
 27 декабря 2017 г. исполнилось 80 лет известному специалисту в
области промышленного катализа, Заслуженному химику России, общественному деятелю, профессору, доктору химических наук Евгению Зиновьевичу Голосману.
27 декабря 2017 г. исполнилось 80 лет известному специалисту в
области промышленного катализа, Заслуженному химику России, общественному деятелю, профессору, доктору химических наук Евгению Зиновьевичу Голосману.
В течение 55 лет профессор Е.З. Голосман работает в Новомосковском филиале государственного института азотной промышленности и продуктов органического синтеза (НФ ГИАП, НИАП, «НИАП-КАТАЛИЗАТОР»), куда он пришел в 1962 г. после окончания Московского химико-технологического института им. Д.И. Менделеева. За эти годы Евгений Зиновьевич создал и возглавил сектор (1966 г.), лабораторию (1971 г.), а затем (1978 г.) хорошо оснащенный приборами и установками большой отдел физико-химических и аналитических исследований, стандартизации и качества в институте, который являлся одним из крупнейших центров страны по разработке, исследованию и наработке промышленных катализаторов. С 2011 г. – главный научный сотрудник «НИАП-КАТАЛИЗАТОР».
Евгений Зиновьевич – действительный член Российской инженерной академии и Международной академии экологии, профессор кафедры химической технологии неорганических веществ Новомосковского института РХТУ им. Д.И. Менделеева.
Под руководством Е.З. Голосмана и при его непосредственном участии создано новое научное направление – химия приготовления оксидных и металлоксидных цементсодержащих катализаторов для широкого круга органических, неорганических и экологических процессов. В соответствии с разработанной малоотходной технологией организовано промышленное производство цементсодержащих катализаторов в катализаторном цехе «НИАП-КАТАЛИЗАТОР», катализаторном производстве Дорогобужского ЗАУ и др.
Е.З. Голосман принимал участие в испытаниях и внедрении в промышленность ряда разработанных катализаторов на химических, металлургических, машиностроительных, нефтехимических заводах. В первую очередь, для процессов метанирования, получения защитных атмосфер, синтеза бутиловых спиртов, анилина, низкотемпературной конверсии оксида углерода и др. на Новомосковской АК «Азот», «Салаватнефтеоргсинтез», Новолипецком, Магнитогорском, Белорецком, Мариупольском, меткомбинатах, Запсибе, Омском заводе «Химпром», Новочеркасском заводе синтетических спиртов, Черкасском, Придонском, Северодонецком, Череповецком, Гродненском, Кирово-Чепецком, Березниковском, Щекинском, Одесском, Невинномысском, Тольяттинском, Куйбышевском, Дорогобужском азотных заводах, «Нижнекамскнефтехим», «Ангарскнефтехим», Кировоградском заводе «Пишмаш», заводе «Микропровод» (Подольск), Северском, Первоуральском трубных заводах, Череповецком сталепрокатном, Волжском и Нижегородском автомобильных заводах, телевизионном заводе «Хроматрон», Львовском, Ленинградском ювелирных заводах, «Тулачермет», Казанском авиационном заводе, Новомосковском гипсовом комбинате, часовом заводе (Москва), «Хром» (Муром), Подольском цементном заводе, Волжском «Оргсинтез», комбинате «Норильскникель», Ефремовском заводе СК, «Аромасинтез» (Калуга), Днепровской (г. Киев) и Юго-Западной (г. Москва) водопроводных станциях, заводе по переработке автопокрышек (Таиланд), РКЦ им М.В. Хруничева, «Чувашкабель» (Чебоксары), Майли-Сайском электроламповом заводе в Киргизии, Ленинабадском комбинате редких металлов (г. Чорух-Дайрон, Таджикистан), Узбекском металлургическом заводе, адронном коллайдере в Швейцарии и др.
Под научным руководством профессора Е.З. Голосмана разработаны и внедрены в промышленность катализаторы разложения озона серии ГТТ и ГТ, созданы высокоэффективный катализатор метанирования НКМ-7, катализаторы очистки газов от закиси азота, катализаторы получения водородсодержащего газа для топливных элементов. Разработанные и синтезированные неплатиновые катализаторы серии НТК-10, КДА, НКМ, НКО, ГТТ, ГТ, ФК, КЦ, НТК-10-7 и др. внедрены и эксплуатируются на 170 заводах и организациях РФ, СНГ и дальнего зарубежья.
