
К 70-летию со дня рождения
18 апреля 2018 года отметил свое семидесятилетие Валентин Николаевич Пармон – академик РАН, вице-президент Российской академии наук, Председатель Сибирского отделения Российской академии наук, научный руководитель Института катализа им. Г.К. Борескова СО РАН, выдающийся ученый и организатор науки.

Валентин
Николаевич – выпускник Московского физико-технического
института. В 1977 г.
был
приглашен в Институт
катализа Сибирского отделения АН СССР. В 1985 г. возглавил
лабораторию каталитических методов преобразования солнечной энергии,
с 1995 по
2015 гг. являлся директором Института катализа
Основными направлениями научной деятельности В.Н. Пармона являются разработка и исследование катализаторов и каталитических процессов, в том числе для преобразования и аккумулирования различных видов энергии, катализ и фотокатализ в природе и в использовании возобновляемых и нетрадиционных энергоресурсов, а также выяснение роли абиогенных каталитических и фотокаталитических процессов в формировании состава атмосферы Земли и зарождении биосферы.
В.Н. Пармоном разработаны научные основы фотокаталитических методов преобразования солнечной энергии в химическую (разложение воды на водород и кислород в искусственных системах). Создано новое научное направление – радиационно-термический катализ. Под руководством В.Н. Пармона сконструированы и испытаны не имеющие мировых аналогов солнечные каталитические реакторы.
В.Н. Пармоном разработан принципиально новый подход к прямому преобразованию ионизирующего излучения в энергию химических топлив. В результате предложен и испытан в лабораторных условиях энергозапасающий и энергопреобразующий процесс “ИКАР”, который может быть эффективно использован для решения проблем ядерной и термоядерной энергетики будущего. Впервые созданы и испытаны не имеющие аналогов катализаторы на основе оксидов урана, комбинирующие функции ядерного топлива и катализатора для запасания химической энергии.
Под руководством В.Н. Пармона разработаны принципиально новые центробежные реакторы для термоударной обработки порошков твердых субстратов и отработана технология получения с помощью этих реакторов порошков активной окиси алюминия. Данная технология используется для промышленного производства новейшего поколения мелкосферических катализаторов дегидрирования легких алканов в олефины, уже нашедших промышленное применение для дегидрирования изобутана в изобутилен в кипящем слое катализатора на предприятии «Тобольск-Нефтехим» нефтехимической компании ОАО «СИБУР-холдинг».
Под руководством В.Н. Пармона разработаны и промышленно внедрены катализаторы нового поколения для производства моторных топлив.
Под руководством В.Н. Пармона в кооперации с зарубежными партнёрами успешно ведутся работы по новым перспективным направлениям энергетики и транспорта (получение высококачественных топлив из возобновляемого растительного сырья, создание компактных генераторов водорода и др.).
В.Н. Пармон – вице-президент Российской академии наук, Председатель Сибирского отделения Российской академии наук, председатель Объединенного ученого совета по химическим наукам РАН, председатель Научного совета по катализу ОХНМ РАН.
В.Н. Пармон – автор и соавтор более 650 научных работ, 6 монографий, 33 обзоров, 6 учебников для вузов, обладатель более 100 авторских свидетельств и патентов.
Более 30 лет Валентин Николаевич преподает в Новосибирском Государственном университете, являясь профессором кафедры физической химии факультета естественных наук. Под его руководством подготовлены 5 докторов наук и 17 кандидатов наук.
В.Н. Пармон
– главный редактор-организатор журнала «Катализ в
промышленности» и Каталитического бюллетеня, а также редактор
журнала “Химия в России” (РХО), член редколлегий журналов
“Успехи химии”, “Кинетика и катализ”,
“Журнала физической химии” (РАН),
ряда международных журналов, Российский национальный представитель в
Европейской федерации каталитических обществ (EFCATS) и Международной
ассоциации каталитических обществ (IACS), член Президиума Российского
химического Общества имени
Заслуги В.Н. Пармона отмечены высокими государственными наградами, среди которых Государственная премия РФ, премия им. В.А. Коптюга СО РАН и НАН Беларуси, премия за инновации в катализе EFCATS, международная премия «Глобальная энергия». Награждён орденом «За заслуги перед Отечеством» IV степени, орденом Почёта, медалью Франциска Скорины Республики Беларусь.
Научный совет по катализу ОХНМ РАН и редакция Каталитического бюллетеня сердечно поздравляют Валентина Николаевича с юбилеем, желают ему крепкого здоровья и новых творческих достижений!
 |
60 лет Институту катализа |

