор должен быть строгим, следует отбросить любой эксперимент или результат, который не помогает донести основную мысль вашего сообщения.
Хотя на первый взгляд может показаться естественным представить результаты в хронологическом порядке их получения, этот порядок не обязательно окажется идеальным для лучшего понимания слушателями того, что вами сделано. Подумайте, когда лучше обсудить основные моменты: в начале, ближе к концу, или распределить их по всему докладу. Вам решать, но лучше выбрать порядок, который, на ваш взгляд, больше устроит аудиторию.
Насколько подробно вам придется остановиться на описании экспериментальных подходов и методов характеризации, зависит от научной подготовленности ваших слушателей. Постарайтесь НЕ отождествлять аудиторию с вашим руководителем, вряд ли большинство слушателей имеет достаточно специальных знаний по вашему предмету. К тому же вряд ли кто-то будет возражать против того, чтобы услышать что-то уже известное, если изложение ведется на хорошем уровне и, возможно, занимательным образом.

4) Начало и введение
В начале, то есть в первых предложениях, вы привлекаете внимание, например, самим научным вопросом либо броским и даже провокационным заявлением. Может быть, стоит тут же привести выводы вашей работы. Старайтесь говорить неторопливо, с интонационными выделениями и следите за аудиторией. Конечно, нужно тщательно подготовить и выучить первые предложения.
Однако, прежде чем произнести первую фразу, хорошо бы начать с обращения: "Господин председатель, леди и джентльмены ...", затем сделать паузу в несколько секунд и обвести взглядом аудиторию, проверяя, проявляют ли люди внимание. Тем самым вы реально побуждаете аудиторию слушать. С помощью этих слов вы также проверяете микрофон, убеждаясь, что важные первые предложения вашего выступления будут услышаны.
В оставшейся части вступления вы очерчиваете предысторию вашего исследования. Помните, что многих слушателей очень заинтересует краткое описание состояния вашей области исследований. Поэтому выделите достаточно времени (по меньшей мере 30% всего выступления) на представление общих аспектов вашей работы. Хорошо, если вы не только четко определите рассматриваемую научную проблему, но и сформулируете выводы своей работы. Тем самым вы позволите аудитории легче следить за ходом рассуждений и предвосхищать результат экспериментов. Иначе говоря, вы предоставите слушателям возможность активно воспринимать ваше сообщение. Не забывайте, что научное выступление - это не детективный рассказ, где развязка происходить лишь в последний момент.
5) Выводы и заключение
Чтобы привлечь внимание всей аудитории, следует четко объявить, что сейчас вы представите выводы. Формулируйте ваши выводы в сопоставлении с вопросами, поднятыми во введении. Исключите все лишние детали. Представив все выводы, можете поблагодарить тех, кто вам помогал (но не соавторов, перечисленных в программе), а также организации-спонсоры. Можно завершить выступление заключительной фразой, повторяющей главную мысль вашего выступления, например:
"Леди и джентльмены, надеюсь, я показал вам, что носитель XY является очень перспективным катализатором превращения метана в синтетический бензин при комнатной температуре". Это то сообщение, которое останется в голове слушателя, которое он должен запомнить, желательно, в сочетании с вашим именем и местом работы.
6) Очень важно хорошо сделать рисунки
Рисунок стоит тысячи слов. Но не всякий рисунок. Иллюстрации, особенно подготовленные с помощью электронных средств, могут быть очень точными и аккуратными, но в то же время могут представлять собой настоящие головоломки. Хороший рисунок отвечает следующим требованиям:
Так, приводя ряд спектров или кривых активности, на каждую кривую вы помещаете понятное обозначение (но не буквы a, b, c, которые объясняются в отдельной подписи!!). Избегайте кодового обозначения образцов, например, "образец AX234/a5", - такие обозначения уместны в лабораторном журнале, но неприемлемы в выступлении (а также в статьях).
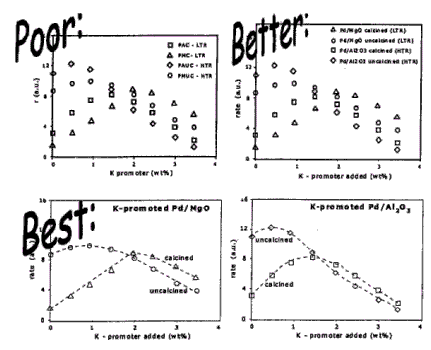
В большинстве случаев не рекомендуется использовать числовые таблицы. Помните, что слушатели прочитывают все, что показано на экране, и во время чтения они меньше внимания уделяют тому, что вы говорите. Не стоит перегружать выступление теоретическими формулами и математическими выкладками. Конечно, иногда они необходимы, но лучше свести использование этих элементов к минимуму. Вы должны понимать, что человеческая память хранит информацию в образной форме. Поэтому четкие рисунки, схемы и диаграммы - это лучший способ передачи информации.
7) Иллюстративные материалы: прозрачки (диапозитивы), слайды или компьютерный проектор?