Профессор Е.З. Голосман внес существенный вклад в подготовку научных кадров: под его руководством выполнено более 200 научно-исследовательских дипломных работ студентов, защищено 12 кандидатских диссертаций. Автор более 600 публикаций, в том числе более 300 статей, четырех монографий, около 100 изобретений и патентов (как российских, так и иностранных), более 300 тезисов докладов научных конференций и др.
Евгений Зиновьевич активно занимается научно-организационной работой. В настоящее время – вице-президент Тульского областного правления Союза научных и инженерных общественных объединений, заместитель председателя Российского химического общества им. Менделеева Тульской области, член центрального правления РХО им. Менделеева. Член редколлегии журнала «Катализ в промышленности» и бюллетеня «Химия в России». Занесен в Книгу Почета НИАП, награжден Почетным Знаком Губернатора Тульской области “Общественное признание”, имеет другие награды. В 2017 году профессор Е.З. Голосман стал лауреатом премии Правительства Российской Федерации в области науки и техники, в 2018 году награжден золотой медалью им. В.Г. Шухова и медалью «Лучший изобретатель Тульской области».
Научный совет по катализу ОХНМ РАН и редакция Каталитического бюллетеня сердечно поздравляют Евгения Зиновьевича с юбилеем, желают ему крепкого здоровья и дальнейшей успешной работы!
 |
Российская академия наук |
Отчет о научно-организационной деятельности в 2017 году
Секретариат Научного совета по катализу ОХНМ РАН (НСК) предлагает Вашему вниманию сводный отчет о деятельности Совета и научных исследованиях в области катализа, выполненных научными коллективами под руководством членов Научного совета по катализу в 2017 году.
Отчет состоит из трех разделов:
Тексты отчетов, полученные от членов НСК и научно-исследовательских коллективов, практически не подвергнуты корректировке.
Организационная деятельность
В 2017 году в рамках научно-организационной деятельности Научного совета по катализу ОХНМ РАН были выполнены следующие мероприятия.
Изданы материалы проведенных конференций.
В работе Конгресса Европакат-13 (27-31 августа 2017 г., Флоренция, Италия) приняли участие три представителя РФ в Европейской федерации каталитических сообществ (акад. В.Н. Пармон, акад. В.И. Бухтияров, д.х.н. А.Ю. Стахеев).
Продолжается сотрудничество с организациями Академий наук РФ и стран СНГ, Министерствами РФ, институтами разных ведомств и другими организациями России, дальнего и ближнего зарубежья по различным вопросам научной, научно-организационной, учебно-преподавательской и общественной деятельности в области катализа. В рамках координации международного сотрудничества ведется совместная работа коллективов, возглавляемых членами НСК, и зарубежных научных центров: например, сотрудничество Института катализа СО РАН с Институтом химии материалов Венского Технического Университета (Австрия), координатор академик В.И. Бухтияров; совместный проект с Институтом химии новых материалов НАН Белоруссии, координатор академик В.Н. Пармон; совместная Российско-Германская лаборатория (Институт катализа СО РАН и Институт Фритца-Хабера, Берлин) под руководством академика В.И. Бухтиярова; взаимодействие Института катализа СО РАН с Университетом Гачон (Республика Корея), координатор проф. РАН О.Н. Мартьянов.
В целях пропаганды и популяризации научных знаний члены НСК дают интервью и публикуют статьи в научно-популярных изданиях (журнал «Стимул», посвященный инновациям в России, журнал «Наука из первых рук», журнал «В мире науки», газета «Наука в Сибири» и др.). В 2017 году членам НСК академику В.В. Лунину и д.х.н. Е.С. Локтевой была присуждена Премия имени В.А. Коптюга за высокие научные достижения и просветительскую деятельность, которую они ведут во многих университетах страны, пропагандируя через свои достижения основополагающие принципы «зеленой химии». В декабре 2017 г. Президиум Сибирского отделения РАН выразил благодарность сотрудникам Института катализа им. Г.К. Борескова за работу в области популяризации научных исследований среди подрастающего поколения.