60-летний юбилей Института катализа им. Г.К. Борескова СО РАН был отмечен праздничной научной сессией «Современные тенденции в химии и катализе», которая прошла 7 июня 2018 года в Новосибирске.
В программу сессии были включены пять пленарных лекций:
В ходе научной сессии было объявлено о присвоении звания «Почетный профессор Института катализа СО РАН» Клоду Миродатосу (Claude Mirodatos) из Института IRCELYON (Лион, Франция), Жильберу Фрома (Gilbert F. Froment) из Гентского университета (Бельгия), Алексу Беллу (Alex Bell) из Калифорнийского университета (Беркли, США) и Роберту Шлёглю (Robert Schlögl) из института Фрица Габера (Общество Макса Планка, Германия). С 2018 года по решению Ученого совета ИК СО РАН это звание введено для иностранных граждан, внесших выдающийся вклад в развитие научных связей ИК СО РАН с крупнейшими зарубежными университетами, научно-исследовательскими институтами и компаниями, проводящими исследования и разработки в области катализа и смежных дисциплин, способствующих повышению международного рейтинга Института и поддерживающих его вне зависимости от международной политической конъюнктуры.
 Финальным
аккордом юбилейного
научного мероприятия стало подписание Договора о стратегическом
сотрудничестве между химическим факультетом Московского
госудаственного университета имени М.В. Ломоносова и Институтом
катализа СО РАН. Сотрудничество между двумя организациями будет
развиваться в области науки, образования, организации конференций,
симпозиумов, семинаров и приглашения ведущих ученых для обмена
опытом.
Финальным
аккордом юбилейного
научного мероприятия стало подписание Договора о стратегическом
сотрудничестве между химическим факультетом Московского
госудаственного университета имени М.В. Ломоносова и Институтом
катализа СО РАН. Сотрудничество между двумя организациями будет
развиваться в области науки, образования, организации конференций,
симпозиумов, семинаров и приглашения ведущих ученых для обмена
опытом.

20-23 мая 2018 г., Москва, Россия
С 20 по 23 мая 2018 года в Институте органической химии им. Н.Д. Зелинского РАН (Москва, Россия) прошла V Международная школа-конференция по катализу для молодых ученых «Каталитический дизайн: от исследований на молекулярном уровне к практической реализации».

Основным организатором школы-конференции выступил Институт катализа СО РАН (Новосибирск, Россия), соорганизаторы школы: Институт органической химии им. Н.Д. Зелинского РАН (Москва, Россия), Институт нефтехимического синтеза им. А.В. Топчиева РАН (Москва, Россия), Московский государственный университет имени М.В. Ломоносова (Москва, Россия), Новосибирский государственный университет (Новосибирск, Россия), Новосибирское отделение Российского химического общества имени Д.И. Менделеева (Новосибирск, Россия), Сибирское отделение Российской академии наук. Школа прошла при финансовой поддержке Российского фонда фундаментальных исследований (РФФИ) и Федерального агентства научных организаций (ФАНО). Спонсорами школы-конференции выступили ООО «Реолгрейд-сервис», фирма SPECS Surface Nano Analysis GmbH (Германия), ООО «УНИКАТ», ООО «ФИЗЛАБПРИБОР».
 Информационную поддержку школе-конференции оказали журналы «Кинетика и катализ», «Нефтехимия», «Катализ в
промышленности».
Информационную поддержку школе-конференции оказали журналы «Кинетика и катализ», «Нефтехимия», «Катализ в
промышленности».
В работе школы приняли очное участие 150 представителей российских и зарубежных научных институтов и промышленных организаций из России, Австрии, Азербайджана, Бельгии, Канады, Великобритании, Германии, Франции, Чили, Чехии, Дании, Норвегии, Польши, Румынии, Словакии, Швейцарии, Нидерландов и США. Было представлено 11 пленарных лекций (30 минут), 4 специальных устных доклада (20 минут), 45 устных докладов (15 мин), 80 стендовых докладов и 39 заочных докладов по следующим направлениям:

 В день открытия школы с пленарными лекциями выступили профессор Владимир Геворгян (Университет Иллинойса, США), профессор Марк
Копер (Университет Лейдена, Нидерланды), академик Валерий Иванович Бухтияров (Институт катализа СО РАН, Новосибирск, Россия) и чл.-корр. РАН
Владимир Александрович Лихолобов (Институт проблем переработки углеводородов СО РАН, Омск, Россия).
В день открытия школы с пленарными лекциями выступили профессор Владимир Геворгян (Университет Иллинойса, США), профессор Марк
Копер (Университет Лейдена, Нидерланды), академик Валерий Иванович Бухтияров (Институт катализа СО РАН, Новосибирск, Россия) и чл.-корр. РАН
Владимир Александрович Лихолобов (Институт проблем переработки углеводородов СО РАН, Омск, Россия).
 Лекция профессора В. Геворгяна была посвящена новым подходам к функционализации С-Н связи,
катализируемой переходными металлами с использованием кремниевого связующего. Были обсуждены механизмы функционализации С-Н связи, а также описаны основные особенности
каждого механизма.
Лекция профессора В. Геворгяна была посвящена новым подходам к функционализации С-Н связи,
катализируемой переходными металлами с использованием кремниевого связующего. Были обсуждены механизмы функционализации С-Н связи, а также описаны основные особенности
каждого механизма.
В докладе профессора М. Копера был представлен теоретический анализ для множественных протонно-электронных реакций переноса, основанный на микроскопической теории протонно-связанных реакций переноса электронов. Также были продемонстрированы последние разработки в
термодинамической теории многоступенчатых реакций переноса электронов.
Академик РАН В.И. Бухтияров в своей лекции рассказал о размерных эффектах металлических частиц, о влиянии размера металлических частиц на активацию и адсорбцию реагентов. Лекция была посвящена влиянию хемосорбции на реактивность поверхностных атомов металла за исключением явлений, происходящих на границе раздела металл-подложка.

