

Академик Кирилл Ильич Замараев (1939 – 1996 гг.) – выдающийся российский ученый, крупный организатор науки, талантливый педагог, внесший большой вклад в развитие химической физики и науки о катализе.
К.И. Замараев родился 20 мая 1939 года в Москве в семье известного советского инженера-химика Ильи Кирилловича Замараева. Начав свою профессиональную подготовку в Московском химико-технологическом институте им. Д.И. Менделеева, Кирилл Ильич перешел в Московский физико-технический институт (МФТИ), чтобы серьезно заняться химической физикой. В 1966 г. он закончил аспирантуру в МФТИ, защитив диссертацию на соискание ученой степени кандидата физико-математических наук. С этого времени К.И. Замараев работал в Институте химической физики им. Н.Н. Семенова АН СССР в должностях младшего, старшего научного сотрудника, затем заведующего лабораторией и одновременно преподавал в МФТИ в качестве доцента.
В 1972 г. им была защищена диссертация «Исследование строения и реакционной способности комплексов переходных металлов при помощи электронного парамагнитного резонанса» на соискание ученой степени доктора химических наук по специальности Физическая химия.
В 1974-1975 годах К.И. Замараев занимался исследовательской работой в США в Корнельском, Стенфордском и Чикагском университетах. Он был в числе первых советских ученых, приглашенных в Америку для долгосрочной работы по программе научного обмена в области химии между СССР и США.
В 1976 г. К.И. Замараев был избран членом-корреспондентом Академии наук СССР. В начале 1977 года по приглашению академика Г.К. Борескова К.И. Замараев переехал в Новосибирск с большой командой молодых специалистов по химической физике, значительно расширив проводившиеся в Институте катализа исследования элементарных каталитических реакций на молекулярно-атомном уровне с применением современных физических и кинетических методов.
С 1977 года К.И. Замараев занимал должность заместителя директора Института катализа СО АН СССР, а в 1984 году возглавил Институт. С этого момента и до последних дней жизни К.И. Замараев являлся его научным руководителем.
В 1987 году К.И. Замараев был избран действительным членом Академии наук СССР.
Много сил и энергии потребовало от К.И. Замараева создание новых организационных форм взаимодействия науки и промышленности. С 1986 по 1992 год он возглавлял межотраслевой научно-технический комплекс «Катализатор». Работа МНТК оказала большое влияние на коллектив Института катализа, усилила его способность работать с промышленными предприятиями. Именно в это время проявились великолепные организаторские качества Кирилла Ильича, сумевшего создать внутри Института высокоэффективный коллектив единомышленников, установивший деловые контакты с отраслевыми организациями, и обеспечить координацию их деятельности на общесоюзном уровне. Эффективная научно-организационная деятельность МНТК открыла двери в промышленность для новейших разработок прежде всего академических институтов, пробиться которым в прежние годы было практически невозможно из-за противодействия отраслевых институтов и работников министерств. За период деятельности МНТК были освоены около 30 новых технологий, на четверть обновлен ассортимент основных промышленных катализаторов и носителей.
К.И. Замараев был настоящим лидером МНТК. Жизненная энергия Кирилла Ильича воплощалась в жизненную силу коллектива Института. Поэтому при обвальном переходе на рыночные отношения в научно-технической сфере Институт катализа смог продемонстрировать высокую устойчивость и эффективность.
В последние годы академик Замараев принимал активное участие в перестройке системы отечественной науки и ее адаптации к условиям рыночной экономики. Кирилл Ильич играл ведущую роль в формировании широкой сети Государственных научных центров Российской Федерации, призванных, совместно с Российской Академией наук, поддерживать основной научный потенциал России.
Являясь признанным лидером российского сообщества специалистов-каталитиков, академик Замараев проводил большую научно-организационную работу, был председателем Научного совета по катализу и его промышленному использованию, Объединенного научного совета по химическим наукам СО РАН.
К.И. Замараев принимал активное участие в подготовке научных кадров. С 1977 года являлся профессором и заведующим кафедрой физической химии Новосибирского государственного университета.
К.И. Замараев проводил активную работу по развитию международного сотрудничества. С конца 60-х годов он был ученым секретарем Национального комитета советских химиков, официально представлявшим интересы нашей страны в Международном союзе теоретической и прикладной химии (ИЮПАК). В 1987-1989 гг. он работал Президентом отделения физической химии, затем в 1989-1993 гг. членом Бюро, а в 1993-1995 годах занимал пост Президента Международного союза теоретической и прикладной химии, а затем пост-Президента.
К.И. Замараев, работая в комитете по публикациям ИЮПАК (1990-1993 г.г.), инициировал серию монографий «Химия для 21-го века» и вместе с Дж.М. Томасом стал редактором первого тома этой серии «Перспективы катализа».
С 1987 г. он возглавлял советскую часть проекта «Катализ» долгосрочной комплексной программы сотрудничества с Национальным центром научных исследований Индии.
О международном признании научных заслуг К.И. Замараева свидетельствует избрание его членом Академии Европы, иностранным членом Индийской и Корейской академий наук, редактором международных научных журналов.