Членами НСК подготовлены и опубликованы в журнале «Катализ в промышленности» прогнозно-аналитические обзоры, которые могут быть использованы для прогноза развития исследований по катализу в РФ: Л.Г. Пинаева, А.С. Носков, В.Н. Пармон «Перспективы прямой каталитической переработки метана в востребованные химические продукты»; К.Н. Сорокина, Ю.В. Самойлова, А.В. Пилигаев, У. Шивакумар, В.Н. Пармон «Новые методы одностадийной переработки полисахаридных компонентов лигноцеллюлозной биомассы (целлюлозы и гемицеллюлоз) в ценные продукты»; А.В. Жужгов, О.П. Криворучко, Л.А. Исупова, О.Н. Мартьянов, В.Н. Пармон «Катализаторы и процесс низкотемпературной конверсии орто-водорода в жидкий пара-водород»; А.С. Харитонов, К.Ю. Колтунов, В.И. Соболев, В.А. Чумаченко, А.С. Носков, С.Е. Кузнецов «Перспективы переработки нефтезаводских газов в высокооктановые кислородсодержащие компоненты моторных топлив».
При активном участии членов НСК создан и утвержден Комплексный план научных исследований «Ресурсо- и энергоэффективные катализаторы и процессы». Его цель – повысить на международном уровне конкурентоспособность отечественной химической науки в области межотраслевых технологий, при этом применяя результаты фундаментальных исследований в конкретных направлениях для последующих исследований полного цикла. Инициаторами проекта выступили ведущие академические институты: Институт катализа им. Г.К. Борескова СО РАН (Новосибирск), Институт нефтехимического синтеза им. А.В. Топчиева РАН (Москва), Институт органической химии им. Н.Д. Зелинского РАН (Москва), Институт химии нефти СО РАН (Томск), Институт проблем химико-энергетических технологий СО РАН (Бийск), Институт проблем переработки углеводородов СО РАН (Омск). Участники программы ставят перед собой задачи развития междисциплинарного многоуровневого подхода в разработке процессов для новых химических технологий межотраслевого назначения. Для решения таких задач, как и для повышения эффективности выполнения фундаментальных исследований мирового уровня по междисциплинарным темам в области катализа, важной является интеграция кадровых, материальных и интеллектуальных ресурсов научных организаций. Кроме того, работа в рамках КПНИ позволит отработать механизм внедрения научных результатов в реальный сектор экономики. Программа имеет широкий круг партнеров, в их числе ведущие российские и зарубежные вузы, госкорпорации, мировые промышленные компании. Приоритетные направления, для которых будут разрабатываться новые катализаторы и технологии – это конверсия природного газа и его компонентов, новые процессы получения высокомаржинальных полупродуктов и продуктов нефтехимии, крупно- и среднетоннажного органического синтеза, получение высокомолекулярных соединений, новых синтетических материалов.
Фундаментальные исследования в области создания новых каталитических систем
и
применения физических методов для их диагностики
Новые каталитические материалы для кислородной и углекислотной конверсии метана в синтез-газ на основе никеля и кобальта, диспергированных в алюмомагниевой гидроталькитной матрице
РГУ нефти и газа (НИУ) имени И.М. Губкина совместно с ИОНХ РАН им. Н.С. Курнакова разработаны новые каталитические материалы для кислородной и углекислотной конверсии метана в синтез-газ на основе никеля и кобальта, диспергированных в алюмомагниевой гидроталькитной матрице. Катализаторы отличаются низким содержанием никеля и кобальта – 2% масс. На Ni-содержащих катализаторах получен выход синтез-газа 90% в кислородной и 97% в углекислотной конверсии метана. Определен состав катализатора, позволяющий избежать зауглероживания при использовании в углекислотной конверсии метана.
академик
А.Г. Дедов, академик И.И. Моисеев, д.х.н., проф. А.С. Локтев,
аспирант И.Е. Мухин
Российский государственный университет нефти
и газа
(Национальный исследовательский университет) имени
И.М. Губкина, г. Москва
чл.-корр.
РАН В.К. Иванов, д.х.н., проф. В.П. Данилов, к.х.н. О.Н.
