15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы
24 декабря 2025
Ученые создают катализаторы эффективной переработки пищевых масел в экологичное авиатопливо

12 июня 2010 г. Президент РФ Дмитрий Медведев вручил Государственную премию Российской Федерации в области науки и технологий за 2009 год на торжественной церемонии в Георгиевском зале Большого Кремлевского дворца
ВАЛЕНТИНУ НИКОЛАЕВИЧУ ПАРМОНУ
"За вклад в развитие теории и практики каталитических методов глубокой переработки углеводородного сырья и использования возобновляемых ресурсов”

Валентин Николаевич Пармон родился 18 апреля 1948 г. в г. Бранденбурге (ГДР). Директор Института катализа Борескова Сибирского отделения РАН, д.х.н., профессор, академик РАН. Награжден орденами Почета (1999) и За заслуги перед Отечеством IV ст. (2007), медалью Франциска Скорины Республики Беларусь (2009), лауреат Премии за инновации в катализе Европейской федерации каталитических обществ EFCATS (2005).
В.Н. Пармон – крупный ученый и признанный лидер в области катализа и фотокатализа, химической кинетики в конденсированных фазах, химической радиоспектроскопии, химических методов преобразования энергии, нетрадиционных и возобновляемых источников энергии, а также термодинамики неравновесных процессов. Глава научной школы в областях: фотохимические и термохимические способы преобразования солнечной энергии. Радиационно-термический катализ. Фотокатализ в глобальной химии земной атмосферы. Абиогенный катализ в природе. Получение наноматериалов. Новые типы химических реакторов. Автор и соавтор более 650 научных работ, 6 монографий, 6 учебников для ВУЗов, обладатель более 100 авторских свидетельств и патентов. Профессор В.Н. Пармон активно участвует в подготовке молодых ученых. Более 25 лет он преподает, а последние 15 лет возглавляет кафедру физической химии факультета естественных наук НГУ. Среди его учеников доктора и кандидаты наук.
В.Н. Пармоном впервые выведено (ставшее классическим) уравнение кинетики туннельных реакций в твердой фазе с равномерным пространственным распределением реагентов, широко используемое специалистами, исследующими природный фотосинтез.
В области фотокатализа и применения катализа для решения энергетических проблем лауреатом разработаны научные основы фотокаталитических методов преобразования солнечной энергии в химическую (разложение воды на водород и кислород в искусственных системах).
В.Н. Пармоном создано новое научное направление – радиационно-термический катализ. Под его руководством сконструированы и испытаны не имеющие мировых аналогов солнечные каталитические реакторы, остающиеся сегодня самыми эффективными из известных (эффективность преобразования солнечной энергии достигает 43% при полезной мощности 2 кВт).
Лауреатом создан принципиально новый подход к прямому преобразованию ионизирующего излучения в энергию химических топлив. В результате предложен и испытан принципиально новый энергозапасающий и энергопреобразующий процесс “ИКАР”, перспективный для решения многих проблем ядерной и термоядерной энергетики будущего. Впервые созданы и испытаны не имеющие мировых аналогов катализаторы на основе оксидов урана, комбинирующие функции ядерного топлива и катализатора для аккумуляции химической энергии.
В.Н. Пармоном разработаны и испытаны новые уникальные композиционные материалы для обратимого аккумулирования низкопотенциального тепла. Ряд разработанных материалов производится серийно и поставляется на промышленные предприятия России в качестве высокоэффективных осушителей специального назначения.
В.Н. Пармон руководит рядом важных инновационных направлений по разработке каталитических технологий для глубокой переработки ископаемого углеводородного сырья и структурной перестройки сырьевой базы химической промышленности и энергетики. Заложенные им подходы развиваются в настоящее время в мире как основные для получения высококачественных углеводородных топлив из возобновляемого растительного сырья.
Под научным руководством лауреата в 2003-2006 гг. разработаны и промышленно внедрены катализаторы нового поколения для производства моторных топлив. Российскими нефтяными компаниями только за 3 года действия проекта было произведено и реализовано дополнительной продукции на сумму более 8,3 млрд. рублей, что в 16 раз превышает объем затраченных бюджетных средств.
Под руководством В.Н. Пармона разработана и прошла опытно-промышленную апробацию первая отечественная технология переработки попутных нефтяных газов в смесь жидких ароматических углеводороводов, позволяющая решать проблему утилизации попутных нефтяных газов.
Кроме того, в последнее десятилетие под его руководством разработаны и переданы для крупномасштабного использования в отечественной промышленности новейшие поколения разнообразных катализаторов, в том числе для получения азотной кислоты (ежегодный эффект – экономия 200 кг платины), получения сверхпрочного полимера СВМПЭ, а в последние два-три года - для гидрирования технических и пищевых жиров с экономическим эффектом (за счет удешевления и возможности расширения производства) более 500 млн. рублей. С прошлого года успешно эксплуатируется первая полногабаритная коммунальная котельная с использованием каталитического сжигания топлив, обеспечившая двукратную экономию угля для снабжения теплом поселка Артышта в Кемеровской области.
Возглавляемый В.Н. Пармоном Институт катализа им. Г.К. Борескова стал одним из лидеров по масштабам инновационной деятельности в стране в области химической промышленности и природоохранных технологий. В кооперации с европейскими партнерами также ведутся успешные работы по новым перспективным направлениям энергетики и транспорта (получение высококачественных топлив из возобновляемого растительного сырья, создание компактных генераторов водорода и др.).
Теперь в истории Института катализа известны две Государственные премии, которыми отмечены выдающиеся заслуги сотрудников в реализации новых химических технологий. Первая, за 1986 г,. присуждена Г.К. Борескову (посмертно) за разработку технологии спецпродукта в коллективе НПО “Алтай” во главе с академиком Г.В. Саковичем. Вторая, за 2009 г., отражает как личный вклад В.Н. Пармона в развитие теории и практики каталитических процессов, так и достижения всего коллектива Института, которым он руководит.
Научный совет по катализу ОХНМ РАН и редакция Каталитического бюллетеня от всей души поздравляют Валентина Николаевича с высокой государственной наградой, желают ему крепкого сибирского здоровья, семейного счастья, талантливых учеников, новых творческих достижений и успешной реализации всего задуманного.

В период с 13 по 16 апреля 2010 года в пос. Листвянка в конф.-зале Байкальского музея СО РАН прошел IV Семинар “Молекулярный дизайн катализаторов и катализ в процессах переработки углеводородов и полимеризации”, приуроченный к 75-летию со дня рождения профессора Юрия Ивановича Ермакова. Основными организаторами семинара выступили Институт катализа им. Г.К. Борескова СО РАН (ИК СО РАН, г. Новосибирск), Институт проблем переработки углеводородов СО РАН (ИППУ СО РАН, г. Омск), Институт химии и химической технологии СО РАН (ИХХТ СО РАН, г. Красноярск), Научный совет по катализу ОХНМ РАН (г. Москва), Байкальский музей ИНЦ СО РАН (БМ ИНЦ СО РАН, пос. Листвянка Иркутской обл.). Финансовую поддержку семинару оказали Российский фонд фундаментальных исследований (РФФИ), ИК СО РАН, ИППУ СО РАН, БМ ИНЦ СО РАН.