Профессор Замараев был главным редактором международного журнала Reaction Kinetics and Catalysis Letters, редактором Journal of Molecular Catalysis, членом редакционных советов многих журналов: Catalysis Reviews, Catalysis Today, Catalysis Letters, Topics in Catalysis, Chemistry – A European Journal, Mendeleev Communications, Chemistry for Sustainable Development, Кинетика и катализ, Успехи химии, Известия Академии наук, Журнал структурной химии и др.
В 1994 году К.И. Замараеву была присуждена Серебряная медаль Королевского химического общества Великобритании как выдающемуся лектору современности.
В 1995 году за работы в области экологического катализа он был награжден престижной немецкой наградой для российских ученых – премией и медалью имени А.П. Карпинского.
К.И. Замараев внес крупный вклад в развитие теории и практики каталитической науки. Основополагающее значение имеют его работы по изучению механизмов каталитических реакций и структуры активных центров, исследования в области фотохимии и разработки реакторов для использования солнечной энергии, а также работы по изучению теоретических основ спинового обмена в растворах и его применения в химии.
Кирилл Ильич начал свою деятельность с исследования методом электронного парамагнитного резонанса строения комплексов переходных металлов в растворе. Хорошо известны его работы по интерпретации сложных спектров ЭПР замороженных растворов металлокомплексов, по определению связи магниторезонансных параметров со строением комплексов металлов. Параллельно им проводились фундаментальные экспериментальные и теоретические исследования спинового обмена в растворах парамагнитных комплексов металлов. Эти работы составили основу широко известных монографий по проблемам спинового обмена, изданных в России и за рубежом.
В середине семидесятых Кириллом Ильичем открыты и исследованы реакции туннельного переноса электрона на большие расстояния между парами реагентов, вмороженными в твердые нейтральные матрицы, была продемонстрирована возможность туннелирования электронов на расстояния 15-30 Å и подробно изучена кинетика этого явления. Результаты этих работ были впоследствии изложены в монографии «Туннелирование электрона в химии», опубликованной в издательствах Наука и Elsevier. В это же время начат интересный цикл работ по изучению роли внешнесферной координации в механизмах каталитических реакций.
Кирилл Ильич был одним из пионеров исследования строения активных центров катализаторов и ключевых интермедиатов гомогенных и гетерогенных каталитических реакций методами ЯМР-, ЭПР- и ИЦР-спектроскопии. В частности, впервые были зафиксированы и охарактеризованы методом многоядерного ЯМР алкилпероксо- и пероксокомплексы молибдена – ключевые интермедиаты эпоксидирования олефинов. Методом твердотельного многоядерного ЯМР было исследовано строение активных центров широкого круга промышленно важных катализаторов, установлена природа соединений, ответственных за каталитические свойства, исследовано координационное окружение элементов, получены данные о природе поверхностных центров, об изменениях химической связи в молекулах при адсорбции, о природе промежуточных продуктов химических реакций. С использованием современных методик ЯМР детально изучены механизмы превращения спиртов и углеводородов на цеолитах, выяснена природа и определено строение интермедиатов, образующихся в этих реакциях. Открыты новые для цеолитного катализа пути превращения углеводородов.
В последние годы Кирилл Ильич уделял большое внимание экологическим проблемам, передовым технологиям. Наиболее известны его работы в области преобразования солнечной энергии в химическую, как основы развития безопасной для окружающей среды энергетики будущего, работы по фотокаталитическим реакциям и их влиянию на процессы, протекающие в атмосфере, а также работы по созданию новых катализаторов и каталитических технологий для защиты окружающей среды.
Работы К.И. Замараева составляют фундамент для развития каталитической химии будущего. Им опубликованы 3 монографии и более 300 научных работ в отечественных и зарубежных изданиях.
Неиссякаемая энергия, целеустремленность, творческая активность, безусловная преданность делу – это те человеческие ценности и идеалы, которые пронес Кирилл Ильич через всю жизнь.
XXIII Международная конференция по химическим реакторам
ХИМРЕАКТОР-23
5-9 ноября 2018 года, Гент, Бельгия
http://conf.nsc.ru/CR_23/en/

XXIII Международная конференция по химическим реакторам ХИМРЕАКТОР-23 традиционно проходила под эгидой Европейской Федерации по химической технологии (EFCE). Организаторами конференции выступили Институт катализа им. Г.К. Борескова СО РАН (Россия) и Университет Гента (Бельгия). Местом проведения конференции стал Конгресс-центр Университета Гента Хет Пэнд – здание, которое славится своими историческими корнями и расположено в самом центре средневекового города Гент. Совместно с конференцией ХИМРЕАКТОР-23 (8-9 ноября) прошла Международная конференция по математическим основам в (био)химической кинетике и инжиниринге MaCKiE 2018, посвященная применению математических методов для изучения и описания кинетических явлений.
За много лет своей истории конференция ХИМРЕАКТОР превратилась в важный международный форум, в котором принимают участие сотни исследователей и инженеров из многих стран мира. Традиционно программа конференции ХИМРЕАКТОР фокусируется на фундаментальных аспектах и практическом применении каталитических процессов и химических реакторов, а также на разработке новых высокоэффективных технологий. Мероприятие проводится в разных странах и городах, где есть крупные исследовательские центры, деятельность которых связана с развитием инновационной химической инженерии.