Чл.-корр. РАН В.А. Лихолобов завершил научную часть первого дня конференции лекцией, посвященной дизайну активных центров как инструменту усовершенствования промышленных катализаторов ‒ композиций «Pt/оксиды алюминия». Был представлен анализ результатов исследований, направленных на улучшение технологических показателей (активность, селективность, стабильность) каталитических композиций «Pt/оксиды алюминия» в процессах дегидроциклизации и дегидрирования индивидуальных алканов. Полученные результаты важны для разработки технологии получения нового поколения платиновых катализаторов дегидрирования алканов.
Во второй день школы-конференции было представлено три пленарных лекции, два специальных устных доклада (20 минут) и ряд устных докладов молодых специалистов по четырем секциям школы.
Открыла второй день школы-конференции лекция директора Института им. Ф. Габера общества Макса Планка (Берлин, Германия) Беатрис Ролдан Куэнья под названием «Операндо нанокатализ». В своей лекции профессор Ролдан рассказала о важнейшей проблеме в области исследования катализа, связанной с получением заданной химической реакционной способности наноматериалов на атомном уровне. В докладе приводились примеры последних достижений в области приготовления, плазма-функционализации и характеризации наноструктурированных пленок и наноразмерных катализаторов с четко определенной морфологией, размером и формой. Обсуждалось решение проблемы определения структуры и состава наноразмерных металлических катализаторов сочетанием микроскопии in situ и operando (АСМ, СТМ, ПЭМ) и спектроскопии (XAFS, РФЭС).

Следующая лекция также была представлена сотрудником Института им. Ф. Габера общества Макса Планка (Берлин, Германия) ‒ доктором Детре Тешнером. Данная лекция была посвящена механизму и кинетике гетерогенной каталитической реакции Дикона. В своем докладе Д. Тешнер описывал различные подходы, используемые для понимания механизма реакции. Был предложен простой феноменологический подход, который заключается в сборе кинетических данных в зависимости от условий процесса, таких как парциальное давление реагентов (и продуктов), температура, тип реактора, время воздействия и т.д. Сочетание детальных кинетических исследований, натурной спектроскопии и DFT-расчетов может позволитьразработку улучшенных катализаторов.

Третью лекцию в этот день прочитал профессор Эрик Хирс (Университет Гронингена, Нидерланды). Он рассказал про био-ароматические соединения, получаемые из биомассы. Группа Э. Хирса проводит исследования, направленные на поиск подходящей каталитической технологии для получения ароматических продуктов из источников биологического происхождения.