В семинаре приняли участие 77 специалистов из 10 городов России (Новосибирск, Омск, Москва, Красноярск, Иркутск, Черноголовка, Пермь, Томск, Нижнекамск, Ангарск) и 1 из Азербайджана (Баку). Из них: 75 – представители академических институтов и ВУЗов, 3 – представители промышленных предприятий. На семинаре были представлены доклады по результатам фундаментальных и прикладных исследований, направленных на изучение процессов синтеза и применения различных типов катализаторов, механизмов их действия для процессов нефте- и газохимии, нефтепереработки, органического синтеза, полимеризации олефинов. Научная программа семинара была сформирована по 3 направлениям: “Катализ в процессах переработки углеводородного сырья”, “Каталитическая полимеризация олефинов”, “Металлокомплексный катализ”. В программе семинара были представлены: ключевые лекции (40 мин) – 11; устные доклады (20 мин) – 18; устные сообщения (10 мин), включая доклады молодых ученых – 25.
На секции “Катализ в процессах переработки углеводородного сырья” в основном были представлены доклады специалистов ИК СО РАН и ИППУ СО РАН. В ключевой лекции В.И. Бухтияров (ИК СО РАН) рассказал о новых методах целенаправленного синтеза дисперсных образцов безхлоридных палладиевых и платиновых катализаторов на наиболее распространенных оксидных носителях с узким и контролируемым распределением наночастиц активного компонента по размерам. В результате были выявлены прямые корреляции между размером частиц активного компонента и каталитической активностью для некоторых процессов полного окисления и гидрирования. Установленные размерные эффекты, оптимизация химического состояния и характера взаимодействия с поверхностью носителя могут быть использованы для разработки более активных и селективных катализаторов окисления и гидрирования при минимальном содержании благородного металла.
Ключевая лекция А.Н. Старцева (ИК СО РАН) была посвящена описанию механизма реакции гидрогенолиза C – S – связи на сульфидных катализаторах гидроочистки в рамках льюисовской кислотно-основной теории.
Создание нового эффективного катализатора олигомеризации этилена в изоалкены С5+ на основе системы NiO/B2O3-Al2O3 было рассмотрено в лекции А.В. Лавренова (ИППУ СО РАН). По результатам оптимизации химического состава нового катализатора и условий его получения показано, что на предлагаемой системе возможно практически полное превращение этилена при выходе жидких продуктов олигомеризации до 90.0 мас%, суммарное содержание алкенов С8+ в которых составляет 89.0 мас%.
Белый А.С. (ИППУ СО РАН) посвятил устный доклад молекулярному дизайну активных центров бифункциональных катализаторов риформинга, изомеризации углеводородов, а также новым технологиям, связанным с вовлечением в переработку нефтяного и попутного газа (процессы Биформинг, Бинар, Экоформинг). В качестве основных результатов, полученных в последние годы, были рассмотрены: установление строения и структуры активных центров катализаторов реакции ароматизации алканов; исследование закономерностей нанесения прекурсоров на топографию их распределения в пористой структуре носителя, разработка приемов регулирования пористой и кристаллической структуры алюмоокисных носителей, разработка и создание новых методов модифицирования кислотных свойств и степени дефектности наночастиц катализатора.
В устном докладе Смоликова М.Д. и соавт. (ИППУ СО РАН) на результатах исследования изомеризации н-гексана на платиносодержащем цирконосульфатном катализаторе в сочетании с результатами ИКС и изотопного обмена, показано: адсорбция водорода на атомах окисленной платины с образованием гидрид-ионов и протонов способствует модификации на поверхности катализатора кислотных центров двух типов - БКЦ и ЛКЦ. Гетеролитическая адсорбция водорода обуславливает регенерацию и образование новых кислотных центров, а также изменяет кислотность существующих центров в условиях осуществления реакции изомеризации н-гексана в среде водорода. Образование гидрид-ионов на окисленной платине также может способствовать ускорению завершающей стадии реакции изомеризации – гидридному переносу и сокращению времени жизни промежуточных карбкатионов изомерного строения.
Дроздов В.А. и соавт. (ИППУ СО РАН) рассмотрел в докладе особенности формирования активных алюмохлоридных комплексов in situ в среде жидкого изобутана из активированного алюминия и трет-бутилхлорида. Впервые показана возможность проведения процесса жидкофазного алкилирования изобутана бутенами при таком способе получения алюмохлоридного катализатора.
Доклад Абасова С.И. и др. был посвящен изучению влияния способов нанесения элементов на реакционную способность связанного кислорода, содержащегося в M, ReOx /Al2O3 (где M = Ni, Co или Pt), и активации реакций превращения метана в бензол и дегидроалкилирования бензола пропаном. Связанный кислород, содержащийся в Pt, ReOx /Al2O3 контактах, приобретает реакционную неоднородность благодаря взаимодействию платины, находящейся в высокодисперсном состоянии, с оксидом рения. Часть этого кислорода участвует в образовании С-С связей из двух молекул метана или связи между пропаном и бензолом, активируя реакции превращения метана в бензол или дегидроалкилирование бензола пропаном.
По направлению “Каталитическая полимеризация олефинов” были представлены ключевые и устные доклады от четырех организаций: Института катализа СО РАН, (г. Новосибирск), Института химической физики РАН (г. Москва) Института проблем химической физики РАН (Черноголовка) и Иркутского Государственного университета. Представленные доклады охватывают широкий круг каталитических систем (гомогенные и нанесенные металлоценовые и постметаллоценовые катализаторы, а также современные высокоэффективные титанмагниевые катализаторы) и многие вопросы, связанные с их синтезом, исследованием состава и применением в процессах полимеризации.
В ключевой лекции Л.А. Новокшоновой (ИХФ РАН) освещены результаты, полученные при исследовании полимеризации этилена на нанесенных цирконоценовых катализаторах (носитель монтморрилонит/метилалюмоксан). На таких системах может быть получен композиционный материал с нанодисперсным метилморрилонитом, обладающий интересными свойствами. Получены данные о неоднородности активных центров этих систем с использованием методики анализа органических продуктов термодесорбции активированных катализаторов.

В ключевой лекции Е.П. Талзи (ИК СОРАН) представлены новые результаты спектральных исследований гомогенных катализаторов различного состава (комплексы Со, V и Cr с различными активаторами) для полимеризации этилена и тримеризации этилена. Методом 1Н и 13С-ЯМР спектроскопии установлено образование в присутствии этилена комплексов Со (I) в качестве предшественников активных центров. В случае полимеризации этилена в присутствии полифенолятных комплексов ванадия с использованием метода ЭПР получены данные о существенном влиянии хлорорганического активатора на стабилизацию парамагнитных соединений V (IV) как возможных предшественников активных центров.
В ключевой лекции В.А. Захарова (ИК СО РАН) рассмотрены процессы образования и функционирования поверхностных гидридов титана, циркония и железа в нанесенных катализаторах различного состава и представлены кинетические данные о роли этих соединений в различных реакциях в процессе полимеризации на этих катализаторах.
Значительная часть устных докладов была посвящена кинетическим и физико-химическим исследованиям гомогенных металлоценовых и постметаллоценовых катализаторов полимеризации, в частности, установлению роли триметилалюмния на формирование и реакционную способность ряда постметаллоценовых комплексов Ti и Zr (Н.М. Бравая, ИПХФ РАН), изучению сополимеризации пропилена с высшими α-олефинами в среде жидкого пропилена на изоспецифических гомогенных металлоценовых катализаторах (П.М. Недорезова, ИХФ РАН), исследованию полимеризации этилена на гомогенных и нанесенных полифенолятных комплексах ванадия (Н.В. Семиколенова, Институт катализа), определению числа активных центров и константы скорости роста при полимеризации этилена на гомогенных бис(имино)пиридильных комплексах Со, V и Cr (А.А. Барабанов, Институт катализа), изучению процессов образования циклопентадиенильных комплексов Ni (I) и их роли в олиго- и полимеризации олефинов (А.И. Вильмс, Иркутский гос. университет).