Председателями конференции ХИМРЕАКТОР-23 выступили профессор Александр Носков (Институт катализа им. Г.К. Борескова СО РАН, Новосибирск, Россия) и профессор Гай Маран (Университет Гента, Бельгия). Международный Научный комитет возглавил академик Валентин Пармон (ИК СО РАН, Новосибирск, Россия), Объединенный Программный комитет –
профессор Андрей Загоруйко (ИК СО РАН, Новосибирск, Россия). Председателем конференции В конференции ХИМРЕАКТОР-23 приняли участие около 200 специалистовиз 31 страны мира. Научная программа включала 5 пленарных лекций, 6 ключевых лекций, 77 устных докладов, 8 флэш-презентаций и около 100
стендовых докладов. 37 участников конференции ХИМРЕАКТОР-23 одновременно являлись участниками конференции Научная программа конференции ХИМРЕАКТОР-23, представленная на заседаниях четырех секций, фокусировалась на следующих научных направлениях: По сложившейся традиции, пленарную сессию открыл доклад, посвященный основателю конференции, профессору
М.Г. Слинько. Почетная лекция памяти профессора М.Г. Слинько была учреждена Программным комитетом конференции ХИМРЕАКТОР в 2008 году. С тех пор она звучит на каждой конференции. Право чтения лекции
по направлениям научной деятельности М.Г. Слинько предоставляется известным ученым мирового уровня, работающим в области химической
технологии, а право выбора лекторов всегда остается за членами Международного Научного комитета конференции. На конференции ХИМРЕАКТОР-23 в Генте эта честь выпала выдающемуся
ученому, профессору Йенсу К.Нёрскову(Технический университет Дании, Люнгбю-Торбек, Копенгаген, Дания).
Основной идеей лекции профессора Йенс К. Нёрскова было то, что одним из драйверов развития новой энергетики и химической промышленности
является получение топлива и ценных химических веществ. Дефицит катализаторов для таких приложений вызывает необходимость прорыва в исследовании фундаментальных основ их создания. В лекции профессора
Нёрскова «Катализ для устойчивого производства топлив и химикатов» были обсуждены подходы к разработке таких катализаторов. Изложенные
результаты продемонстрировали углубленное понимание свойств разрабатываемых катализаторов на атомно-молекулярном уровне.
Конкретные примеры включали электрохимическое разделение воды, реакции восстановления двуокиси углерода и активации молекулярного азота. Работы по этому
направлению проводились докладчиком как в академических организациях (Стэнфордский университет, США; Технический университет Дании), так и в промышленных компаниях, таких как
Исследовательский центр IBM имени Томаса Дж. Уотсона и компания Haldor Topsoe. Его научные усилия фокусировались на разработках катализаторов, которые
с помощью солнечного света превращают воду и углекислый газ в жидкое топливо.
Продолжил пленарную сессию профессор Вемури Балакотайя из Университета Хьюстона (Хьюстон, Техас, США). Его лекция «Дизайн
автотермического реактора для парциального каталитического окисления» была посвящена автотермическим реакторам, в которых поддержание
оптимального температурного режима осуществляется за счет теплоты химической реакции без использования внешних источников энергии.
<Профессор <Балакотайя дал обстоятельный анализ трех типов автотермических режимов в зависимости от областей множественных стационарных состояний, генерируемых либо принудительным
(конвективным) теплообменом между реагентами и продуктами с внутренним или внешним теплообменником (тип I), либо периодическим изменением потока (тип II), либо достаточной теплопроводностью
(тепловое обратное смешивание) в реакторе (тип III). Далее его лекция была посвящена проектированию и анализу реакторов с автотермическим режимом III типа в
контексте процессов каталитического парциального окисления. Профессор Дионисос Влахос из Делавэрского университета (Ньюарк, Делавэр, США) в лекции «Многоуровневый мост между инжинирингом химических процессов и вычислительным
катализом» предложил алгоритмы многомасштабного моделирования для разработки энергоэффективных процессов и создания более эффективных
катализаторов с повышенной активностью и селективностью в этих процессах. Разработка надежных и эффективных методов расчета физических свойств материалов
на основе «первых принципов», их применение к конкретным задачам физического материаловедения и анализ полученных результатов
представляются важной и актуальной задачей современной науки. В докладе обсуждалось, как совместить высокую точность расчетов с низкими вычислительными затратами<,
как оценивать и уменьшать ошибки в многоуровневых моделях, как определять активный центр катализатора и предсказывать новые комбинации активных
центров для повышения активности и селективности катализаторов. В лекции профессора Дионисоса Влахоса были обсуждены концепция «небольших данных», энтропийные и энтальпийные
корреляции, количественная оценка коррелятивной неопределенности, применение методов машинного обучения в катализе и оптимизации
катализаторов на атомном уровне. На примерах реакции разложения аммиака и электрокаталитической реакции восстановления кислорода было
продемонстрировано успешное совместное применение спектральных методов, машинного обучения и квантовой химии для построения модели,
адекватно воспроизводящей микроструктуру окружения активного центра в катализаторе. По мнению докладчика, описанный подход может повышать эффективность существующих технологий как минимум на порядок.