Основное внимание было уделено использованию двух технологий: каталитическому пиролизу и каталитической гидрообработке в сочетании с использованием остатков биомассы, таких как лигнин. Были представлены типичные особенности обеих технологий и отмечены последние результаты в данной области, с особым акцентом на выходы био-ароматических веществ.
Устные доклады проходили параллельно в двух залах. В первом зале прошли доклады по первой и четвертой секциям, которые были посвящены, соответственно, приготовлению катализаторов и роли катализа в защите окружающей среды. Первую секцию 20-минутным докладом открыл профессор Давид Симаков (Университет Ватерлоо, Канада), исследовательская группа которого занимается изучением термокаталитической конверсии CO2 в возобновляемые синтетические топлива.
Никлас Розенталь Беннедсен (Датский технический университет, Дания) рассказал про гетерогенный катализатор на основе неблагородного металла для гидросилилирования кетонов.
Доклад Марии Марковой (Тверской государственный университет, Тверь) был посвящен разработке новых катализаторов, полученных гидротермальным методом. Данный метод позволяет формировать наночастицы металлов и их соединений в порах носителя.
В работе Ирины Тюлюковой (Институт катализа СО РАН, Новосибирск) рассматривалось влияние среды синтеза на морфологию и текстуру кристаллов SAPO-11.
Екатерина Горохова (Технологический институт сверхтвердых и новых материалов, Москва) рассказала о параметрах пептизации, используемой для контроля свойств катализаторов Фишера-Тропша.
Доклад Людмилы Николаевны Степановой (ИППУ СО РАН, Омск) был посвящен разработке экономически эффективных и экологически безопасных технологий преобразования лигноцеллюлозной биомассы в топливо и химические вещества. Целью ее работы являлся синтез Mg (Ni,Li)Al-LDH различными методами, исследование их структурных и текстурных свойств и установление взаимосвязи между природой гидрогенизирующего металла (Pd, Ni), основностью носителя (MgAl и LiAl) и каталитическими свойствами катализаторов гидрирования на основе соответствующих LDH.
Марина Валерьевна Бухтиярова (Института катализа СО РАН, Новосибирск) рассказала про N-метилирование аминов в присутствии катализаторов на основе Cu-слоистых двойных гидроксидов.
В докладе Константина Радиковича Валеева (Институт катализа СО РАН, Новосибирск) было рассказано про новые CuAl и CuFeAl керамометаллические катализаторы для реакции паровой конверсии СО.
Кевин Плонер (Университет Инсбрука, Австрия) отметил необходимость постепенного отказа от ископаемых видов энергии и перехода к возобновляемым источникам энергии. Основная проблема заключается в накоплении и распределении этой энергии. Возможным решением является превращение CO2 в метанол, который впоследствии может подвергаться различным превращениям. В своей работе он проводил исследование Ag- и Co3O4-нанесенных ферритов титана-стронция в паровом риформинге метанола.
Александр Штыка (Лодзинский технический университет, Польша) рассказал о предварительной обработке углеродных нанотрубок, влиянии обработки на их физико-химические свойства и каталитические характеристики в окислительном паровом риформинге метанола.
Татьяна Юрьевна Кардаш (Институт катализа СО РАН, Новосибирск) рассказала о дезактивации и улучшении стабильности активной фазы в оксидных катализаторах VMoNbTe для окислительного дегидрирования этана.
Доклад Валентины Юрьевны Трегубенко (Институт проблем переработки углеводородов СО РАН, Омск) был посвящен триметаллическим катализаторам нефтяного риформинга. В своей работе она изучала свойства функции металла и влияние порядка добавления прекурсоров металлов на Pt-Sn-Zr/γ-Al2O3-Cl.
Последний доклад первой секции сделала Дарья Сергеевна Хабарова (Самарский национальный исследовательский университет), он был посвящен гидротермальному синтезу катализаторов окисления, содержащих платину и хром, на металлическом носителе.
Четвертую секцию, посвященную защите окружающей среды, открыл Роман Игоревич Дралюк (Институт катализа СО РАН, Новосибирск). В своей работе он изучал использование гетерогенных катализаторов Фентона на основе ферросиликатов в реакции окисления фенола в жидкой фазе, а также влияние кристалличности, пористости и типа железа гетерогенных катализаторов на их каталитическую активность.
В докладе Марии Владимировны Грабченко (Томский государственный университет, Томск) была описана разработка катализаторов глубокого окисления вредных соединений.
Следующим был доклад Марии Валерьевны Алексеевой (Институт катализа СО РАН, Новосибирск). Она рассказала о роли стабильности катализатора в гидрообработке продуктов пиролиза биомассы.
И.Ю Каплин (Московский государственный университет имени М.В. Ломоносова, Москва) в своей работе синтезировал катализаторы для окисления СО и сажи (MOx-Ce0,8Zr0,2O2 (M = Cu или Mn)) различными методами. Было предположено, что использование и дополнительная модификация стандартного метода синтеза позволяют улучшить конверсию в случае окисления CO, однако заметного повышения активности окисления сажи не происходит.