В ряде докладов были представлены результаты кинетических и физико-химических исследований полимеризации олефинов на нанесенных титанмагниевых катализаторах, в частности, данные о влиянии электронодонорных стереорегулирующих соединений различного состава на молекулярную массу и молекулярно-массовое распределении полипропилена (Г.Д. Букатов, Институт катализа), результаты разработки нового поколения нанесенных титанмагниевых и ванадиймагниевых катализаторов для полимеризации этилена и сополимеризации этилена с α-олефинами (Т.Б. Микенас, Институт катализа), данные о возможностях синтеза изотактического полибутена на нанесенных титанмагниевых катализаторах в сочетании с полидентатными оксидами фосфинов (О.И. Кудинова, ИХФ РАН).
В части докладов основное внимание уделено исследованию возможностей получения новых полимерных материалов и изучению их молекулярной структуры и физико-механических свойств. В докладе Л.Н. Бревнова (ИХФ РАН) представлены данные о синтезе полиолефинов на цирконоценовых катализаторах, иммобилизованных на слоистых носителях с получением полимерных композиций, содержащих наноразмерные алюмосиликатные структуры и данные об их свойствах. Особенности молекулярной структуры сополимеров этилена с α-олефинами, получаемых на полицентровых нанесенных катализаторах циглеровского типа, рассмотрены в докладе М.А. Мацько (Институт катализа). Данные о структуре и свойствах композитных полимерных материалов, получаемых последовательной гомополимеризацией этилена и сополимеризацией этилена с α-олефинами на гомогенных и нанесенных металлоценовых катализаторах, представлены в докладе Т.М. Ушаковой (ИХФ РАН).
В целом в этой секции были представлены доклады по различным аспектам каталитической полимеризации олефинов с использованием катализаторов различного типа и состава и охватывающие методы получения этих катализаторов, физико-химические исследования процессов их формирования и состава активных центров, кинетические данные о процессах полимеризации, результаты изучения молекулярной структуры полимеров и получения полимерных материалов с различными свойствами.
Традиционно секция металлокомплексного катализа представлена широким многообразием каталитических систем и исследуемых объектов. В.А. Лихолобов (ИППУ СО РАН) в своей ключевой лекции рассказал о развитии идеологии получения нанесенных металлических катализаторов через стадию закрепления металлокомплексного предшественника. При этом были рассмотрены теоретические основы и практические результаты использования методов получения катализаторов, содержащих дисперсные частицы металлов. Основное внимание сосредоточено на системах, где в качестве носителей использовались силикагель, γ-Al2О3 и углерод. Продемонстрировано, что при использовании в качестве носителя силикагеля высокая дисперсность металла может быть достигнута при использовании комплексов металлов, лиганды которых могут легко отщепляться под действием гидроксильных групп силикагеля. При использовании в качестве носителя α-Al2O3 процесс взаимодействия металлокомплекса с поверхностью носителя может сопровождаться не только образованием “поверхностного алюмината”, но и дополнительной координацией иона металла с низкокоординированными ионами алюминия. Эта особенность взаимодействия приводит к возможности достижения более высокой дисперсности частиц металла и их более высокой термостабильности. При использовании в качестве носителя углеродных материалов главную роль в закреплении предшественников металлических частиц играет взаимодействие донорно-акцепторного типа, реализующееся за счёт наличия на поверхности углеродных материалов ненасыщенных углерод-углеродных связей. В заключение лекции были приведены примеры применения нанесённых металлических катализаторов, характеризующихся узким распределением частиц металла по размеру, для решения ряда задач теории и практики катализа.
Интересная ключевая лекция была сделана В.П. Ананиковым (Институт органической химии им. Н.Д. Зелинского РАН, Москва), в которой рассматривался вопрос о взаимосвязи между гомогенными, гетерогенными и наноразмерными каталитическими системами при проведении реакций в жидкой среде (органические растворители, вода, ионные жидкости, расплавыи др.). Ключевое внимание было уделено описанию процесса вымывания атомов с поверхности наночастиц и его влиянию на активность и селективность катализаторов. В качестве примеров образования связей углерод-углерод были рассмотрены широко известные реакции кросс-сочетания, для которых получены данные с использованием трех типов каталитических систем.
Проф. Варгафтик М.Н. с соавт. (Институт общей и неорганической химии им. Н.С. Курнакова РАН, Москва) прочел ключевую лекцию о синтезе гетеробиметаллических комплексов палладия(II), в которых атомы PdII связаны с атомами переходных (MnII, CoII, NiII, CuII, AgI, AuIII), пост-переходных (ZnII), щелочноземельных (CaII, SrII, BaII) и редкоземельных (CeIV, NdIII, EuIII, SmIII, TmIII, YbIII) металлов c ацетатными или пивалатными мостиковыми группами. В этих соединениях атом палладия прочно связан с атомом дополнительного металла карбоксилатными мостиками и находится на расстоянии, близком к сумме ковалентных радиусов. Полученные комплексы и продукты их превращений могут представить интерес в качестве исходных соединений для получения каталитически активных нанокластеров и наноматериалов.
В ключевой лекции Г.В. Лисичкина (Московский государственный университет им. М.В. Ломоносова, Москва) были последовательно рассмотрены результаты химического модифицирования поверхности гидроксилированных подложек (преимущественно оксидных), наноалмазов детонационного синтеза, массивных и высокодисперсных благородных металлов, наночастиц ионных кристаллов, обсуждены основные пути практического использования таких материалов в качестве сорбентов и катализаторов.
В устном докладе З.П. Пай (Институт катализа, Новосибирск) проанализированы возможности осуществления каталитического окисления природных субстратов перекисью водорода в мягких условиях при проведении реакции в двухфазной системе. А.Ф. Шмидт (Иркутский Университет) в своем докладе предложил оригинальный подход определения области протекания каталитической реакции – гетерогенный или гомогенный, который проиллюстрировал на примере двух реакций с участием палладия. М.И. Шилина (Московский государственный университет) продемонстрировала, что метатезис алканов на биметаллических комплексах переходных металлов с галогенидами алюминия протекает на координационно-ненасыщенных ионах переходного металла. В.В. Сараев (Иркутский Университет) изучил механизм превращения циклооктадиена в присутствии никель – борфторидной каталитической системы. П.Б. Крайкивский (Иркутский Университет) рассказал о гидридных комплексах никеля с P- и N- донорными лигандами, которые выделены в кристаллическом состоянии и всесторонне охарактеризованы.
Тематика кратких сообщений была также разнообразна. Доклад О.Б. Бельской (Институт проблем переработки углеводородов, Омск) посвящен изучению процесса формирования платиновых центров в матрице гидроксидов со слоистой структурой. Е.Г. Жижина (Институт катализа, Новосибирск) рассказала о бифункциональных свойствах растворов полиоксомолибдатов. Д.И. Кочубей (Институт катализа, Новосибирск) сообщил о методе эксфолиации слоистых дисульфидов. Доклад Д.В. Мамонтова (Томский государственный университет) посвящен конструированию поверхности серебросодержащих катализаторов парциального окисления. В.В. Скудин (Российский химико-технологический университет, Москва) рассказал о каталитически активных мембранах неокислительного дегидрирования пропана. В докладе Б.Н. Кузнецова (Институт химии и химической технологии, Красноярск) речь шла о закреплении металлокомплексов на микрокристаллической целлюлозе. А.А. Курохтина (Иркутский государственный университет) рассказала об использовании интегральных зависимостей для определения лимитирующей стадии каталитической реакции. В сообщении Т.В. Бухаркиной (Российский химико-технологический университет) изучена кинетика окисления этилбензола воздухом в присутствии биметаллического катализатора.
С.В. Вержичинская (Российский химико-технологический университет) рассказала об окислительной полимеризации непредельных кислот.