Краснобаева, к.х.н. Т.А. Носова
Институт общей и неорганической
химии им. Н.С. Курнакова РАН, г. Москва
Новый катализатор получения п-ксилола конверсией изобутанола
РГУ нефти и газа (НИУ) имени И.М. Губкина разработан новый катализатор получения п-ксилола конверсией изобутанола – микро-мезопористый композит MFI/MCM41, промотированный цинком и хромом. Выход п-ксилола составляет 7%, его содержание в жидких углеводородах – продуктах конверсии изобутанола – 17% масс., изомерная чистота – 78%. Использование в качестве сырья изобутанола биогенного происхождения позволит реализовать подход к получению п-ксилола, соответствующий принципам «зеленой химии».
академик
А.Г. Дедов, академик И.И. Моисеев, д.х.н., проф.
А.С. Локтев, аспирант А.А. Караваев
Российский государственный университет нефти и газа
(Национальный
исследовательский университет) имени И.М. Губкина, г. Москва
Димерные стерически объемные арилоксиды диизобутилалюминия. Взаимосвязь строения и активирующей способности в полимеризации олефинов
Синтезирован ряд арилоксидов
диизобутилалюминия со стерически объемными арилокси-лигандами. Новые
соединения охарактеризованы рентгеноструктурным анализом и
представляют собой димеры. Согласно квантово-механическим
исследованиям (DFT
расчет) устойчивость димеров уменьшается с увеличением стерических
размеров арилокси-лигандов. Активирующая способность новых соединений
в катализе полимеризации олефинов возрастает с уменьшением
стабильности димеров. Истинными активаторами металлоценовых
катализаторов являются мономерные формы арилоксидов
диизобутилалюминия. Среди синтезированных арилоксидов в стандартных
условиях активаторами металлоценовых катализаторов оказались только
два соединения, с наиболее стерически объёмными арилокси-лигандами
(AlDPP и AlМTBP).
Aкадемик С.М. Алдошин, к.х.н. Е.Е. Файнгольд,
к.х.н. И.В. Жарков, к.х.н. А.Н. Панин,
к.х.н. О.Н. Бабкина, к.х.н. С.Л. Саратовских,
к.х.н. Н.М. Бравая, к.ф.-м.н. Г.В. Шилов
Институт проблем химической физики РАН, г. Черноголовка
Влияние добавок калия и кальция на каталитическую активность системы Ce0.8Zr0.2O2 в окислении CO
Впервые исследовано влияние добавок калия и кальция на каталитическую активность системы Ce0.8Zr0.2O2 в окислении CO. Методом РФА показано, что образцы Ce0.8Zr0.2O2 и Ce0.8Zr0.2O2-Ca,K содержат смешанный оксид церия-циркония, при этом наличие отдельных фаз соединений калия и кальция в модифицированной системе не обнаружено. Методами низкотемпературной адсорбции-десорбции азота, РФЭС и ТПВ установлено, что система Ce0.8Zr0.2O2-Ca,K, несмотря на более низкую удельную площадь поверхности по сравнению с Ce0.8Zr0.2O2, включает больше активного кислорода поверхности, в роли которого могут выступать пероксидные и супероксидные комплексы, образующиеся при хемосорбции кислорода. Это может служить причиной более высокой эффективности системы Ce0.8Zr0.2O2-Ca,K по сравнению с немодифицированным оксидом. Полученные результаты подтверждают, что присутствие зольных примесей Ca и K может служить одной из причин высокой активности в каталитическом окислении CO биоморфных смешанных оксидов Ce0.8Zr0.2O2, полученных осаждением солей предшественников на темплат – древесные опилки с последующим отжигом темплата. Эти результаты имеют большое значение для разработки высокоэффективных катализаторов очистки выхлопных газов.
д.х.н. Е.С. Локтева, к.х.н. Е.В. Голубина, И.Ю.