Светлана Александровна Селищева (Институт катализа СО РАН, Новосибирск) занималась исследованием модифицированных Cu-содержащих катализаторов в процессе гидрирования фурфурола. Было показано, что промотирование монометаллических медных катализаторов другими металлами (Ca, Fe и Al) может повысить степень конверсии фурфурола до 96% при увеличении селективности образования фурфурилового спирта до 100%.
Во втором зале участники школы-конференции заслушали доклады пятой секции «Катализ в тонком органическом синтезе, нефтехимии и переработке природного газа», а также шестой секции «Катализ для энергетически эффективных процессов, фотокатализ и электрокатализ».
Профессор Иван Васильевич Кожевников (Университет Ливерпуля, Великобритания) сделал доклад «Дегидратация легких спиртов гетерополярными кислотными катализаторами в газовой фазе».
Далее с устными докладами выступили молодые специалисты из разных стран. Доклад Татьяны Отрощенко (Институт катализа им. Лейбница, Германия) был посвящен катализаторам альтернативного типа (материалам на основе циркония) для неокислительного дегидрирования легких алканов. Были представлены факторы, влияющие на характеристики катализаторов.
Мария Артемова (РГУНГ им. И.М. Губкина, Москва) рассказала про металлические катализаторы, нанесенные на углеродные нанотрубки галлуазита для парциального окисления ароматических соединений.
Доклад Екатерины Андреевны Артюха (Институт катализа СО РАН, Новосибирск) был посвящен однореакторному синтезу вторичных ароматических аминов в проточном реакторе с использованием нанесенных медных катализаторов.
Надежда Немыгина (Тверской государственный университет, Тверь) рассказала о средствах оптимизации свойств катализаторов в реакции кросс-сочетания Сузуки. В ходе работы было установлено, что биметаллические катализаторы Pd-Au позволяют заметно (более чем в два раза) повысить каталитическую активность по сравнению с монометаллическими и устойчивость при многократном использовании.
Сергей Александрович Черняк (Московский государственный университет имени М.В. Ломоносова, Москва) обсудил в своем докладе особенности структуры и эволюции кобальтовых катализаторов на углеродных нанотрубках в синтезе Фишера-Тропша.
Также катализаторам Фишера-Тропша была посвящена работа Екатерины Владимировны Кульчаковской (Технологический институт сверхтвердых и новых материалов, Москва). В работе изучали эффект добавления цеолита в каркасный кобальт-содержащий катализатор Фишера-Тропша.
Сергей Тен (Томский государственный университет, Томск) в своей работе изучал наночастицы металлов, иммобилизованные в MOF UiO-66 для селективного окисления пропиленгликоля.
Вечернюю устную сессию открыл профессор Михаил Нечаев (Московский государственный университет имени М.В. Ломоносова, Москва). Его доклад был посвящен N-гетероциклическим карбеновым комплексам переходных металлов. В своем докладе М. Нечаев рассказал о синтезе и структуре этих комплексов и их применении в катализе.
Молодой специалист из Чехии Мария Котова (Высшая школа химической технологии, Прага) обсудила гидрирование бутилового сорбата с использованием рутениевого катализатора.
Доклад Алексея Александровича Филиппова (Институт катализа СО РАН, Новосибирск) был посвящен определению энергии активации и порядка реакции в реакциях переноса водорода в ментоне, катализируемых скелетным никелем.
Его коллега по институту Михаил Михайлович Шмаков (Институт катализа СО РАН, Новосибирск) рассказал об изучении влияния ароматической замещенной структуры на каталитическую активность фторированных органических соединений бора.
Роберт ванн Путтен (Технический университет Делфта, Нидерланды) обсудил гомогенные Mn-содержащие катализаторы для эффективного гидрирования эфиров.
Иван Сергеевич Голубев (Московский государственный университет имени М.В. Ломоносова, Москва) изучал влияние содержания цеолита на активность и селективность катализаторов гидрокрекинга NiW/Y-ASA-Al2O3.
Роман Геннадиевич Кукушкин (Институт катализа СО РАН, Новосибирск) проводил исследование каталитического парового крекинга тяжелой нефти в суспенизонном режиме в присутствии дисперсных катализаторов.
Работа Романа Сергеевича Брайко (Институт катализа СО РАН, Новосибирск) была посвящена парциальному окислению метана в синтез-газ в присутствии структурированного катализатора на основе пористого никеля.
Исследование Анны Юрьевны Куренковой (Институт катализа СО РАН, Новосибирск) было направлено на изучение влияния гидротермальной обработки на фотокаталитическую активность фотокатализаторов Cd1-xZnxS.
Анастасия Юрьевна Шарова (Национальный исследовательский Нижегородский государственный университет им. Н.И. Лобачевского) рассказала про фотокаталитическое разложение 4-нитрофенола с использованием органо-неорганических сополимеров поли(оксида титана), промотированного наночастицами Ag и Au.
В этот день прошла стендовая сессия. Было представлено 80 стендовых докладов по всем научным секциям школы-конференции.