Во время работы семинара состоялся Круглый стол по теме “Катализаторы нефтепеработки и нефтехимии” под руководством к.т.н. И.Д Резниченко, генерального директора ОАО “Ангарский завод катализаторов и органического синтеза”. Алиев Р.Р. (ОАО “Всероссийский научно-исследовательский институт по переработке нефти”, Москва) выступил на нем с обзорным устным докладом “Состояние и перспективы производства отечественных катализатров нефтепереработки”. Участники Круглого стола отметили большое значение разработок отечественных ученых для необходимой модернизации российских катализаторных производств и нефтеперерабатывающих предприятий. В докладе проведен анализ отечественного рынка катализаторов и загруженность катализаторных предприятий. За последние 15 лет производство катализаторов в нашей стране уменьшилось по некоторым направлениям в 7–10 раз. В частности, из 9 установок крекинга с работающим микросферическим катализатором 7 установок полностью используют импортные катализаторы. В стране не производится в промышленных условиях катализатор гидрокрекинга газойлей. Несколько лучше ситуация с производством катализаторов гидроочистки: около 60% установок используют катализаторы Ангарска, Новокуйбышевска и Рязани. Ряд российских компаний, несмотря на более дешевые отечественные катализаторы, предпочитают закупать импортные. В результате отечественный рынок катализаторов в целом на 60–65% занят зарубежными катализаторами. В качестве основного решения сложившейся ситуации участники Круглого стола предложили добиваться целенаправленного получения государственной поддержки для развития, модернизации действующих и создания новых отечественных катализаторных предприятий.
Подводя итоги семинара, участники отметили, что научное направление, связанное с созданием научных основ приготовления нанесенных катализаторов путем целенаправленного синтеза поверхностных соединений, инициатором которого был д.х.н., профессор Ю.И. Ермаков, продолжает развиваться в научных коллективах многих академических институтов и ВУЗов. По мнению участников, семинар был проведен на хорошем научно-организационном уровне: научная общественность и представители промышленности получили информацию о новейших достижениях в области металлокомплексного катализа; наметились новые деловые контакты, обещающие в дальнейшем плодотворное сотрудничество.

Семинар прошел в конф.-зале великолепного Байкальского музея СО РАН, сотрудники которого оказали радушный прием участникам. В перерывах участники запросто “общались” с удивительными обитателями 7 крупных аквариумов музея и “звездами Байкала” – семейством артистичных нерп, а также смогли виртуально погрузиться в “Батискафе” на дно озера. В день заезда участники посетили Музей архитектурно-деревянного зодчества под открытым небом “Тальцы”, покатались на собачьих упряжках и даже чуточку ощутили себя каюрами. По окончании семинара многие участники отправились в яркое и запоминающееся путешествие по льду Байкала на катерах-амфибиях “Хивус” на воздушной подушке. Апрельская погода тоже способствовала успешному туру: весна встретила зеркальным искрящимся льдом, удивительной голубизной ледяных глыб, а организаторы тура угостили всех вкусной омулевой ухой!
В.А. Захаров, А.Н. Старцев, А.В. Лавренов, Л.Я. Старцева
Фото: А.А. Спиридонов
Профессор, д.х.н. Дмитрий Юрьевич Мурзин (из Университета Або Академи, Турку, Финляндия), прочел курс лекций для студентов, аспирантов и молодых ученых в Новосибирске (11-18 мая 2010 г.)
| 11 мая | “Промышленная органическая химия – Quo vadis?” |
| 17 мая | Основные принципы кинетики гетерогенно-каталитических реакций” |
Цикл лекций “Cовременные технологии каталитических процессов” | |
| 12 мая | “Основные принципы химической технологии. Структура химико-технологической системы. Анализ. Примеры подходов к составлению ХТС” |
| 13 мая | “Современные технологии получения синтез-газа и аммиака” |
| 14 мая | “Современные технологии в основном органическом синтезе на примере реакций каталитического окисления” |
| 17 мая | “Технологические подходы к переработке биомассы” |
| 18 мая | “Технологические аспекты дизайна химических продуктов и материалов с заданными свойствами” |
Курс лекций по химической технологии, прочитанный проф. Д.Ю. Мурзиным в Институте катализа, был рассчитан на аспирантов и студентов старших курсов. Одна из лекций затрагивала основные принципы химической технологии и была посвящена структуре химико-технологических систем и их анализу.
В качестве примера вначале был рассмотрен известный случай Бхопальской катастрофы, когда из-за аварии на химическом заводе Union Carbide в индийском городе Бхопал 3 декабря, произошел аварийный выброс паров метилизоцианата, повлёкший смерть, по крайней мере, 18 тысяч человек, из них 3 тысячи человек погибли непосредственно в день трагедии, и 15 тысяч – в последующие годы. По различным данным, общее количество пострадавших оценивается в 150–600 тысяч человек, что позволяет считать бхопальскую трагедию крупнейшей в мире техногенной катастрофой по числу жертв. Кроме исключения грубейших ошибок в эксплуатации, аварии можно было бы избежать при выборе другой технологии, не связанной с образованием метилизоцианата, а основанной на фосгенировании нафтола.
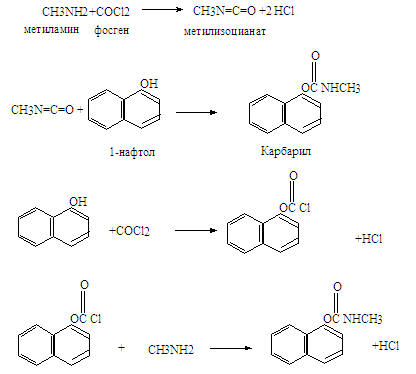
В докладе была обсуждена методология подхода к анализу и дизайну химико-технологических процессов, учитывающая цели, мощности, доступность сырья, расположение потребителей, альтернативные пути, вопросы безопасности и т.д..
Рассмотрены принципы концептуального дизайна, включающего ответы на следующие вопросы: требуется периодический или непрерывный процесс, каковы оптимальные режимы, какие условия протекания процесса опасны, как будет достигнута температура реакции, какой тип реактора предпочтителен, есть ли необходимость в предобработке сырья, какие операции будут проводиться для выделения продуктов.
Значительное внимание в лекции было уделено рассмотрению применения различных реакторов и взаимосвязи типа и формы катализатора и реакторов.
В заключение на примере производства азотной кислоты было показано, как основные знания по химии, термодинамике, кинетике и катализу могут быть применены для создания химико-технологических схем.
J.A. Moulijn, M. Makkee, A. van Diepen, Chemical Process Technology, Wiley, 2001
По вопросам организации курса лекций просим обращаться:
Professor Dmitry Yu. Murzin
Laboratory of Industrial Chemistry and Reaction Engineering
Abo Akademi University
Biskopsgatan 8
20500, Turku/Åbo, Finland
ph: + 358 2 215 4985
fax:+ 358 2 215 4479
e-mail: dmurzin@abo.fi
https://www.abo.fi/student/en/tekniskkemi

С 14 по 17 марта 2010 года в живописном уголке Верхней Баварии, в стенах бывшего Бенедиктинского монастыря, а ныне Клостера Зеона, в котором в настоящее время располагается Культурно-учебный центр Верхней Баварии, состоялся Второй Немецко-Российский семинар “Связь между реальным и модельным катализом”. Семинар был организован Институтом Фрица Хабера (Берлин, Германия) и Институтом катализа им. Г.К. Борескова СО РАН (Новосибирск, Россия). Генеральным спонсором семинара выступил Институт Фрица Хабера.