Большой интерес у аудитории вызвала лекция Клейтона Садлера, представителя компании UOP (Де Плейнс, Иллинойс, США), «Метанол
в олефины: концепция коммерциализации». Поскольку мировой спрос на легкие олефины неуклонно растет, особенно на развивающихся рынках, возникла потребность в новых альтернативных
технологиях. Легкие олефины являются исходным сырьем для производства многочисленных химических продуктов. Стоимость производства легких
олефинов из нефтяных источников постоянно возрастает в связи с ограниченностью и высокой стоимостью нефтяных ресурсов. Превращение
метанола в олефины является способом для производства легких олефинов, этилена и пропилена из сырья, полученного из источников на
ненефтяной основе. В этом случае упрощается технологическая схема и снижаются капитальные затраты. Метанол широко добывается из
природного газа и угля в местах с их большими запасами. Используя метанол, полученный из этих экономически выгодных сырьевых
материалов, технология обеспечивает низкие производственные затраты для этилена и пропилена, что является критически важной составляющей.
В лекции было рассказано о развитии этой технологии от концепции фундаментальных основ процесса до ее коммерциализации в UOP. Основное
внимание было уделено выбору реактора и регенератора, а также моделированию процесса.
Заключительную лекцию «Выбор и настройка растворителей для устойчивого химического процесса»
представил известный специалист в области химической технологии, профессор Кай Сундмахер из Института динамики сложных технических систем им. Макса Планка
(Магдебург, Германия). Во многих химических процессах растворители используются для растворения или разбавления газообразных, жидких или твердых веществ. Большинство
растворителей являются жидкостями при атмосферных условиях. При выборе подходящего растворителя для химического процесса необходимо учитывать несколько аспектов, в частности: 1) полную функциональность
растворителя в процессе, 2) общие годовые затраты при использовании растворителя в процессе и 3) влияние растворителя на окружающую
среду, здоровье и безопасность. Если раньше такой выбор базировался на эмпирических и полуэмпирических методах, то сегодня для этого
применяются современные подходы, в том числе основанные на квантово-химических расчетах, которые, тем не менее, должны
сопровождаться оптимально спланированными экспериментами. Профессор Кай Сундмахер в своей лекции продемонстрировал общий процесс выбора растворителя и технологического процесса,
проиллюстрировал это примерами на инновационных реакциях, представляющих промышленный интерес, а именно превращением
длинноцепочечных олефинов из возобновляемых ресурсов в амины посредством гидроформирования и последующего восстановительного
аминирования. Обе реакции протекают в жидкой фазе с использованием гомогенных катализаторов на основе переходных металлов. Пленарную лекцию профессора Уильяма Грина (Массачусетский Технологический Институт, Кембридж, Массачусетс, США) «Создание
и использование больших кинетических моделей: ошибка механизма и распространение оператора», представленную на <Международной
конференции по математическим основам в (био)химической кинетике и инжиниринге MaCKiE-2018, слушали участники обеих конференций. Его
лекция была посвящена концепциям компьютерного химического кинетического моделирования, проблемам применения точных моделей для конструирования химических реакторов. Ключевые лекции на конференции ХИМРЕАКТОР-23 представили профессор Манос Маврикакис, Университет Висконсина (Мэдисон, США); профессор Хосе
Карлос Лопес, Университет Порто (Португалия); профессор Евгениуш Молга, Варшавский технологический университет (Польша); профессор
Марио Монтес, Университет Страны Басков (Сан-Себастьян, Испания); доктор Бенедикт Куэно, Европейский центр исследований и повышения
квалификации в области научных вычислений (Тулуза, Франция); доктор Марко ван Гетем, компания Technip Benelux B.V. (Зутермер, Нидерланды).
Активность, с которой прошли сессии устных и стендовых докладов, подтверждает интерес всей аудитории к тематике и высокий уровень докладов. Перед
стендовой сессией желающими были представлены 8 пятиминутных флэш-презентаций, в которых авторы отразили ключевые моменты своих
стендовых докладов. Они имели возможность продемонстрировать слайды и пригласить аудиторию к обсуждению представляемого ими на стенде
материала.
В рамках программы конференции ХИМРЕАКТОР-23 прошли специальные сессии двух Европейских проектов: проект BIOLEUM, решающий одну из востребованных задач термохимической конверсии биомассы – получение жидкого и газообразного топлива и ценных химических продуктов методом быстрого пиролиза, и проект IMPROOF,
направленный на повышение энергоэффективности печей парового крекинга при одновременном снижении выбросов парниковых газов и NOx. На заседаниях этих проектов было представлено 11 устных докладов.
В рамках конференции MaCKiE 2018 состоялся Круглый стол по новым методам анализа переходных данных в каталитической кинетике. Он прошел в он-лайн режиме совместно со специалистами Вашингтонского университета в Сент-Луисе, США. Труды конференции Химреактор-23 традиционно будут опубликованы в специальном тематическом выпуске Chemical Engineering Journal (Elsevier Science). Труды конференции MaCKiE 2018 будут опубликованы <в специальном выпуске Международного журнала химической кинетики (Wiley Online Library).
На приветственном брифинге, в котором принимают участие председатели конференции, члены Научного совета, пленарные, ключевые лекторы и
председатели научных заседаний, обсуждались вопросы модернизации тематических направлений конференции ХИМРЕАКТОР, перспектив ее дальнейшего развития, выбора места проведения следующих
мероприятий. Было получено несколько предложений по месту проведения следующей, XXIV Международной конференции по химическим реакторам ХИМРЕАКТОР-24.