Третий день конференции открыл профессор Евгений Пидько (Делфтский технический университет, Нидерланды, Университет ИТМО, Санкт-Петербург). Его лекция была посвящена вычисли- тельному катализу – проблеме равновесия между модельной точностью и точностью метода в вычислительных исследованиях на промышленных каталитических системах.

Михаил Юрьевич Синев (Институт химической физики имени Н.Н. Семенова, Москва) в своей лекции рассказал об окислительных превращениях легких алканов в ценные продукты с точки зрения кинетики реакции. Акцент был сделан на сложные взаимодействия между структурными особенностями и природой химических процессов, участвующих в окислительно-восстановительных циклах (удаление и расходование кислорода).

Устные доклады во второй день были разделены на две группы. В первом зале участники школы-конференции прочитали доклады, относящиеся ко второй секции «Характеристики и in situ методы исследования катализаторов». Параллельно прошли устные доклады третьей секции «Механизмы и кинетика каталитических реакций».
Симон Пеннер (Университет Инсбрука, Австрия), постоянный участник школы-конференции, в своем 20-минутном докладе рассказал про управление наноструктурированной фазовой границей металл-оксид для контроля селективности реакций с участием метанола.
Работа Агнес Цечени (Делфтский технический университет, Нидерланды) была посвящена моделированию структуры Fe-содержащих пористых катализаторов селективного окисления метана.
Следующий доклад прочитала Александра Михайловна Зима (Институт катализа СО РАН, Новосибирск), он был посвящен окислению ароматической С-Н связи на Fe(V)-оксо интермедиатах с аминопиридиновыми лигандами.
Павел Александрович Коц (Московский государственный университет имени М.В. Ломоносова, Москва) рассказал о механизме альдольной конденсации на Sn и Zr-BEA цеолитах.
В докладе Михаила Вячеславовича Полынского (Университет ИТМО, Санкт-Петербург) обсуждались проблемы реакции Негиши. В своей работе он использовал DFT моделирование, чтобы показать, что ZnX2 может реагировать с ключевыми Ni- и Pd-катализаторами в реакции Негиши, таким образом изменяя нормальный каталитический процесс.
Последней в данной секции выступила Анна Аркадьевна Курохтина (Иркутский государственный университет, Иркутск). Ее работа посвящена изучению дифференциальной селективности реакции прямого арилирования гетероароматических соединений по С-Н-связи.
Профессор Себастен Пауль (Университет Лилля, Франция) рассказал о передовых высокопроизводительных технологиях дизайна катализаторов, получаемых в рамках реализации проекта REALCAT. Основная цель данного проекта – развитие инновационных технологий во всех отраслях промышленного катализа с акцентом на заводы по производству биотоплива.
Устные 15-минутные доклады начались с презентации Норберто Кёпфле (Университет Инсбрука, Австрия). Он рассказал о применении ex и in situ методов для выявления активных центров интерметаллического предшественника катализатора Pd/Zr в сухом риформинге метана.
Доклад Андрея Валерьевича Бухтиярова (Институт катализа СО РАН, Новосибирск) был посвящен моделям биметаллических Pd-Me/HOPG (Me=Ag;Cu) катализаторов. Он рассказал о приготовлении таких катализаторов и методах исследования (СТМ/РФЭС).
Ольга Александровна Булавченко (Институт катализа СО РАН, Новосибирск) также занимается in situ РФА и РФЭС исследованиями. В своей работе она изучала процесс восстановления смешанных Mn-Zr и Mn-Co оксидных катализаторов окисления СО.
Следующий доклад сделала Екатерина Пономарева (Институт химической физики имени Н.Н. Семенова, Москва). В докладе обсуждалось понимание природы реакционноспособного кислорода в смешанном оксидном катализаторе Na2WO4-MnxOy/SiO2.
Завершили научную сессию два доклада сотрудников Института катализа СО РАН. Андрей Александрович Сараев рассказал об in situ XAS и РФА исследованиях CuFeAl композитных катализаторов окисления CO. Елизавета Александровна Деревянникова обсудила локальную структуру допированного Pt CeO2 катализатора, исследованную методами PDF и EXAFS.

Последний день конференции открыла лекция профессора Клода Миродатоса (Институт
катализа и защиты окружающей среды в Лионе, Франция). Его лекция была посвящена подходам, позволяющим предсказать необходимый дизайн катализатора в зависимости от механизма реакции. Профессор Миродатос
отметил, что механизм реакции, который контролирует элементарные поверхностные реакции, также сочетается с процессами тепло- и массопереносов, которые тесно связаны с типом рассматриваемого
реактора для заданной реакции.
 В своей лекции он предложил несколько стратегий для нахождения необходимых свойств катализатора для проведения той или иной реакции, а также возможности улучшения данных свойств.
В своей лекции он предложил несколько стратегий для нахождения необходимых свойств катализатора для проведения той или иной реакции, а также возможности улучшения данных свойств.
Профессор Юнни Олсби (Университет Осло, Норвегия) прочитала лекцию, посвященную пониманию селективности процесса превращения метанола в углеводороды на молекулярном уровне. В докладе профессора Олсби обсуждались современные подходы и представления о химии процесса переработки метанола в углеводороды. Понимание термодинамики и кинетики может быть использовано также для управления селективностью процесса.
Завершилась школа-конференция интеллектуальной игрой «Научный квиз». Участники проявили интерес к новому виду взаимодействия. При этом формирование сборных команд дало возможность для неформального обсуждения научных тем между участниками из разных городов и стран.

Для участников конференции были проведены экскурсии в институты-соорганизаторы (Институт органической химии им. Н.Д. Зелинского РАН, Институт нефтехимического синтеза им. А.В. Топчиева РАН, Московский государственный университет имени М.В. Ломоносова).
V Международная школа-конференция по катализу для молодых ученых "Каталитический дизайн: от исследований на молекулярном уровне к практической реализации" была ориентирована на молодых ученых, аспирантов и студентов, занятых в различных областях исследования катализа.