Каплин
Московский государственный университет имени
М.В. Ломоносова,
Химический факультет, г. Москва
Высокоселективная каталитическая деоксигенация липидов растительных масел
В рамках концепции «Зеленая химия» на основе гетерометаллического карбоксилатного платино-оловянного комплекса (PPh4)3[Pt(SnCl3)5], используемого в качестве предшественника, разработаны катализаторы [Pt-Snx]/Al2O3 селективной деоксигенации сложных эфиров, в том числе, растительных масел рапса и микроводорослей с количественным выходом углеводородов с числом атомов углерода, равным исходным углеводородным фрагментам эфиров. где R1 и R2 – углеводородные фрагменты эфиров.
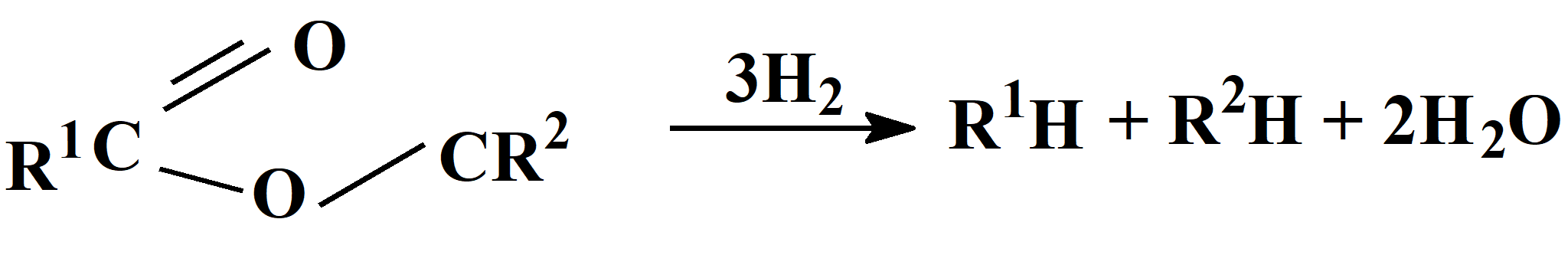
В процессе гидродеоксигенации при исчерпывающем превращении исходного сырья селективность достигает 97-99% с практически полным подавлением образования продуктов крекинга и оксидов углерода, приводящих к потере углеродной массы. Разработанный процесс перспективен для получения компонентов дизельного топлива на базе возобновляемого сырья. Изучение эволюции структуры катализаторов методами ПЭМ, РФЭС, EXAFS и квантово-химического анализа показало, что высокая селективность обусловлена формированием на поверхности γ-Al2O3 наноразмерных интерметаллических частиц PtSn3±δ и SnOx, на которых осуществляется координация субстратов и селективное восстановление атомов кислорода эфиров при температуре 350-370С. Активность катализатора практически не снижалась в длительном опыте, проводимом в течение 100 ч.
д.х.н., проф. М.В. Цодиков, к.х.н. П.А. Жарова,
к.х.н. А.В. Чистяков
Институт нефтехимического синтеза им. А.В. Топчиева РАН, г. Москва
С.С. Шаповалов, д.х.н., проф. А.А. Пасынский
Институт общей и неорганической химии им. Н.С. Курнакова РАН, г. Москва
Плазменно-каталитический риформинг лигнина в синтез-газ, стимулированный микроволновым излучением
Разработан способ плазменно-каталитического углекислотного риформинга органической массы лигнина в присутствии наноразмерных ферримагнитных частиц оксида железа, сформированных непосредственно на поверхности лигнина, в синтез-газ при стимулировании микроволновым излучением с частотой 2, 45 ГГц. Показано, что за 10 мин протекания процесса при индуцированной облучением при температуре 750-800С конверсия лигнина составляет 65% при селективности в образовании синтез-газа состава Н2/СО 1 до 94%. Изучение эволюции структуры катализатора методами рентгеновской дифракции, ПЭМ, мессбауэровской спектроскопии и низкотемпературных измерений магнитных свойств показало, что в ходе СВЧ облучения образуются полифункциональные наноразмерные частицы в конфигурации ядро-оболочка, в которых ядро состоит из ферримагнитных частиц магнетита с размером 3-5 нм и оболочка состоит из нестехиометрического карбида железа с размером 1-2 нм. В этом составе железосодержащие частицы обладают эффективным поглощением микроволнового облучения, генерируя плазму, и проявляют высокую каталитическую активность, обеспечивая скоростное протекание углекислотного риформинга лигнина. Разработанный способ обеспечивает эффективный метод получения энергоносителей путем переработки древесных отходов с утилизацией парникового газа.