Закрытие конференции ХИМРЕАКТОР-23 включало вручение дипломов медиа-партнера мероприятия – Королевского химического общества Великобритании молодым ученым за лучшие устные и стендовые доклады. Следующая конференция, ХИМРЕАКТОР-24, пройдет в 2020 году в Милане (Италия). Материал подготовили





Т.В. Замулина, А.Н. Загоруйко, А.С. Носков
(Институт катализа СО РАН, Новосибирск)
Strong, adhesive polymers made via stereochemical control
Using a chiral anion catalyst to prepare poly(vinyl ethers) transforms them from niche materials to practical plastics
Taking a cue from chemistry that’s common in the pharmaceutical industry, chemists have used chiral anions to control stereochemistry when making poly(vinyl ethers). The resulting strong, adhesive polymers could find use in lightweight composites for making bicycles, boats, and cars, for example, according to Frank A. Leibfarth, a chemist at the University of North Carolina, Chapel Hill who spoke about the work Tuesday at the American Chemical Society national meeting in Orlando during a session in the Division of Polymer Chemistry.
Until now, poly(vinyl ethers) have been pretty niche products, Leibfarth said, because they’ve been atactic, meaning that the stereochemistry of the ether sidechains coming off the polymer backbone has been random. The resulting polymers are viscous liquids at room temperature and are primarily used as adhesives.

When the stereochemistry of the sidechains of poly(isobutylvinyl ether) is random, the polymer is a viscous liquid (left). When 91% of the sidechains have the same stereochemistry, the polymer is a solid (right).
Leibfarth and postdoc Aaron J. Teator thought they could improve the properties of poly(vinyl ethers) by controlling the stereochemistry of the ether side chains so that they’re all identical, or, as polymer chemists say, isotactic. Leibfarth said he was inspired by the work of Nobel Prize-winning chemists Karl Ziegler and Giulio Natta, who developed a catalyst that produces isotactic polyolefins, such as polypropylene.
“This switch from atactic polypropylene to isotactic polypropylene essentially created a multibillion dollar industry because atactic polypropylene is almost useless,” Leibfarth said. But Ziegler-Natta catalysts become poisoned in the presence of Lewis basic heteroatoms like oxygen, which means they can’t be used to make oxygen-rich poly(vinyl ethers).
To control stereochemistry when making poly(vinyl ethers), Leibfarth and Teator turned to chiral anion catalysis, which has been used to control the stereochemistry of small molecules since 2000. The cationic polymerization takes place in a solvent with a low dielectric constant so that the cation at the end of the growing polymer chain tightly pairs with a chiral anion that dictates which side of the chain the next monomer will add to, resulting in an isotactic polymer (shown).
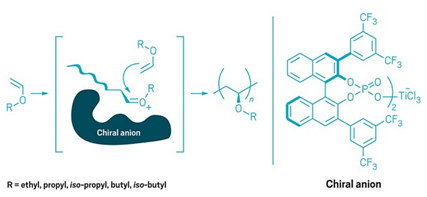
As the poly(vinyl ether) chain grows, a chiral anion blocks one side, dictating the stereochemistry of the monomer's addition.
The isotactic poly(vinyl ethers) have excellent properties, Leibfarth said. They’re similar to commercial polyolefins but adhere more strongly to polar substrates, such as glass. Leibfarth and Teator also reported the work in Science at the end of March (DOI: 10.1126/science.aaw1703) and have filed a provisional patent application on the process (62/719, 240).
Geoffrey W. Coates, a polymer chemist at Cornell University, called the work “a tour-de-force that combines mechanistic insight, synthesis, stereochemistry, and polymer properties.” He adds, “Given the relative lack of work in stereocontrolled cationic polymerization since it was originally reported by chemist Calvin Schildknecht over 70 years ago, it is great to have such an exciting advance that will revive research in this important area of polymer chemistry.”
Boron helps to gently couple dinitrogen
Organoboron compounds offer a direct route for synthesizing nitrogen chains that may be used in explosives and pharmaceuticals
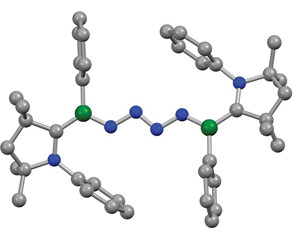
An N4 chain sits sandwiched between two borylene moieties.
Dinitrogen tends to be a loner. Extreme conditions, such as intense radiation in the ionosphere, are needed to coerce two or more N2 molecules to form chains. Various pharmaceuticals and explosives made by humans contain three- and four-atom nitrogen chains. To make these compounds, chemists have to use an indirect route. They first split dinitrogen through a high-temperature and high-pressure industrial process to produce ammonia and amines. Then they stitch together those N1 compounds into the nitrogen chains.
A new study describes a direct and gentle way to make compounds with nitrogen chains using dinitrogen. Marc-André Légaré and Holger Braunschweig of Julius-Maximilians University Würzburg and coworkers report that an organoboron compound can stitch together two N2 molecules under near-ambient conditions to form a complex in which an N4 chain bridges two boron moieties (Science 2019, DOI: 10.1126/science.aav9593).