В семинаре приняло участие 15 ученых из Германии, 27 из России и один из Азербайджана, которые представляли не только академические организации и ВУЗы, но и промышленные предприятия. Научная программа семинара включала 6 пленарных лекций и 32 устных сообщения (20 мин.), представленных на 3-х секциях:
С пленарными докладами выступили д.х.н. А.Г. Степанов и к.х.н. И.В. Юданов из Института катализа, а также профессора J. Lercher, J. Sauer, R.W. Fischer и M. Muhler из университетов Германии.

В Культурно-учебном центре участникам был предоставлен не только удобный конференц-зал, но и обеспечено комфортное проживание и питание, что способствовало активному неформальному общению всех участников семинара. И в перерывах между докладами, и в вечернее свободное время российские и немецкие ученые могли обсуждать планы дальнейшего сотрудничества, как в области фундаментальных исследований, так и в сфере реальных каталитических процессов (т.е. установления взаимосвязи между модельным и реальным катализом для оптимизации и улучшения промышленных каталитических процессов). Сегодня это имеет особенное значение, поскольку именно предприятия промышленного катализа смогли бы обеспечить достойное финансирование современных дорогостоящих научных исследований, а также способствовать внедрению перспективных научных разработок.

В первый день семинара состоялись две пленарных лекции. Научную программу открыл профессор J. Lercher из Технического университета города Мюнхена с лекцией, посвященной изучению механизма действия бифункциональных и “концертных” катализаторов конверсии углеводородов. Вторая лекция И.В. Юданова из Института катализа была посвящена применению квантово-химических расчетов для исследования каталитической активности металлических нанокластеров. На ряде примеров докладчик продемонстрировал возможности использования метода функционала плотности для изучения природы размерного эффекта и структурной чувствительности в гетерогенных каталитических реакциях. В лекции были рассмотрены особенности адсорбции СО, механизм разложения метанола на кластерах, содержащих ~100 атомов Pd, а также структура PdAu нанокластеров.

В последующие два дня было представлено еще четыре пленарных лекции. Профессор J. Sauer из Берлинского Университета имени Гумбольдта представил анализ новейших результатов теоретических исследований процессов активации C-H связей углеводородов оксидами переходных металлов. Профессор R.W. Fischer (Süd-Chemie AG, Bruckmühl, Germany) в лекции акцентировал внимание на необходимости более плотного совмещения фундаментальных исследований с реальным катализом. Для демонстрации плодотворного сотрудничества промышленных и академических структур докладчик привел пример разработки и практического применения Ru-содержащих катализаторов. Лекция профессора M. Muhler (Ruhr-University Bochum, Germany) была посвящена последним достижениям в области исследования природы активных центров синтеза метанола на поверхности катализаторов Au/ZnO. А.Г. Степанов из Института катализа продемонстрировал возможности применения метода ЯМР спектроскопии твердого тела в режиме in situ для получения детальной информации о механизмах превращения углеводородов на твердых кислотных катализаторах, а также для исследования процессов диффузии углеводородов в порах цеолитов и зауглероживания активных центров. В заключение докладчик представил оригинальные данные о механизме совместной ароматизации метана и высших алканов.

Сессию устных докладов открыл профессор И.В. Коптюг из Международного Томографического центра (Новосибирск, Россия). В своем докладе автор представил новую концепцию применения ядерного магнитного резонанса для in situ исследований механизмов реакций гетерогенного гидрирования параводородом. В дальнейшем тема развития и применения различных in situ методов звучала в докладах И.Э. Бекк, И.Г. Даниловой, В.В. Каичева, А.В. Матвеева, И.П. Просвирина из Института катализа, а также A. Knop-Gericke и A. Trunschke (Fritz-Haber-Institut der MPG, Berlin, Germany). В большинстве работ приводились примеры in situ исследований различных гетерогенных реакций с применением методов РФЭС, XANES и масс-спектрометрии. Так, например, в докладе И.Э. Бекк подробно обсуждались результаты исследований реакции полного окисления метана на высокодисперсных алюмоплатиновых катализаторах. Данная задача возникла недавно в связи с проблемой очистки выхлопных газов автомобилей, использующих в качестве топлива природный газ. К близкой тематике относился также и доклад А.В. Матвеева, посвященный in situ исследованиям процессов деактивации катализаторов на основе Pt, Pd и Rh в реакции окисления пропилена.
В заключение следует отдельно отметить доклады А.А. Велигжанина (Российский научный центр “Курчатовский институт”, Москва, Россия), Е.А. Лашиной (Институт катализа, Новосибирск, Россия) и Д.А. Пичугиной (Московский государственный университет, Москва, Россия). А.А. Велигжанин рассказал о структурных исследованиях катализаторов, проводимых в Курчатовском центре синхротронного излучения и нанотехнологий с применением методов рентгеновской дифракции и малоугловой рентгеновской дифрактометрии, РФА, XANES и EXAFS, в том числе, в режиме in situ. В докладе Е.А. Лашиной были представлены результаты математического моделирования автоколебаний в реакции окисления метана на никеле. Доклад Д.А. Пичугиной был посвящен квантово-химическим исследованиям природы активных центров на поверхности золотых наночастиц. Данная работа весьма актуальна в свете обнаруженной уникальной каталитической активности высокодисперсного золота.
В последний день семинара для участников была организована интересная и интенсивная культурная программа. Знакомство с весенней Баварией началось с посещения Замка Нойшванштайн и закончилось обзорной экскурсией по Мюнхену. Столица Баварии Мюнхен – один из уютнейших и красивейших городов Германии.
По окончании семинара участникам был предложен 2-х дневный пост-тур для знакомства с Зальцбургом и Веной.
При подведении итогов участники семинара отметили хороший уровень представленных докладов, плодотворность проведенных дискуссий и одобрили решение о проведении следующего III Cеминара “Связь между реальным и модельным катализом” в России через 3 года.
В.В. Каичев, И.П. Просвирин

ПОДВИЖНИК
Памяти Владимира Сергеевича Бескова
14.08.1937 – 11.06.2010
11 июня 2010 трагически погиб заведующий кафедрой Общей химической технологии, доктор технических наук, профессор, почётный химик СССР, почётный химик РФ, Владимир Сергеевич Бесков.
Владимир Сергеевич Бесков – известный учёный в области промышленного катализа, математического моделирования химических процессов и реакторов, аэродинамического моделирования каталитических процессов, внесший большой научный вклад в теорию и практику химической технологии. Увлечённо занимался интенсификацией химических процессов.
Он был ближайшим и любимым учеником Михаила Гавриловича Слинько. С их именами связано становление и развитие методов математического моделирования каталитических процессов в Институте катализа Сибирского отделения и в СССР.
Он пришел в Институт катализа в марте 1960 г. после окончания физико-химического факультета Московского химико-технологического института им. Д.И. Менделеева.
В 1964 г. он защитил кандидатскую диссертацию. В 1972 г. – успешно защитил докторскую по теме “Реакторы большой мощности с неподвижным катализаторным слоем”. Один из оппонентов – академик Г.И. Марчук дал исключительно высокую оценку представленной работе.
За время работы в Институте (1960-1974 гг.) В.С. Бесков вырос в одного из ведущих специалистов в области математического моделирования каталитических процессов, возглавив работы по моделированию процессов в неподвижном слое. Занимался расчетами и оптимизацией процессов окисления двуокиси серы, конверсии природного газа, синтеза аммиака, окисления метилового спирта, процессами полимеризации, окисления фталевого ангидрида, вопросами устойчивости реакторов, процессами переноса в пористом зерне катализатора и многими другими.
В.С. Бесков непосредственно участвовал в реализации научных результатов в химической промышленности, являлся членом Научно-технических советов министерств химической и нефтехимической промышленности СССР, был членом Научного совета по катализу со времен СССР (при Госкомитете СМ по науке и технике) и оставался им до
В феврале 1974 г. он перешел на работу в Москву, сначала в ГИАП, а затем в МХТИ им. Д.И. Менделеева.
В Государственном институте азотной промышленности (ГИАП) в период с 1974 по 1981гг. В.С. Бесков возглавил лабораторию математического моделирования и принимал самое активное участие в моделировании различных технологических процессов отрасли и в создании автоматизированной системы научных исследований (АСНИ).
С июля 1981 года по 11 июня 2010 года В.С. Бесков возглавлял кафедру Общей химической технологии МХТИ (РХТУ)
им. Д.И. Менделеева, являясь славным продолжателем традиций, созданных его предшественниками: академиком АН СССР Н.М. Жаворонковым, профессором П.М. Лукьяновым, профессором Д.А. Кузнецовым, профессором А.Г. Амелиным.
Для Владимира Сергеевича научная и педагогическая работы были неразделимы. В течение двадцати восьми лет он читал курсы “Общая химическая технология”, “Химические процессы и реакторы”, специальный курс “Гетерогенный катализ и каталитические процессы”. В основу построения курсов положены научные методы: математическое моделирование и системный анализ химико-технологических систем.
Много сил и времени Владимир Сергеевич уделял совершенствованию учебного процесса. Им талантливо разработан ряд новых курсов по химической технологии для общеинженерного образования студентов высших учебных заведений, обучающихся как по химико-технологическим направлениям, так и для других направлений подготовки бакалавров и дипломированных специалистов. Разработан принципиально новый лабораторный практикум по химической технологии на основе компьютерного моделирования химико-технологических процессов.
Учебник В.С. Бескова “Общая химическая технология” и учебное пособие “Примеры и задачи по общей химической технологии” (В.И. Игнатенков, В.С. Бесков), изданные в ИКЦ “Академкнига”, получили всеобщее признание в педагогических и научных кругах. ИКЦ “Академкнига” был отмечен специальной грамотой за издание комплекта учебных пособий В.С. Бескова. Вершина педагогической деятельности В.С. Бескова – УМК, своеобразная трилогия (учебник, задачник, практикум), посвящённая инженерной дисциплине “Общая химическая технология”.
Методические основы, структура и содержание курса ОХТ, разработанного в РХТУ им. Д.И.Менделеева, стали типовыми и легли в основу аналогичных курсов в других вузах РФ и стран СНГ.
К значительным научным результатам, полученным В.С. Бесковым и его коллегами и существенно развившим представления о природе промышленного гетерогенного катализа, можно отнести обнаруженные новые явления в катализе и выявление их электрофизической природы: “каталитическая плазма” – прослежено её влияние на каталитическую реакцию; “каталитический работающий слой как каталитический фильтр”; разработанные методы построения кинетических моделей сложных многокомпонентных реакций в нефтехимической переработке; разработанные способы формования блочных катализаторов различной сложной геометрической формы непосредственно из активной катализаторной массы.
На кафедре, возглавляемой В.С. Бесковым, сложилась благоприятная творческая атмосфера, что позволило восьми сотрудникам кафедры защитить докторские диссертации: 1987 г. – А.П. Тихонов, 1991 г. – А.Н. Кабанов, 1993 г. – А.В. Беспалов, 1996 г. – Г.М. Семенов, 1997 г. – А.С. Федосеев, 2000 г. – В.Б. Сажин, 2003 г. – В.И. Ванчурин,