д.х.н.,
проф. М.В. Цодиков, О.В. Арапова
Институт
нефтехимического синтеза им. А.В. Топчиева РАН, г. Москва
Исследование оксидного катализатора NaWMn/SiO2 окислительной конденсации метана
Методами рентгеновской фотоэлектронной спектроскопии, рентгенофазового анализа и сканирующей электронной микроскопии, совмещенной с локальным элементным анализом, изучено состояние активных компонентов смешанного оксидного катализатора NaWMn/SiO2 окислительной конденсации метана. Показано, что обратимое выделение (десорбция при 800-900С) и поглощение кислорода (реокисление при 600С) сопровождаются резкими изменениями химического и фазового состава системы. Присутствующее в исходном (окисленном) состоянии фазы Na2WO4 и Mn2O3 исчезают. При этом в образце фиксируется фаза MnWO4, а также не описанная ранее в литературе фаза, по видимому аморфная, содержащая в своем составе натрий и кислород, а также марганец в степени окисления (2+). После реокисления образца его фазовый состав возвращается к исходному состоянию. Предложен и обоснован механизм действия данного катализатора, особенностью которого является протекание основных окислительно-восстановительных превращений с участием жидкой фазы (расплава), присутствующей на поверхности носителя (SiO2) и содержащей ионы Na+, O2–, OH–, CO32, Mn2+/Mn3+ и, возможно, WO42–. Ее наличие в условиях стационарного катализа объясняет высокую активность и селективность данного катализатора.
д.х.н.
М.Ю. Синев, к.х.н. Е.А. Пономарева, к.х.н. И.М. Синев, к.х.н.
В.И. Ломоносов,
к.х.н. З.Т. Фаттахова, к.х.н. Д.П. Шашкин, Ю.А.
Гордиенко
Институт химической физики им. Н.Н. Семенова РАН, г. Москва
Применение импульсного метода изучения автоколебательных режимов протекания гетерогенных каталитических реакций
В лаборатории гетерогенного катализа ИХФ РАН впервые был предложен импульсный метод изучения автоколебательных режимов протекания гетерогенных каталитических реакций. Возможность применения этого метода была показана на примере изучения автоколебаний скоростей реакций окисления метана и окисления СО на никелевой фольге. Установлено, что в определенных условиях при подаче газовой реакционной смеси не непрерывным потоком, а серией импульсов можно наблюдать периодическое изменение величины импульсов отклика, частота которого не связана с частотой подачи исходных импульсов газа. Это явление было названо «модулированными» колебаниями с периодом, равным числу импульсов, через которое наблюдается повторение одинаковой серии откликов системы на последовательность импульсов. С помощью применения импульсного метода удалось оценить баланс по кислороду и углеводороду в каждом импульсе, а также наблюдать за цветом поверхности Ni фольги. Это дало возможность получить новую информацию о механизме колебаний скорости реакции окисления метана на никелевой фольге. В частности, были установлены 5 различных стадий колебательного процесса. Кроме того, применение импульсного метода позволило разделить изменения температуры Ni фольги, связанные с протеканием собственно реакции окисления метана и связанные с изменением отражательной способности поверхности фольги.
к.х.н. В.Ю. Бычков, Ю.П. Тюленин, д.х.н. М.М.
Слинько, д.х.н., проф. В.Н. Корчак
Институт
химической физики им. Н.Н. Семенова РАН, г. Москва
Гетерогенизированные никелевые и палладиевые катализаторы для реакций с участием норборнадиена
Каталитические процессы с участием норборнадиена открывают исключительные возможности для синтеза широкого круга труднодоступных полициклических углеводородов. Методами адсорбционного закрепления и поверхностной сборки впервые получены гетерогенизированные никелевые и палладиевые катализаторы для реакций с участием норборнадиена. Осуществлён их скрининг в реакциях циклодимеризации и аллилирования норборнадиена, определены основные технологические параметры процессов. Полученные системы характеризуются высокой удельной активностью, селективностью и продолжительностью работы. На основании предварительных данных, гетерогенизированные катализаторы по этим показателям не уступают лучшим гомогенно-каталитическим системам.