The study follows work published last year by the Würzburg group in which they succeeded in binding a single dinitrogen molecule between organoboron ligands. This general area of research, known as nitrogen fixation, seeks ways to use highly abundant atmospheric nitrogen in chemical synthesis.
The team made the new nitrogen-chain complex by first synthesizing a dihaloorganoborane precursor via standard methods and then reducing it with a solution of KC8 in the presence of dinitrogen at roughly 4 bar (four times atmospheric pressure) and -30 °C. The group determined the product’s structure using X-ray diffraction and various spectroscopy methods. By teaming up with theoreticians at Goethe University Frankfurt, the chemists studied the molecule’s unusual bonding. They found that the borylene moieties bind dinitrogen similar to how transition metals do—by forming end-on N2-bridging complexes—yet the boron compound’s reactivity is like that of main-group elements.
Légaré says the group is now working to incorporate the nitrogen chains into organic molecules for use in synthesizing pharmaceutical compounds.
“The beauty of this study is the simplicity of the transformation, in which one reduced boron species can lead to spontaneous coupling of N2 under fairly mild conditions,” says Frédéric-Georges Fontaine, a catalysis specialist at Laval University.
Fontaine remarks that chemists might expect such a transformation to be mediated by a transition metal complex or alkaline earth species, but this one is done by a fairly simple boron molecule. He describes the work as an important step in the trend to develop metal-free transformations. This trend has shown that main group elements, used under the right set of conditions, can replace conventional catalysts, which can be costly and challenging to prepare. “The prospect of eventually including N2 as a reagent in metal-free catalysis is really exciting,” he adds.
Chemists uncover rules of thumb to help with computational screening of MOF catalysts
Relationship between active-site formation energy and bond-breaking energetics can be plugged into algorithms that search for efficient methane-activation catalysts
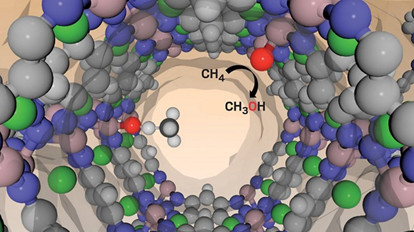
A theoretical study has uncovered a key relationship that can be used to search large numbers of MOFs for promising catalysts that can convert methane to methanol. Forming a metal-oxo species to which methane docks is key to that reaction.
By studying a large set of metal-organic frameworks (MOFs), researchers have uncovered a simple relationship between the energetics of a reactive site species and the probability of driving methane oxidation in these porous crystalline materials. The discovery provides rules-of thumb that can be incorporated into computational algorithms for screening large numbers of MOFs quickly and cost-effectively in search of catalysts that can activate methane under moderate reaction conditions.
Vast reserves of natural gas, of which roughly 95% is methane, remain untapped because the resource sits in locations that are too remote for the gas to be shipped economically. For years, researchers have worked to develop energy- and cost-effective methods for converting the gas to liquids, which can be transported more easily than gas. Commercial processes for transforming methane to methanol, hydrocarbons, and other liquids already exist. But most of those methods start by converting methane to synthesis gas, a mixture of CO and hydrogen. That process runs at high temperatures (>600 °C) and is economically viable only at very large scales.
Recent studies show that MOFs are promising catalysts for activating methane under mild conditions. But because this class of materials numbers in the tens of thousands, the task of finding or designing the best MOF for the job—for example, converting methane to methanol—is daunting. At the American Chemical Society national meeting in Orlando on Sunday, Andrew S. Rosen reported on an advance that may improve computer-based methods for screening MOF libraries for promising methane activation catalysts.
Rosen, who works with Northwestern University’s Justin M. Notestein and Randall Q. Snurr, highlighted his group’s work in a symposium organized by the Division of Catalysis Science and Technology.
By investigating MOF chemistry via quantum chemical calculations, the researchers found that a MOF’s ability to break the strong C−H bond in methane—the first step in converting the gas to methanol--can be directly related to how easy it is to oxidize the MOF’s surface metal atoms. That oxidation step is critical because it forms the metal-oxo species that functions as the catalyst’s active site (ACS Catal. 2019, DOI: 10.1021/acscatal.8b05178).
Rosen noted that the study also uncovered a periodic trend: MOFs with later transition metals form less stable but more reactive metal-oxo sites than ones with early transition metals. That finding highlights a key catalysis tradeoff. If a reactive site or intermediate is too reactive, it can dissociate before undergoing the desired catalytic reaction. If it’s too stable, it will be too inert to form the product.
By combining this new correlation with earlier work on methane activation, this group has developed an equation that can describe both the activity toward methane activation and the ability to form the active site, remarked University of Delaware’s Dionisios G. Vlachos, a catalysis specialist. This allows chemists to predict a range of suitable catalyst candidates for methane activation. He added, “Clearly, this study will stimulate interest from computationalists and experimentalists alike.”
Stir bar contamination may inadvertently catalyze reactions
Traces of metal nanoparticles embedded in used magnetic stirrers can interfere with chemical reactions

Used stir bars contain traces of metal that can catalyze reactions.