Среди участников Международной конференции “Катализ: теория и практика”,
посвященной 100-летию со дня рождения Г.К. Борескова (Новосибирск, 4-8 июля 2007 г.)
В.А. Собянин, Н.З. Ляхов, В.С. Бесков, З.Р. Исмагилов
В.С. Бесковым опубликовано более 500 научных работ, обзоров и методических пособий и указаний. Получено около 50 авторских свидетельств и патентов на изобретения. Он автор 15 монографий, учебников и учебных пособий, в том числе, таких как “Моделирование каталитических процессов на пористых зернах” (О.А. Малиновская, В.С. Бесков, М.Г. Слинько), “Моделирование каталитических процессов и реакторов” (В.С. Бесков, В. Флокк). Последнюю можно поставить в один ряд с трудами таких известных ученых, как Х. Крамерс и Р. Вестертерп, Р. Арис, И.И. Иоффе и Л.М. Письмен в области теории и практики химических реакторов. Р. Вестертерп неоднократно приглашал В.С. Бескова для выполнения совместных научных работ в Голландию, но Владимир Сергеевич был самозабвенно предан кафедре ОХТ и не мог оставить её на длительный срок.
Под руководством В.С. Бескова защищено 46 кандидатских и 10 докторских работ.
В.С. Бесков выступал на многих международных и национальных конференциях по катализу, химическим процессам и реакторам. Принимал самое активное участие в организации и проведении четырнадцати (из 17 организованных Институтом катализа) конференций по химическим реакторам "ХИМРЕАКТОР".
В.С. Бесков имел репутацию и признание в названных научных направлениях, являлся членом рабочей группы по химическим реакторам Европейской федерации инженерной химии, научных советов РАН по катализу и теоретическим основам химической технологии, пяти диссертационных советов РХТУ им. Д.И. Менделеева и ряда других организаций.
Диссертационный Совет по защите докторских и кандидатских диссертаций по специальности “Технология неорганических веществ” РХТУ им. Д.И. Менделеева, возглавляемый Бесковым В.С. более 20 лет, по оценке ВАК РФ, является одним из самых действенных и работоспособных в России.
Сотрудники кафедры потрясены невосполнимой потерей. В нашей памяти навсегда останется светлый образ обаятельного, скромного, высокоодарённого, неутомимого труженика Владимира Сергеевича!!!
Многочисленные друзья и коллеги Владимира Сергеевича по Институту катализа будут помнить его всегда.
Коллектив кафедры Общей химической технологии РХТУ им. Д.И. Менделеева
Коллеги из Института катализа СО РАН
GREENER METHYLATIONS
Dimethyl carbonate is a “superior” methylating reagent in a high-yield synthesis of industrially important methyl ethers, according to a research team led by Derek J. Irvine and Martyn Poliakoff of the University of Nottingham, in England (Org. Process Res. Dev., DOI: 10.1021/OP900307w). Ethers are traditionally made via the Williamson ether synthesis, which employs an alcohol and sodium hydroxide to form an alkoxide that subsequently couples with an alkyl halide. But that method requires toxic alkyl halides as reagents and generates undesirable inorganic salts as by-products. Building on their expertise in continuous-flow supercritical CO2 reactor technology, the Nottingham researchers showed that solid-acid catalysts, such as γ-alumina, mediate the selective reaction of nontoxic dimethyl carbonate with l-octanol and other primary alcohols to form methyl ethers in supercritical CO2 solvent (170-270 °C, 100 bar). The research was sponsored by specialty chemical manufacturer Croda as part of its effort to identify greener technologies for making ingredients that go into cosmetics, toiletries, and other consumer products.—SR
WWW.CEN-ONLINE.ORG FEBRUARY 15, 2010
Carbenes made easy
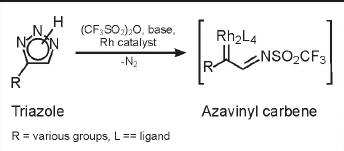 Chemists have found a straightforward way to make rhodium azavinyl carbenes, a new family of reactive species for organic synthesis. To complement the existing tool kit of carbenes, which are high-energy intermediates c apable of selective reactions, Valery V. Fokin and coworkers at Scripps Research Institute recently developed tunable azavinyl carbenes with unique synthetic capabilities (J. Am. Chem. Soc. 2009, 131, 18034). But making them involves sul-fonyl azides and diazo compounds, which require very careful handling. Fokin, Neil Grimster, and Li Zhang have now devised a new route that gets around the hazardous compounds. From stable NH-triazoles, the one-pot procedure generates carbenes using triflic anhydride, a pyridine base, and a rhodium catalyst (J. Am. Chem. Soc., DOI: 10.1021/ja910187s). The rhodium azavinyl carbenes react with alkenes, yielding cyclopropanes or dihydropyrroles with good stereoselectivity.—CD
Chemists have found a straightforward way to make rhodium azavinyl carbenes, a new family of reactive species for organic synthesis. To complement the existing tool kit of carbenes, which are high-energy intermediates c apable of selective reactions, Valery V. Fokin and coworkers at Scripps Research Institute recently developed tunable azavinyl carbenes with unique synthetic capabilities (J. Am. Chem. Soc. 2009, 131, 18034). But making them involves sul-fonyl azides and diazo compounds, which require very careful handling. Fokin, Neil Grimster, and Li Zhang have now devised a new route that gets around the hazardous compounds. From stable NH-triazoles, the one-pot procedure generates carbenes using triflic anhydride, a pyridine base, and a rhodium catalyst (J. Am. Chem. Soc., DOI: 10.1021/ja910187s). The rhodium azavinyl carbenes react with alkenes, yielding cyclopropanes or dihydropyrroles with good stereoselectivity.—CD
WWW.CEN-ONLINE.ORG FEBRUARY 15, 2010
Amides made efficiently from aldehydes
Copper-catalyzed reaction of aldehydes with amine hydrochloride salts provides an efficient new route to amide bond formation, according to Woo-Jin Yoo and Chao-Jun Li of McGill University, Montreal (J. Am. Chem. Soc., DOI: 10.l021/ja064315b).
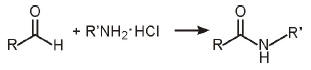 The carbonyl-nitrogen amide moiety is ubiquitous in pharmaceuticals and polymers, the researchers note,
and amide bond formation is necessary to couple peptides in protein synthesis. Amides are usually formed
by reacting carboxylic acids with an amine, they add, but this and other approaches are limited by costly
transition-metal catalysts and the number of available substrates. Building on their previous work on
copper-catalyzed oxidative esterification of aldehydes, Yoo and Li reacted various benzaldehydes with alkyl,
aryl, or glycine ester amine hydrochlorides in acetonitrile solvent (shown) in the presence of CuI
with AgIO3 as the catalyst, tert-butyl hydroperoxide as the oxidant,
and CaCO3 as a base. They believe their method is the first example of
producing amides and peptides from aldehydes and simple amines.
The carbonyl-nitrogen amide moiety is ubiquitous in pharmaceuticals and polymers, the researchers note,
and amide bond formation is necessary to couple peptides in protein synthesis. Amides are usually formed
by reacting carboxylic acids with an amine, they add, but this and other approaches are limited by costly
transition-metal catalysts and the number of available substrates. Building on their previous work on
copper-catalyzed oxidative esterification of aldehydes, Yoo and Li reacted various benzaldehydes with alkyl,
aryl, or glycine ester amine hydrochlorides in acetonitrile solvent (shown) in the presence of CuI
with AgIO3 as the catalyst, tert-butyl hydroperoxide as the oxidant,
and CaCO3 as a base. They believe their method is the first example of
producing amides and peptides from aldehydes and simple amines.
WWW.CEN-ONLINE.ORG SEPTEMBER 25, 2006
IMAGING INDUSTRIAL-TYPE CATALYSTS
By using a novel electron microscopy method, an international team has recorded images of industrial-type nanostructured molybdenum disulfide catalyst particles with element specificity and single-atom resolution (An-gew. Chem. Int. Ed. 2010, 49, 2708). Previous studies that yielded atomic resolution details of MoS2, a hydrotreating catalyst used to strip sulfur from petroleum, typically focused on model single-crystal-supported specimens that were prepared under pristine vacuum conditions. In contrast, the current study — led by Stig Helveg of Danish catalyst manufacturer Topsøe , Christian Kisielowski of Lawrence Berkeley National Laboratory, and coworkers — focuses on MoS2 samples synthesized on graphitic carbon powder via the same methods used to manufacture catalysts for oil refineries.
 The researchers analyzed the samples with a recently developed transmission electron microscopy technique that enabled them to distinguish between one- and two-layer-thick regions of the particles and determine the chemical identity of the atoms throughout those regions. The results, which the team notes are in “excellent agreement” with computer simulations, could help establish new relationships between the catalyst’s function and atomic structure and could offer possibilities for improving catalyst formulations, they say.-MJ
The researchers analyzed the samples with a recently developed transmission electron microscopy technique that enabled them to distinguish between one- and two-layer-thick regions of the particles and determine the chemical identity of the atoms throughout those regions. The results, which the team notes are in “excellent agreement” with computer simulations, could help establish new relationships between the catalyst’s function and atomic structure and could offer possibilities for improving catalyst formulations, they say.-MJ
By combining experimental (left) and simulated microscopy results, researchers pinpoint single- and double-layer regions in MoS2 catalyst particles (right).
WWW.CEN-ONLINE.ORG APRIL 5, 2010
EPA TARGETS BISPHENOLA
REGULATION: Agency will examine levels of plastics chemical in water supply and effects on wildlife
EPA HAS ADDED bisphenol A (BPA) to its list of chemicals targeted for possible regulation. The agency announced its action plan for BPA on March 29, citing concerns about potential environmental impacts of the widely used plastics chemical.
In the plan, EPA will use its authority under the Toxic Substances Control Act (TSCA) to gather information on the concentrations of BPA on surface, ground, and drinking water. The agency will also require manufacturers to provide data on the reproductive and developmental effects of BPA on aquatic organisms and wildlife.
EPA estimates that in the U.S., the amount of BPA released into the environment exceeds 1 million lb annually. The proposed rules are expected this fall.
In January, FDA announced that it has “some concern” about the potential health effects of BPA on infants and children (C&EN, Jan. 25, page 8). FDA is currently studying the health impacts of BPA from food packaging and ways to reduce exposure to the chemical.
“We share FDA’s concern about the potential health impacts from BPA,” Steve Owens, assistant administrator of EPA’s Office of Prevention, Pesticides & Toxic Substances, said in a statement. EPA will also examine ways to reduce exposures to BPA and assess the safety of alternatives. EPA does not, however, intend to initiate rulemaking under TSCA on the basis of risks to human health at this time.
EPA’s decision comes amid pressure from environmental activists and members of Congress to expedite regulatory action on BPA. In early March, Sen. Charles E. Schumer (D-N.Y.) sent a letter to EPA Administrator Lisa P. Jackson urging EPA to take immediate action against BPA as part of the agency’s efforts to reform its chemicals management program.
The American Chemistry Council, a trade group that represents the chemical industry including several BPA manufacturers, emphasized in a statement that EPA is not proposing any regulations because of concerns about human health. The group said it looks forward to working with EPA on modernizing TSCA “in a way that allows EPA to better prioritize chemicals for review.’— BRITT ERICKSON
WWW.CEN-ONLINE.ORG APRIL 5, 2010
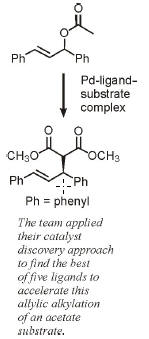
SURVIVAL OF THE WEAKEST
CATALYST DISCOVERY: Least stable intermediates can lead to the best catalysts
Evolution may be based on “survival of the fittest,” but when it comes to selecting the best catalysts, that dictum doesn’t always hold.
A research team has shown that one can select the best catalysts from combinatorial libraries of candidates by instead using the principle of “survival of the weakest” — that is, the most unstable catalytic intermediates make for the best catalysts. The approach could make it possible to discover catalysts more quickly for syntheses of drugs and other products.
It has been difficult to find selection methods to separate the cream of the crop (the fastest catalysts) from the dross when screening libraries to identify highly active catalysts. Now, theoretical chemist F. Matthias Bickelhaupt of Free University of Amsterdam, catalysis specialist Joost N. H. Reek of the University of Amsterdam, and coworkers have developed an innovative selection strategy for such experiments based on survival of the weakest intermediates (Nat Chem., DOI: 10.1038/nchem.614).
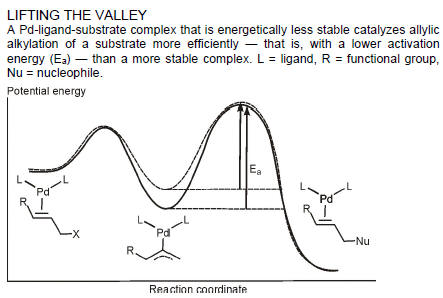
A Pd-ligand-substrate complex that is energetically less stable catalyzes allylic alkylation of a substrate more efficiently — that is, with a lower activation
energy (Ea) — than a more stable complex. L = ligand, R = functional group, Nu = nucleophile.
The researchers mix limited amounts of palladium — bound to the substrate of an allylic alkylation reaction — with an overabundance of several organic ligands. In the equilibrium mixture, different ligands bind to Pd, forming catalysts that vary in their ability to accelerate alkylation of the substrate. The ligands that form the most stable Pd-ligand-substrate complexes are most abundant in solution, and those that form unstable complexes are scarce.
Using electrospray mass spectrometry (ESI/MS) to determine the least abundant and therefore least stable complexes, the researchers identified ligands that form the most active catalysts. The study thus evaluates catalysts not in the conventional way — by determining their ability to lower the activation barrier, the energy required to make the reaction go — but instead by finding the least stable intermediates, which are closer energetically to the top of the barrier and can therefore more easily traverse it.
 The work “highlights the value of smart combinatorial approaches for the development of highly efficient catalysts,” says catalysis specialist Helma Wennemers of the University of Basel, in Switzerland.
The work “highlights the value of smart combinatorial approaches for the development of highly efficient catalysts,” says catalysis specialist Helma Wennemers of the University of Basel, in Switzerland.
“This is a clever demonstration of successful catalyst screening using simple ESI/MS methodology,” says polymer chemist Krzysztof Matyjaszewski of Carnegie Mellon University.
Groups such as Matyjaszewski’s and that of catalyst screening specialist Andreas Pfaltz of the University of Basel have identified good catalysts from mixtures before. But the new study represents one of the first screening examples in which “catalyst systems are in equilibrium and communicate,” Reek says. “It’s a Darwinian selection in which the weakest survive, whereas most others have monitored the properties of mixtures of catalysts that are not communicating.”
Dynamic combinatorial chemistry expert Sijbren Otto of the University of Groningen, in the Netherlands, says, "We understand initial states much better than transition states,” which are transient species at the tops of activation barriers. Manipulating the energies of reactant intermediates“ may, for selected systems, be a much easier way to find catalysts than attempting to interact selectively with much more elusive transition states,” Otto says.
Using the new technique to detect least abundant species in large libraries and screen substantially different ligand classes in the same mixture, however, could be difficult, Otto adds. Nevertheless, he says, “the approach holds considerable promise for catalyst development and discovery.” — STU BORMAN
/WWW.CEN-ONLINE.ORG/">WWW.CEN-ONLINE.ORG APRIL 5, 2010
Лучшие книги по катализу