При показе прозрачек с помощью простого проектора особых проблем обычно не возникает. Как правило, рисунки хорошо видны, слушателям легко их прочесть, комнату не нужно затемнять, что помешало бы при желании вести записи. Вам, как докладчику, использование прозрачек предоставляет возможность в любой момент внести в рисунок изменения или даже написать на нем что-то во время выступления.
Слайды не обеспечивают такой гибкости. Конечно, оптимально подготовленные слайды в сочетании с высококачественным проектором предоставляют прекрасную зрительную поддержку вашего выступления. К сожалению, качество многих слайд-проекторов далеко от оптимального, и к тому же, многие докладчики показывают неудачные слайды. Кроме того, может заесть проекционный механизм, слайды могут оказаться перевернутыми вниз головой,возможны сбои в управлении проектором и т.д. Еще одно неудобство использования слайдов - это необходимость затемнения лекционной комнаты, при этом слушателям трудно делать записи. А если изложение недостаточно увлекательно, кто-то может и заснуть...
В последнее время становится популярным компьютерный показ с использованием лучевого проектора. Несомненно, это прекрасная возможность создания впечатляющих эффектов. Однако большинство переносных лучевых проекторов не обеспечивают достаточной яркости в больших конференц-залах, и лишь немногие конференц-центры имеют встроенные высококачественные проекторы. Кроме того, программное обеспечение предоставляет такое разнообразие возможностей, что лекторы часто создают неприемлемые цветовые сочетания и неудачное фоновое оформление. На самом деле, старомодные средства не так уж плохи...

8) Общение вместо представления
Ваше выступление выиграет, если вы будете излагать материал тем же повседневным языком, которым пользуетесь в лаборатории, давая разъяснения студенту. Нет никакой необходимости использовать более формальный язык. На самом деле, формальный язык вовсе не нужен, поскольку мешает слушателям понять смысл сообщения. Не пытайтесь поразить аудиторию причудливыми выражениями, формальными конструкциями, узкопрофессиональным жаргоном или излишними сокращениями. Думайте о выступлении в терминах общения, не рассматривайте его как постановку литературной пьесы. Слушателей порадует, если за вашим рассказом будет легко следить.
9) Хронометраж совершенно необходим
Теперь наступает момент истины: укладывается ли подготовленный вами материал в заданные временные рамки? Выяснить это можно единственным способом: возьмите секундомер и начинайте. Обычно такой опыт разочаровывает. Во-первых, вы можете обнаружить, что предложения просто не подходят. В таких случаях мое решение - сесть и написать первую часть ясными, короткими предложениями. Во-вторых, скорее всего, окажется, что материала слишком много. Следовательно, придется урезать материал, но я все же надеюсь, что вы не слишком сократите общее введение. Помня о кривой уровня внимания, лучше выбросить несколько не самых важных моментов в середине сообщения. Никогда не сокращайте за счет введения и выводов!
Тщательный хронометраж вашего выступления крайне важен. Превышение времени - это оскорбление аудитории и следующего за вами докладчика, особенно если сессии идут параллельно. Нет ничего более обескураживающего, чем ситуация, когда председатель вынужден прервать вас прежде, чем вам удастся изложить выводы!
10) Вы нервничаете? Надеюсь, это так!

Немногие из нас родились талантливыми ораторами. Почти каждый волнуется перед выступлением. У начинающих нервозность может легко превратиться в неуверенность, вызванную ощущением неопытности.
Часто начинающие воспринимают свое волнение как знак того, что они совершенно не способны хорошо выступить. Это неправильно. Все симптомы нервозности, такие как частое сглатывание, дрожь, потение и др., это признаки того, что ваш организм готовится к чему-то важному. Гимнасты, актеры, музыканты и ... опытные ораторы научились распознавать эти симптомы и правильно их оценивать. Их тревожит, если эти симптомы отсутствуют!
Опыт - это то, что приходит со временем, из практики и анализа своих и чужих выступлений. Никто не упрекнет вас за то, что вы новичок. Однако, если вы постараетесь избежать типичных ошибок, которые делают начинающие (и даже опытные ораторы), это будет хорошим началом вашей карьеры докладчика. Если вы знаете и понимаете основные принципы и умеете их применять, скорее всего, вам удастся выступить значительно лучше по сравнению со средним уровнем выступлений на международных встречах. Таким образом, недостаток опыта не столь важен, если ваше сообщение хорошо подготовлено и вы стараетесь избежать ошибок, перечисленных в этой брошюре.
По сути, рассмотренные десять шагов сводятся к двум принципиальным моментам: 1) какую основную мысль я хочу донести до слушателей, 2) как помочь аудитории лучше понять эту мысль. Понимание того, как люди слушают и запоминают - ключ к подготовке выступления, которое многим понравится.
Окончание статьи в следующем номере.