The humble stir bar is a ubiquitous workhorse of the chemistry lab that has swirled its way into researchers’ hearts. But it seems that magnetic stirrers have also been mixing things up in a different way—by smuggling rogue metals into chemists’ reaction flasks (ACS Catal. 2019, DOI: 10.1021/acscatal.9b00294).
Stir bars are typically coated with polytetrafluoroethylene (PTFE), a durable and inert polymer. But they can quickly become discolored, especially when exposed to catalytic metals such as palladium. So Valentine P. Ananikov of the N. D. Zelinsky Institute of Organic Chemistry gathered 60 used stir bars from other labs in his institute and took a closer look at what had caused the stains.
Electron microscopy revealed that the bars’ surfaces were littered with scratches, dents, and cracks that often contained a tangle of polymer filaments. These filaments trapped metal nanoparticles or microparticles, including palladium, gold, platinum, cobalt, or iron, which Ananikov’s team identified by X-ray spectroscopy. “It appears that almost all used stir bars in labs doing intensive catalysis and synthesis experiments are contaminated to varying degrees,” Ananikov says.
The researchers also found that these metals could leach into solution and interfere with reactions. Ananikov’s team tested used stir bars in the palladium-catalyzed Suzuki-Miyaura reaction, which couples aryl halides with boronic acids. First they used a palladium catalyst and a fresh stir bar to carry out a series of these reactions; then they repeated the experiments with no catalyst and a contaminated stir bar. In several cases, the dirty stir bar alone delivered a significant amount of the coupling product, and in one case it even matched the yield from the palladium catalyst. Running the reaction with no catalyst and a brand new stir bar gave no products at all.
Chemists who work with palladium catalysts generally know that dirty stir bars can affect their reactions, but it’s important to understand the extent of the problem, says synthetic chemist Mimi Hii of Imperial College London. “It’s been talked about anecdotally for a while, so it’s really nice to see a systematic study on it.” Because Hii’s group has found palladium to be active at concentrations as low as 5 parts per million, the researchers cleanse their stir bars of palladium with aqua regia, a mixture of nitric acid and hydrochloric acid. Others are not so thorough—indeed, many chemists clean their stir bars with nothing more than a quick scrub and a rinse with acetone and water, Ananikov says.
“I was surprised that there was enough metal there to catalyze reactions,” says Vladimir Gevorgyan, an organic chemist at the University of Illinois at Chicago. He points out that trace amounts of metal can sometimes interact with other metal catalysts to alter their reaction mechanism, so the problem is unlikely to be restricted to palladium chemistry. “I think it’s more general than that,” he says.
Significant stir bar damage can appear within weeks of use, and Ananikov’s theoretical calculations suggest that damaged PTFE binds metal particles much more strongly than pristine PTFE. He advises chemists to use new stir bars when they report reactions that apparently need very low concentrations of metal catalysts, or even none at all. “It’s a cautionary tale for anyone working in catalysis,” Hii says.
Chemical & Engineering News
|
June
11-13, 2019 23rd Annual Green Chemistry & Engineering Conference and 9th International Conference on Green and Sustainable Chemistry Reston, VA (USA) |
http://gcande.org |
|
June
15-17, 2019 2019 Sino-Russian High-Level International Symposium on Catalysis Harbin, China |
wuwei@hlju.edu.cn |
|
June
16-20, 2019 6th Nano Today Conference Lisbon, Portugal |
https://www.elsevier.com/events/conferences/nano-today-conference |
|
June
17-20, 2019 3rd International Conference on Applied Surface Science Pisa, Italy |
https://www.elsevier.com/events/conferences/ international-conference-on-applied-surface-science |
|
June
18-21, 2019 1st Summer School “Catalysis: from understanding to applications” Albi, France |
https://schoolcat2019.sciencesconf.org |
|
June
19-23, 2019 22nd International Conference on Chemical Thermodynamics in Russia (RCCT 2019) St. Petersburg, Russia |
http://rcct2019.spbu.ru |
|
June
23-26, 2019 5th Russian-German Seminar on Catalysis “Bridging the Gap between Model and Real Catalysis. Synchrotron Radiation in Catalysis” Novosibirsk, Russia |
http://conf.nsc.ru/rgs-2019/ |
|
June
24-28, 2019 5th EuChemS Inorganic Chemistry Conference Moscow, Russia |
http://www.igic.ras.ru/docs/news/tcirkulyar_evrohim_2019.pdf |
|
June
26-30, 2019 6th European Conference on Environmental Applications of Advanced Oxidation Processes (EAAOP-6) Portoroz-Portorose, Slovenia |
http://eaaop6.ki.si |
|
1-4 июля 2019 г. VII Всероссийская школа-конференция молодых ученых «Органические и гибридные наноматериалы» Иваново, Россия |