д.х.н., проф. В.Р. Флид, асп. С.А. Дураков, асп. В.В.
Замалютин
Московский
технологический университет, г. Москва
Разработка Fe содержащих катализаторов гидрирования
Впервые показано, что оксидные наночастицы Fe2O3 демонстрируют высокую активность в гидрировании ацетиленовых субстратов. Наилучшие результаты достигнуты при использовании (NH4)3[Fe(C2O4)3] в качестве прекурсора, причем для данного катализатора не требуется предварительного восстановления в водороде. Смешанные Pd-Fe-O частицы оказались наиболее активными в гидрировании ацетиленов и нитросоединений; их активность превосходит активность чисто палладиевых систем и они на порядок более активны, чем катализатор Линдлара. Эти исследования показывают перспективы использования нетоксичных и дешевых катализаторов в промышленных процессах.
проф., д.х.н. Л.М. Кустов, А.А. Шестеркина, к.х.н.
Е.В. Шувалова, к.х.н. О.А. Кириченко
Институт
органической химии им. Н.Д. Зелинского РАН, г. Москва
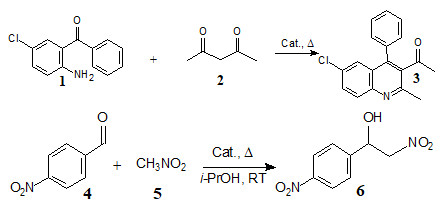
Гибридный катализатор с иерархической микро/мезопористой структурой на основе наночастиц металл-органического каркаса MOF-199, инкапсулированных в матрице мезопористого силиката
Новый
гибридный материал получен на основе металл-органического каркаса
MOF-199
(Cu3(BTC)2,
BTC
= бензол-1,3,5-трикарбоксилат) и
мезопористого силиката. В процессе синтеза матрица мезопористого
силиката является структурообразующим агентом, который
способствует формированию малых наночастиц MOF-199
(4-8 нм) внутри каналов силиката. Каталитические свойства нового
гибридного наноматериала исследованы в процессах, широко применяемых
в тонком органическом синтезе: в реакции Фридлендера (реакция
2-амино-5-хлоробензофенона с ацетилацетоном) и нитроальдольной
конденсации Анри (реакция нитрометана с 4-нитробензальдегидом):
проф., д.х.н. Л.М. Кустов, д.х.н. В.И. Исаева,
д.х.н. В.В. Веселовский
Институт органической химии им.
Н.Д. Зелинского РАН, г. Москва
Создание биокатализатора на основе иммобилизованной глюкооксидазы
Исследована активность биокатализаторов на основе фермента глюкооксидазы, иммобилизованной на Al2O3 и SiO2. В качестве модификатора поверхности исследовались полиэлектролиты, а наиболее эффективным сшивающим агентом оказался глутаровый альдегид. Использование таких биокатализаторов является перспективным направлением биотехнологического производства пищевых добавок и лекарственных средств в соответствии со стандартом GMP (Good Manufacturing Practice). Предлагаемый биокаталитический способ получения глюконовой кислоты характеризуется высокой эффективностью (85%), а полученный биокатализатор стабилен в течение 10 рабочих циклов.
асп. Е.П. Голикова, к.х.н. Н.В. Лакина, к.х.н. О.В.
Гребенникова,
д.х.н., проф. В.Г. Матвеева, д.х.н., проф. Э.М.
Сульман
Институт нано- и биотехнологий Тверского государственного
технического университета, г. Тверь
Гидролитическое гидрирование целлюлозы в условиях субкритической воды с использованием рутенийсодержащих катализаторов
Исследован процесс гидролитического гидрирования целлюлозы в среде субкритической воды в присутствии Ru-содержащих катализаторов на основе сверхсшитого полистирола (СПС) MN-270 и его функционализированных аналогов: NH2-HPS (MN-100) и SO3H-HPS (MN-500). Показано, что замена традиционного углеродного носителя на СПС увеличивает выход главных продуктов конверсии целлюлозы – полиолов, важных веществ для химической промышленности. Катализаторы исследованы методом просвечивающей электронной микроскопии (ПЭМ), в том числе, ПЭМ высокого разрешения, произведена оценка пористости. Каталитические исследования показали, что катализатор, содержащий 1% Ru на основе СПС MN270, наиболее активен. Суммарный выход сорбита и маннита составил 50% при конверсии целлюлозы 85%.