Springer books are available as eBooks for PCs & portable devices, as well as in printed format.
University libraries with access to our
eBook collection can now offer their patrons an extra format.
MyCopy , paperback edition for 24.95 USD/EUR
More books in Catalysis
Become part of the largest eBook Collection in Chemistry by publishing your next book with Springer.
We encourage you to send your book proposals to our editors.
Don't Have Access Yet?
Simply recommend the Springer eBooks collection to your librarian!
Thank you for your continued interest in Springer`s publications.
Yours sincerely
Andrea Wurm
Associate Product Manager Springer
Самые яркие статьи по катализу из журналов
Hottest articles in Catalysis Selected from the journals
ChemCatChem
Mesoporous Silica Nanosphere-Supported Chiral Ruthenium Catalysts: Synthesis, Characterization, and Asymmetric Hydrogenation Studies
David J. Mihalcik, Wenbin Lin
Studies on Dehydrogenation of Ethane in the Presence of CO2 over Octahedral Molecular Sieve (OMS-2) Catalysts
Lei Jin, Justin Reutenauer, Naftali Opembe, Monique Lai, Daniel J. Martenak, Scott Han, Steven L. Suib
Perrhenate Esters in New Catalytic Reactions
Stéphane Bellemin-Laponnaz
Vanadium-Containing Oxynitrides: Effective Catalysts for the Ammoxidation of 3-Picoline
Christiane Janke, Jörg Radnik, Ursula Bentrup, Andreas Martin, Angelika Brückner
ChemSusChem
The Effect of Alkaline Earth Metal Ion Dopants on Photocatalytic Water Splitting by NaTaO3 Powder
Akihide Iwase, Hideki Kato, Akihiko Kudo
Gold-Catalyzed Aerobic Oxidation of 5-Hydroxymethylfurfural in Water at Ambient Temperature
Yury Y. Gorbanev, Søren K. Klitgaard, John M. Woodley, Claus H. Christensen, Anders Riisager
Catalytic Partial Oxidation of Methanol and Ethanol for Hydrogen Generation
Keith L. Hohn, Yu-Chuan Lin
Investigations of the Conversion of Inorganic Carbonates to Methane
Dinesh Jagadeesan, Muthusamy Eswaramoorthy, C. N. R. Rao
Advanced Synthesis & Catalysis
Construction of Heterocycle Scaffolds via Transition Metal- Catalyzed sp2 C — H Functionalization
Ming Zhang
Angewandte Chemie International Edition
Nanostructured Zinc Oxide Nanorods with Copper Nanoparticles as a Microreformation Catalyst
Yan-Gu Lin, Yu-Kuei Hsu, San-Yuan Chen, Yu-Kai Lin, Li-Chyong Chen, Kuei-Hsien Chen
Enantioselective Gold-Catalyzed Allylic Alkylation of Indoles with Alcohols: An Efficient Route to Functionalized Tetrahydrocarbazoles
Marco Bandini, Astrid Eichholzer
Catalytic Oxidative Synthesis of Nitriles Directly from Primary Alcohols and Ammonia
Takamichi Oishi, Kazuya Yamaguchi, Noritaka Mizuno
Chemistry - A European Journal
Microwave-Assisted Cross-Coupling and Hydrogenation
Chemistry by Using Heterogeneous Transition-Metal Catalysts:
An Evaluation of the Role of Selective Catalyst Heating
Muhammed Irfan, Michael Fuchs, Toma N. Glasnov, C. Oliver Kappe
Auto-Tandem Catalysis: A Single Catalyst Activating
Mechanistically Distinct Reactions in a Single Reactor
Naoya Shindoh, Yoshiji Takemoto, Kiyosei Takasu
Chemistry – An Asian Journal
Catalytic ß-Boration/Oxidation of 1-AzadienesHelvetica Chimica Acta
Structures of the Reactive Intermediates in Organocatalysis with Diarylprolinol Ethers
Uroš Grošelj, Dieter Seebach, D. Michael Badine, W. Bernd Schweizer, Albert K. Beck, Ingo Krossing, Petra Klose, Yujiro Hayashi, Tadafumi Uchimaru
Chemistry & Biodiversity
Synthesis of Novel Diselenide-Linked Porphyrin Dimers under Phase-Transfer Catalysis Condition and Their Interactions with DNA
Zhi Xue, Daniel Wei-Jing Kwong, Ling-Wei Xue, Qing Liu, An-Xin Hou, Wai-Kwok Wong
Macromolecular Rapid Communication
High-Throughput Screening in Olefin-Polymerization Catalysis: From Serendipitous Discovery Towards Rational Understanding
Vincenzo Busico, Roberta Pellecchia, Francesco Cutillo, Roberta Cipullo
Macromolecular Chemistry and Physics
Determining Initiator Efficiency in Radical Polymerization by Electrospray-Ionization Mass Spectrometry
Michael Buback, Holm Frauendorf, Fabian Günzler, Felix Huff, Philipp Vana
Starch – Stärke
Formation of Resistant Starch in Corn Starch and Estimation of its Content from Physicochemical Properties
Sung-Kon Kim, Jae-Eun Kwak
Electroanalysis
Electrocatalytic Reduction of Hydrogen Peroxide by Nanostructured Bimetallic Films of Metalloporphyrins
M. Hamer, R. R. Carballo, I. N. Rezzano
ZAAC – ZZeitschrift für anorganische und allgemeine Chemie
Hydrothermal Synthesis, Structure Characterization, Catalytic Property of Four Inorganic-Organic Hybrid Phosphomolybdates
Qi Chen, Yan Cui, Qi Sun, Wenjun Pan, Qiang Chen, Yan Xu
Fuel Cells
Determination of the Potentiostatic Stability of PEMFC Electro Catalysts at Elevated Temperatures
V. A. T. Dam, K. Jayasayee, F. A. de Bruijn
Small
Colloidal Nanoparticles as a Wireless Booster for Electroenzymatic Reactions
Sahng Ha Lee, Keehoon Won, Hyun-Kon Song, Chan Beum Park
Advanced Functional Materials
Cu(II)-Azabis(oxazoline)-Complexes Immobilized on Superparamagnetic Magnetite@Silica-Nanoparticles: A Highly Selective and Recyclable Catalyst for the Kinetic Resolution of 1,2-Diols
Alexander Schätz, Markus Hager, Oliver Reiser
European Journal of Inorganic Chemistry
Mono-, Bi- and Tridentate N-Heterocyclic Carbene Ligands for the Preparation of Transition-Metal-Based Homogeneous Catalysts
Rosa Corberán, Elena Mas-Marzá, Eduardo Peris
Applied Organometallic Chemistry
Aminophosphines: their chemistry and role as ligands and synthons
Janarthanan Gopalakrishnan
Conversion of carbon dioxide to cyclic carbonates using diimine Ru(II) complexes as catalysts
Mahmut Ulusoy, Engin Çetinkaya, Bekir Çetinkaya
Transition-metal nanoparticles: synthesis, stability and the leaching issue
Laura Durán Pachón, Gadi Rothenberg
Обзорный журнал по химии
Review Journal of Chemistry
Главный редактор - академик Н.С. Зефиров,
Российская академия наук.
тел.: +7(495) 939-02-90
факс.: +7(495) 939-02-90
e-mail: zefirov@org.chem.msu.ru
Ответственный секретарь – Яшин Н.В.,
Химический факультет МГУ им. М.В. Ломоносова
тел. +7(495) 939-51-55
факс.: +7(495) 939-02-90
e-mail: yashin-n@org.chem.msu.ru
Приглашаем Вас стать автором нового обзорного рецензируемого журнала по химии, учрежденного компанией Pleiades Publishing, лидером в издании российской научной периодики, при поддержке Международной ассоциации академий наук.
Журнал объединит обзорные работы авторов из России и СНГ, а также их коллег из всех других стран и будет издаваться в двух версиях - на русском и английском языках.
Журнал будет издаваться четыре (шесть) раз в год и в нем будут публиковаться критические обзоры современного состояния проблем химии и смежных междисциплинарных областях знаний.
Журнал будет печатать обзоры не только по приглашению редколлегии, но по инициативе авторов. Публикации будут осуществляться на гонорарной основе.
Журнал будет издаваться в электронном и печатном виде. Распространение русской версии Обзорного журнала по химии будет производиться через eLibrary.ru, которая на сегодняшний день является самым крупным на территории бывшего СССР поставщиком научной информации онлайн. Английская версия Review Journal of Chemistry будет доступна по всему миру через Springerlink.com в рамках программы Russian Library of Science. Таким образом, Ваша публикация будет не только соседствовать с обзорными работами ведущих специалистов из России и стран СНГ в рамках этого нового журнала, но и будет охвачена глобальным поиском, в который войдут ведущие публикации специалистов всего мира, а также будет индексироваться во всех престижных базах данных.
Российский конгресс по катализу
“РОСКАТАЛИЗ”
3-7 октября 2011 года, Москва
http://conf.nsc.ru/RUSCATALYSIS-2011
Российский конгресс по катализу “РОСКАТАЛИЗ”
проводится с целью объединения ученых и специалистов в направлении инновационного развития химического комплекса и глубокой переработки сырьевых ресурсов России.ОРГАНИЗАТОРЫ КОНГРЕССА
СЕКЦИИ КОНГРЕССА
Секция 1. Физико-химические основы каталитических процессов
Секция 2. Научные основы производства катализаторов
Секция 3. Перспективные каталитические процессы
Секция 4. Промышленные катализаторы и каталитические процессы
Круглый стол: Образование и катализ
В рамках конгресса состоится симпозиум “Органический синтез”, посвященный 150-летию со дня рождения Н.Д. Зелинского.
Секции симпозиума: Основной органический синтез; Тонкий органический синтез.
Контактный адрес: http://conf.nsc.ru/RUSCATALYSIS -2011
(e-mail: star@catalysis.ru)
Уважаемые Дамы и Господа!
Компания “VitaNova Association” приглашает Вас принять участие в Международном Форуме “Современные пути развития НПЗ”, который состоится 5-8 сентября 2010 г. в КО “Ривьера” (г. Анапа)
Вопросы развития НПЗ в настоящее время являются наиболее актуальными, в связи с переходом России на новые стандарты качества топлива. Для обеспечения российского рынка бензином требуемого качества в ближайшие годы необходимо провести реконструкцию российских НПЗ, а также изменить номенклатуру применяемых в бензинах присадок.
Учитывая особую важность тематики, компания “VitaNova Association” прглашает обсудить состояние и перспективы развития отрасли в широком кругу экспертов и заинтересованных специалистов.