http://ivanovo.ac.ru/about_the_university/science/conferences/ organic_VII.php?sphrase_id=34684 |
|
1-5
июля 2019 г. .XIII Международная школа молодых ученых и специалистов имени А.А. Курдюмова «Взаимодействие изотопов водорода с конструкционными материалами» Саров, Россия |
https://ihism.org |
|
July
5-12, 2019 50th General Assembly & 47th IUPAC World Chemistry Congress Paris, France |
https://www.iupac2019.org |
|
July
8-11, 2019 14th International Conference on Materials Chemistry (MC14) Birmingham, UK |
http://www.rsc.org/events/detail/31760/14th-international- conference-on-materials-chemistry-mc14 |
|
July
15-19, 2019 Catalysis Fundamentals and Practice Summer School University of Liverpool, UK |
https://www.liverpool.ac.uk/chemistry/events/catalysis-summer-school-2019 |
|
July
17-19, 2019 Energy Security and Chemical Engineering Congress 2019 (SChE 2019) Penang, Malaysia |
http://esche.ump.edu.my/index.php/en |
|
July
22-27, 2019 Summer School “ Making Business with Green Chemistry & Sustainable Energy” Sarteano, Siena province, Italy |
www.eric-aisbl.eu/sarteano |
|
July
26-28, 2019 Mendeleev 150: 4th International Conference on the Periodic Table endorsed by IUPAC Saint Petersburg, Russia |
http://mendeleev150.ifmo.ru |
|
August
4-8, 2019 36th International Conference of Solution Chemistry Xining, China |
http://icsc2019.csp.escience.cn/dct/page/1 |
|
August
18-23, 2019 European Congress on Catalysis (14th EuropaCat) Aachen, Germany |
www.europacat2019.eu |
|
August
23-24, 2019 Russian-German Workshop on Energy-Related and Environmental Catalysis and operando Spectroscopy Karlsruhe, Germany |
http://www.itcp.kit.edu/grunwaldt/english/index.php |
|
September
2-6, 2019 5th International Conference “Catalysis for Renewable Sources: Fuel, Energy, Chemicals” (CRS-5) Crete, Greece |
http://conf.nsc.ru/CRS5 |
|
September
2-6, 2019 1st International Conference on Noncovalent Interactions (ICNI) Lisbon, Portugal |
http://icni2019.eventos.chemistry.pt |
|
4-6
сентября 2019 г. V Всероссийская научно-практическая конференция молодых ученых и специалистов «Материалы и технологии XXI века» Бийск, Россия |
post@frpc.secna.ru kubasov_artem@mail.ru 8 962 804 9888 |
|
September
9-11, 2019 Euro Conference on Catalysis & Chemical Engineering Advancements 2019 Rome, Italy |
https://scientonline.org/catalysis-conferences/index.php |
|
9-13
сентября 2019 г. XXI Менделеевский съезд по общей и прикладной химии Санкт-Петербург, Россия |
http://mendeleev2019.ru/ |
|
September
9-13, 2019 XI International Conference on Chemistry for Young Scientists Saint Petersburg, Russia |
http://mendeleev.spbu.ru/ |
|
September
11-14, 2019 7th International Conference on Semiconductor Photochemistry Milano, Italy |
https://www.sp7.unimi.it |
|
16-25
сентября 2019 г. XXXI Симпозиум «Современная химическая физика» Туапсе, Россия |
www.chemicalphysics.ru |
|
September
23-27, 2019 5th International Congress on Catalysis for Biorefineries (Catbior 2019) Turku / Åbo, Finland |
http://www.catbior2019.fi |
|
1-4
октября 2019 г. XXIII Всероссийская конференция (с международным участие м)и II Всероссийская молодежная школа-конференция «Рентгеновские и электронные спектры и химическая связь» Воронеж, Россия |
https://rescb.ru |
|
2-4
октября 2019 г. Международная научная конференция «Актуальные проблемы современной химии», посвященная 90-летию Института нефтехимиических процессов им. академика Ю.Г. Мамедалиева Баку, Азербайджан |
http://nkpi.az/?lang=ru
nkpi@nkpi.az |
|
6-10
октября 2019 г.
Международный Российско-Казахстанский Симпозиум «Углехимия и экология Кузбасса» Кемерово, Россия |
http://www.iccms.sbras.ru/ccsymp-2019 |
|
October
7-11, 2019 XI International Conference “Mechanisms of Catalytic Reactions” (MCR-XI) Sochi, Russia |
http://conf.nsc.ru/mcr2019/en/ |
|
7-11
октября 2019 г. XII Всероссийская школа-конференция молодых ученых «Теоретическая и экспериментальная химия жидкофазных систем» (Крестовские чтения 2019) Иваново, Россия |
http://krestov.isc-ras.ru/ |
|
October
10-13, 2019 III International School-Conference “Applied Nanotechnology & Nanotoxicology” (ANT-2019) Sochi, Russia |
http://conf.nsc.ru/ant-2019/en/ |
|
October
13-17, 2019 19th National Congress on Catalysis of China (19th NCC) Chongqing, Chinа |
http://19ncc.medmeeting.org/8591?lang=en |
|
October
20-23, 2019 5th International Symposium on Innovative Materials and Processes in Energy Systems (IMPRES2019) Kanazawa, Japan |
http://www.knt.co.jp/ec/2019/impres2019/index.html |
|
|
|
|
June
28 – July 3, 2020 The World Conference on Carbon (CARBON 2020) Kyoto, Japan |
http://www.tanso.org/carbon2020 |
|
July
5-9, 2020 48th World Polymer Congress (MACRO2020) Jeju Island, Korea |
http://www.macro2020.org |
|
August
16-21, 2020 12th Triennial Congress of the World Association of Theoretical and Computational Chemists (WATOC 2020) Vancouver, Canada |
http://watoc2020.ca |