Участники школы-конференции получили уникальную возможность обсудить свои исследования с ведущими специалистами. Участники отметили высокий уровень проведения школы-конференции, актуальность приглашенных докладов и изъявили желание участвовать в последующих школах-конференциях, проводимых Институтом катализа СО РАН. Следующая школа-конференция должна пройти в 2021 году.
Материал подготовили
М.С. Суворова, М.В. Бухтиярова,
М.В. Алексеева, О.О. Заикина
(Институт катализа СО РАН, Новосибирск)
New solar-powered electrolysis system avoids briny bugbears like chlorine production
Harvesting hydrogen gas from water through electrolysis could lead to a renewable source of fuel. For a small island nation like Singapore, though, fresh water is a precious resource. So electrolysis researchers there have turned their attention to the sea. They have now developed a catalyst that helps to electrolyze seawater with record-breaking efficiency, generating oxygen and hydrogen that could eventually feed fuel cells (Adv. Mater. 2018, DOI: 10.1002/adma.201707261).
The system is powered by solar electricity, producing hydrogen from sunlight with an overall efficiency of 17.9%. “As far as we know, that’s the highest efficiency for seawater,” says Bin Liu of Nanyang Technological University, who was part of the research team.
The oceans contain a vast store of hydrogen atoms, but freeing them by electrolysis is a huge challenge. In an electrolyzer, the current used to split briny water usually turns chloride ions into unwanted chlorine gas, while other ions like calcium and magnesium form insoluble precipitates that clog vital catalysts on the electrodes. The electrolysis reactions can also cause pH changes that corrode electrodes.
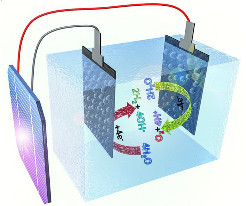 A cobalt hexacyanoferrate catalyst helps to generate bubbles of oxygen
gas at the anode (yellow arrow, right) of a solar-powered seawater
electrolyzer. Meanwhile, a nickel molybdenum sulfide catalyst at the
cathode produces hydrogen gas (arrow, left). Solar panel shown at far
left.
A cobalt hexacyanoferrate catalyst helps to generate bubbles of oxygen
gas at the anode (yellow arrow, right) of a solar-powered seawater
electrolyzer. Meanwhile, a nickel molybdenum sulfide catalyst at the
cathode produces hydrogen gas (arrow, left). Solar panel shown at far
left.
Liu’s team had previously developed a nickel molybdenum sulfide catalyst that lowered the voltage needed to generate hydrogen gas at a cathode from seawater (Sci. Adv. 2015, DOI:10.1126/sciadv.1500259). Their new catalyst relies on earth-abundant elements to oxidize seawater at an anode, producing oxygen gas, protons, and electrons.
To make the anode, the researchers grew nanoneedles of basic cobalt carbonate on carbon fiber cloth. Then they dipped the cloth in 2-methylimidazole, which formed a thin layer of a cobalt-imidazole metal organic framework (MOF) on the outside of the needles. Adding sodium ferrocyanide transformed that layer into cobalt hexacyanoferrate, which inherited the porous nanostructure of the MOF and formed 20-nm-thick catalytic shells around the conducting nanoneedles.
With a commercial triple-junction solar cell to supply electricity, the team tested the system using local seawater, adding nothing more than a phosphate buffer to maintain a neutral pH. After 100 hours of continuous operation, the electrolyzer had made hydrogen and oxygen, but no chlorine at all. Moreover, its electrodes and catalysts were intact, and its output had fallen by just 10%. In contrast, an electrolyzer that used conventional catalysts of platinum and iridium oxide to split the local seawater lost activity much more rapidly, and also produced some chlorine.
“The fact that it is selective for oxygen evolution rather than chlorine evolution is very significant,” says Michael E. G. Lyons, an electrocatalysis researcher at Trinity College Dublin. “It’s a very tricky thing to do.”
Peter Strasser at the Technical University of Berlin, who has worked on seawater electrolysis, points out that the system has a very low current density. To make useful amounts of hydrogen, the system would need a much higher current density, which could trigger chlorine evolution or other unwanted side reactions. “Problems arise when you go to high current densities,” he says.
Liu says that initial tests at higher current densities have not produced any chlorine. But he acknowledges that the system’s performance could be improved. Using fresh water, for example, solar-powered electrolyzers have reached solar-to-hydrogen efficiencies of more than 30% (Nat. Commun. 2016, DOI: 10.1038/ncomms13237). Liu’s team is now working with researchers at the Dalian Institute of Chemical Physics to develop their system into a prototype device for generating hydrogen.
Heme proteins make all possible isomers of prized 3-membered rings
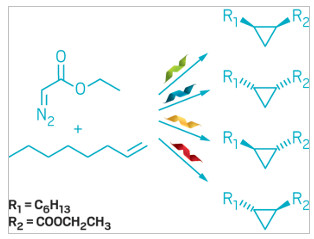
Each
engineered heme protein catalyzes formation of a different
cyclopropane stereoisomer.
With some engineering in the lab, a quartet of iron-containing heme proteins from microbes can convert inert alkenes into each possible stereoisomer of cyclopropanes, which are valuable motifs in medicinally active compounds (ACS Cent. Sci. 2018, DOI:10.1021/acscentsci.7b00548). Previous engineered proteins needed help from an artificial cofactor to complete this feat. This work suggests that heme proteins are perfectly capable of doing this chemistry on their own.