К работе на Форуме приглашены: представители ведущий российских и зарубежных нефтяных и нефтехимическихкомпаний, среди которых: Shell, “Ассоциация нефтепереработчиков и нефтехимиков”, “Атырауский НПЗ”, “Ачинский НПЗ”, “ВНИИ НП”, “Волжский Оргсинтез”, “Газпром нефть”, “Газпром переработка”, “Газпром”, “КИНЕФ”, “КОРТЕС”, “ЛИНИК”, “ЛУКОЙЛ”, “Московский НПЗ”, “Омский НПЗ”, “Комсомольский НПЗ”, “Роснефть”, “РуссНефть”, “Рязанская НПК”, “Салаватнефтеоргсинтез”, “СИБУР”, “Славнефть-ЯНОС”, “Сургутнефтегаз”, “Сызранский НПЗ”, “ТАИФ”, “ТАНЕКО”, “Татнефть”, “ТНК-ВР”, “Транснефтепродукт”, “Укртатнафта”, “Хабаровский НПЗ”, Представители Госорганов.
На повестке Форума:
Надеемся, что Вы сочтете возможным принять участие в работе Форума.
Дополнительную информацию о предстоящем мероприятии Вы можете получить в Оргкомитете. Для регистрации необходимо заполнить заявку на участие и прислать на e-mail:
info@forum-npz.ru.
С уважением,
Оргкомитет форума
tel: (495) 211-74-32
mob: 8 (916) 623-66-30
http:// www.forum-npz.ru
Глубокоуважаемые коллеги!
Приглашаем Вас и сотрудников вашей организации принять участие в работе ежегодной конференции РХО им. Д. И. Менделеева: “ИННОВАЦИОННЫЕ ХИМИЧЕСКИЕ ТЕХНОЛОГИИ И БИОТЕХНОЛОГИИ НОВЫХ МАТЕРИАЛОВ И ПРОДУКТОВ”.
Организаторы конференции: Российское и Московское химическое общество им. Д. И. Менделеева, Российский Союз химиков, Российский химико-технологический университет им. Д.И. Менделеева. Председатель оргкомитета – академик П. Д. Саркисов. Конференция состоится 28-29 сентября 2010 года в Москве во время проведения Международной химической ассамблеи “ICA - 2010”.
В рамках работы трёх секций:
на конференции планируется обсудить научные основы методов интенсификации и модернизации процессов химической технологии и биотехнологии с целью разработки инновационных технологий широкого спектра неорганических, органических и гибридных материалов и продуктов, в том числе и для фармацевтики.
Будут рассмотрены: разработка новых катализаторов и каталитических процессов; совершенствование процессов разделения смесей за счет физико-химической интенсификации; процессы переработки сложных органических композиций, нефти и газового конденсата; разработки новых полимерных, керамических, стеклокристаллических материалов; электрохимические технологии и биотехнологии.
Как направлению, открывающему широкие возможности для создания новых материалов, продуктов, лекарственных форм с уникальными свойствами, также будет уделено внимание разработке нанотехнологических процессов.
Тезисы, заявка на участие в конференции и копия квитанции (или платежного поручения) об оплате в электронном виде должны поступить в Оргкомитет по электронной почте (conf@muctr.ru) не позднее 10 сентября 2010 года. Тезисы докладов, переданные факсом, не принимаются.
По вопросам оплаты оргвзноса и тезисов обращаться:
МХО им. Д.И. Менделеева,
107045, Москва, Колокольников пер., д. 17/20.
Тел./факс: (495) 625-86-00, 742-04-22;
e-mail: mxo@asvt.ru, www.mmxo.ru
С уважением,
президент РХО им. Д. И. Менделеева,
академик П. Д. Саркисов
председатель оргкомитета
ФОРУМ СО2. Переработка больших объемов СО2 в материалы и топлива.
Dear Colleague,
How can we transform large volumes of CO2 into materials and fuels needed for quality of life and sustainable development?
The 2010 edition theme of the International Scientific Forum on CO2 chemistry and biochemistry is devoted to Large-volume CO2 recycling.
It will be held on Sept 27-28, 2010 in Lyon, France.
The registration is now open, until the July 26th for poster submission and September 3rd without poster to http://co2forum.cpe.fr/
We hope we will have the pleasure to welcome you in Lyon.
For the organizing committee
Alessandra QUADRELLI Chair
Sustainable Development Chair CPE Lyon
Claude FUSSLER, Vice Chair
UN Global Compact
PROGRAMME
|
Monday 27.
Registration and welcome coffee – posters hang up |
|
Participants welcome and Introduction Gérard Pignault, CPE Lyon. |
|
SESSION 1 CO2 : the context |
|
Chair: Claude Fussler, Global Compact, United Nations. |
|
Climate mitigation – the scientific context Rajendra Pachauri, Intergovernmental Panel on Climate Change. |
|
Climate mitigation – the social and political context Janos Pasztor, United Nations. |
|
CO2 capture and carbon finance – windows of opportunity |
|
Questions to speakers’ round table and Chair’s conclusions |
|
SESSION 2 CO2 to carbonates and chemicals |
|
Chair: Isabelle Rico-Lattes, CNRS, France. |
|
CO2 as a C1–building block |
|
CO2 to carbonates |
|
Mineral carbonation for CO2 Storage |
|
SESSION 3 CO2 to polymers and chemicals |
|
Chair: Thomas Müller, Catalytic Center RWTH Aachen, Germany. |
|
Conversion of CO2 for coating applications |
|
Electrochemical conversion of CO2 |
|
CO2 as a feedstock for polyurethane production |
|
Questions to speakers’ round table and Chairs’ conclusions |
|
POSTER Poster Session and Cocktail |
|
Tuesday 28. SESSION 4 CO2 to fuels |
|
Chair : Jean-Marie Basset, KAUST and CPE Lyon. |
|
Beyond Oil and Gas: Methanol Economy by Recycling of Carbon Dioxide |
|
CO2 Enzymatic carboxylation and reduction to methanol |
|
CO2, formic acid and hydrogen economy |
|
|
|
SESSION 5 Bioroutes to CO2 conversion |
|
Chair : Slavik Kasztelan, IFP, France. |
|
Microalgae Pilot plants for CO2 sequestration |
|
Bioconversion of CO2 by photochemical materials |
|
Photosynthetic efficiency in production of microalgae |
|
Questions to speakers’ round table and Chairs’ conclusions |
|
SESSION 6 Enabling technologies |
|
Chair : Sophie Jullian, AXELERA and IFP, France. |
|
Chemical industry, white biotechnology and renewables |
|
Solar energy engineering |
|
SESSION 7 Outlook and Conclusions |
|
Chair : Paul-Joël Derian, Rhodia. |
|
The Future of Energy Conversion: A Perspective from the Chemical Industry |
|
Energy conversion and renewables: The potential and; limits of CO2 large scale capture and recycling |
|
Questions to speakers’ round table and Chairs’ conclusions |
|
Closure |
Euro Petroleum Consultants Ltd
НЕДЕЛЯ НЕФТЕПЕРЕРАБОТКИ, ГАЗА И НЕФТЕХИМИИ В МОСКВЕ
20-24 сентября 2010
Грандъ Отель Мариотт, Москва
Уважаемые коллеги,
Мы рады сообщить, что программы конференций, которые входят в Неделю Нефтепереработки, Газа и Нефтехимии в Москве, доступны на нашем сайте
www.EuroPetro.com Регистрация на мероприятия уже открыта!
Информационно наполненная программа мероприятий позволит Вам получить исчерпывающую информацию о:
• Состоянии газовой, нефтехимической и нефтеперерабатывающей отраслей
• Последних технологиях и новейших разработках, практических примерах их применения
• Недавно завершившихся важных проектах отрасли
Мы ожидаем, что Неделю Нефтепереработки, Газа и Нефтехимии посетит 450-500 делегатов: руководство и главные специалисты нефтегазовых компаний, ГХК, НПЗ и НХК России и стран СНГ, ведущие мировые поставщики технологий и услуг отрасли.
По Вашему личному запросу, мы можем предоставить Вам список участников в 2009.
НЕДЕЛЯ ВКЛЮЧАЕТ СЛЕДУЮЩИЕ МЕРОПРИЯТИЯ:
IGTC 2010 | 20 - 21 сентября
2ая Международная газовая технологическая конференция и выставка
Высоко информативное и продуктивное собрание профессионалов газовой отрасли, включая презентации компаний ИТЕРА, СИБУР, Саратовский НПЗ, ExxonMobil, UOP, Siirtec Nigi, GTC Technologies, Flowserve, Русгазинжиниринг и др.
RPTC 2010 | 21 - 22 сентября
9ая Конференция и выставка по технологиям нефтехимии России и стран СНГ
Фокусируется на последних тенденциях рынков и технологических инновациях в области олефинов, полиолефинов и ароматики; способы повышения эффективности и производительности будут рассматриваться в презентациях компаний СИБУР, CMAI, Nexant, Новоуренгой Газпром, Foster Wheeler, Axens, UOP, Lummus Novolen, Lyonedellbasell, INEOS, GTC, Zeolyst и др. Запланированы интерактивные дискуссии на актуальные промышленные темы.
RRTC 2010 | 23 - 24 сентября
10ая Юбилейная Конференция и выставка по
технологиям нефтепереработки России и стран СНГ
В 10ый раз мы проводим самую популярную конференцию по теме технологий нефтепереработки для России и стран СНГ.
- Докладчики компаний CRITERON, AXENS, КИНЕФ, БАШНЕФТЬ, UOP, Foster Wheeler, Honeywell, Albemarle, BASF, KBR, Air Liquid и другие
- Компании CRITERION и Shell Global Solution традиционно приглашают всех делегатов 23 сентября на Гала Коктейль для плодотворного делового общения в сопровождении живой музыки в изящной зоне у фонтана в Градъ отеле Марриотт.
- Приглашаем всех делегатов присоединиться к празднованию юбилея конференции RRTC, где наши самые постоянные участники будут награждаться подарками.
СКИДКИ
• Специальные цены для ГХК, НПЗ и НХК России и стран СНГ
• Для делегатов, участников выставки, спонсоров или рекламодателей двух и более конференций
• При регистрации до 6 августа
• Для групп делегатов
СПОНСОРЫ
CRITERION, SHELL GLOBAL SOLUTIONS, HONEYWELL, ALFA LAVAL, AXENS, UOP, EXXONMOBIL, INVENSYS, DUPONT, РИФИНГ, ALBEMARLE, CLG, CD TECH, ELLIOTT EBARA GROUP
По вопросам маркетинговых возможностей обращайтесь на Moscow@europetro.com
Регистрация в группе LinkedIn
Специально для делегатов конференции создана группа в Интернет-сообществе LinkedIn. Участники конференции получат доступ к группе и возможность общения с другими участниками до и после мероприятия, планирования деловых встреч и переговоров. Вы также можете присоединиться к Первому русскоязычному Интернет-сообществу для профессионалов в области нефтепереработки, нефтехимии и газа. Для регистрации в группах, пожалуйста, пройдите по ссылкам.
Мы будем рады приветствовать Вас на мероприятиях, которые вновь обещают быть успешными!
С уважением и наилучшими пожеланиями,
Мария Зайцева
Директор по конференциям
Euro Petroleum Consultants Ltd [EPC]
Тел.: +7 (495) 517 77 09 / Факс: +7 (495) 662 33 87
Email: Maria_Zaytseva@EuroPetro.com
Web: www.EuroPetro.com