Building cyclopropanes with protein catalysts is not new, says Frances H. Arnold, the California Institute of Technology professor who led the work. However, prior heme protein catalysts made by her group and others worked best on relatively reactive alkenes. “These proteins are being commercialized, and our clients want more challenging cyclopropanations,” including transformations of unactivated alkenes, she says.
So graduate student Anders M. Knight and colleagues used directed evolution, which simulates natural selection, to find promising candidates. They optimized four heme-containing proteins from bacteria and archaea, each of which produced a different cyclopropane stereoisomer from the unactivated alkene 1-octene.
The cyclopropane-making reaction, a carbene transfer, takes place inside Escherichia coli cells and works in the presence of alcohols and other groups that might normally interfere with the reaction. Caltech has filed a provisional patent application on the technology.
“This work shows that the diversity of heme proteins in nature, coupled with the power of directed evolution, can be a route to novel stereoselective catalysts,” says John Hartwig of the University of California, Berkeley. His team has carried out this chemistry using proteins with a nonnatural iridium cofactor. So far, the new heme proteins convert terminal alkenes only, but Hartwig thinks with more work, they could convert internal alkenes too.
Knight agrees. “These active sites are very tunable,” he says. Arnold adds, “I hope that as we do more difficult target substrates, it’ll push people in industry to give this a try.”
Chemical & Engineering News
International Symposium on Zeolites and Microporous Crystals (ZMPC 2018) Yokohama, Japan |
|
|
8th Tokyo Conference on Advanced Catalytic Science and Technology (TOCAT8) Yokohama, Japan |
|
|
Nordic Symposium on Catalysis Copenhagen, Denmark |
|
|
XII International Symposium on Heterogeneous Catalysis Sofia, Bulgaria |
|
|
7th EuCheMS Chemistry Congress Liverpool, UK |
|
|
15th Eurasia Conference on Chemical Sciences (EURASIA-15) Rome, Italy |
|
|
8th IUPAC International Conference on Green Chemistry (8th ICGC) Bangkok, Thailand |
|
|
2nd Edition of Global Conference on Catalysis, Chemical Engineering & Technology (CAT-2018) Rome, Italy |
|
|
14-я российская конференция “Физико-химические проблемы возобновляемой энергетики” Черноголовка, Россия |
|
|
13th International Conference on Solid State Chemistry (SSC 2018) Pardubice, Czech Republic |
|
|
XXX Симпозиум “Современная химическая физика” Туапсе, Россия |
|
|
XII Международная конференция молодых ученых по нефтехимии Звенигород, Россия |
|
|
IV Междисциплинарный симпозиум по медицинской, органической и биологической химии и фармацевтике (МОБИ-ХимФарма2018) Новый Свет, Крым, Россия |
|
|
10th International Conference on Environmental Catalysis & 3rd International Symposium on Catalytic Science and Technology in Sustainable Energy and Environment Tianjin, China |
|
|
14th International Conference on Fundamental and Applied Aspects of Physical Chemistry “Physical Chemistry 2018” Belgrade, Serbia |
|
|
V Международная научная школа-конференция молодых ученых “Катализ: от науки к промышленности” Томск, Россия |
|
|
X Международная конференция “Химия нефти и газа” Томск, Россия |
|
|
XXXI Международная научно- техническая конференция “Химические реактивы, реагенты и процессы малотоннажной химии” (РЕАКТИВ-2018) Минск, Республика Беларусь |
|
|
VII Международный российско-казахстанский симпозиум “Углехимия и экология Кузбасса” Кемерово, Россия |
|
|
Международная научно- практическая конференция “Инновативные перспективы развития нефтепереработки и нефтехимии” Баку, Азербайджан |
(99412) 902-476 (99412)902-443 azmea_nkpi@box.az ipcp_lab3@mail.ru |
|
XV Российская ежегодная конференция молодых научных сотрудников и аспирантов с международным участием “Физико-химия и технология неорганических материалов” Москва, Россия |
|
|
15th International Conference on Micro Reaction Technology (IMPET 2018) Karlsruhe, Germany |
|
|
XXIII International Conference on Chemical Reactors (CHEMREACTOR-23) Ghent, Belgium |
|
|
International Conference on Mathematics in (bio)Chemical Kinetics and Engineering (MaCKiE-2018) Ghent, Belgium |
|
|
III Всероссийская молодежная конференция “Проблемы и достижения химии кислород- и азотсодержащих биологически активных соединений” Уфа, Республика Башкортостан, Россия |
(347) 229-96-86 |
|
International Conference on Phosphorus, Boron and Silicon 2018 Barcelona, Spain |
|
|
International Symposium on Catalysis and Fine Chemicals 2018 (C&FC 2018) Bangkok, Thailand |
|
|
|
|
|
3rd International Conference on Catalysis and Chemical Engineering (Catalysis-2019) Houston TX, United States |
|
|
3rd International Congress on Catalysis and Chemical Science Singapore, Singapore |
|
|
International Symposium on Green Chemistry (ISGC) La Rochelle, France |
|
|
5th International Conference on Polygeneration (ICP 2019) Fukuoka, Japan |
(The website will be open in early May 2018) |
|
14th International Symposium on Macrocyclic and Supramolecular Chemistry (ISMSC2019) Lecce, Italy |
|
|
6th European Conference on Environmental Applications of Advanced Oxidation Processes (EAAOP-6) Portoroz-Portorose, Slovenia |
|
|
50th General Assembly & 47th IUPAC World Chemistry Congress Paris, France |
|
|
14th EuropaCat – European Congress on Catalysis “Catalysis without Borders” Aachen, Germany |
|
|
5th International Congress on Catalysis for Biorefineries (Catbior 2019) Turku / Åbo, Finland |
|