д.х.н., проф. В.Г. Матвеева, д.х.н., проф. Э.М.
Сульман, к.х.н. О.В. Манаенков, к.х.н. А.Е. Филатова, к.х.н.
О.В. Кислица, к.х.н. А.И. Сидоров, к.х.н. В.Ю. Долуда, д.х.н.,
проф. М.Г. Сульман, д.х.н., проф. Е.В. Ребров
Институт нано- и биотехнологий Тверского государственного технического университета, г. Тверь
Промотирующий эффект гидроксидов щелочных металлов на каталитические свойства палладиевых наночастиц, стабилизированных в полимерной матрице, в процессе селективного гидрирования тройной связи
Установлено, что постимпрегнация наночастиц Pd, стабилизированных в сверхсшитом полистироле, гидроксидами натрия или калия оптимальной концентрации значительно повышает каталитическую активность в реакции селективного гидрирования тройной связи 2-метил-3-бутин-2-ола при атмосферном давлении водорода. Гидроксид щелочного металла ускоряет трансформацию остаточной соли Pd(II) в наночастицы Pd(0) и снижает период индукции реакции. Кроме того, селективность по 2-метил-3-бутен-2-олу повышается с использованием K-и Na-содержащих катализаторов с 97.0 до 99.5%. Этот эффект был приписан взаимодействию ионов щелочных металлов с поверхностью наночастиц палладия, что приводило к разделению центров и изменению адсорбции реагентов.
к.х.н. Л.Ж. Никошвили, к.х.н. А.В. Быков, магистр
Т.Е. Худякова,
д.х.н., проф. В.Г. Матвеева, д.х.н., проф. Э.М.
Сульман
Институт нано- и биотехнологий Тверского государственного технического
университета, г. Тверь
Синтез и свойства новых парамагнитных комплексов Ni(I) и Ni(II)-H
Впервые путем электролиза комплексов Ni(II) с 1,5-диаза-3,7-дифосфациклооктановыми лигандами [Ni(PPh2NR2)2]2+ синтезированы новые парамагнитные комплексы Ni(I) и Ni(II)-H – малоизученные ключевые интермедиаты реакций выделения/окисления водорода с участием синтетических гидрогеназ. Показана их высокая каталитическая активность в реакции выделения водорода из протонодонорных сред. Свойства этих интермедиатов были изучены методами циклической вольтамперометрии, спектроэлектрохимии УФ/ИК и ЭПР при комнатной температуре и в замороженном растворе, предложены несколько типов механизмов в зависимости от природы заместителей при атомах азота аминометилфосфина.
академик О.Г. Синяшин, д.х.н. Ю.Г. Будникова, к.х.н.
В.В. Хризанфорова,
д.х.н., проф. А.А. Карасик, к.х.н. В.И.
Морозов
Институт органической и физической химии им. А.Е.
Арбузова ФИЦ КазНЦ РАН, г. Казань
Новые однореакторные методы синтеза замещенных пирролов и пиразинов с участием катализатора Cp2TiCl2
В развитие фундаментальных и прикладных исследований в области синтеза практически важных гетероциклических соединений разработан новый однореакторный метод конструирования замещенных пирролов и пиразинов с выходами более 70%, основанный на применении разработанной авторами многокомпонентной реакции терминальных ацетиленов с EtAlCl2 и органическими нитрилами под действием катализатора Cp2TiCl2 (10 мол %) в условиях реакции (20С, тетрагидрофуран – растворитель, 8 ч). Доступность исходных мономеров и реагентов, простота осуществления данного метода, высокие выходы целевых пирролов и пиразинов, универсальность способа создают реальные предпосылки для использования предложенного метода как в лабораторной практике, так и химической промышленности.
чл.-корр. РАН У.М. Джемилев, к.х.н. Л.О. Хафизова,
к.х.н. М.Г. Шайбакова, асп. Н.А. Рихтер
Институт
нефтехимии и катализа РАН, г. Уфа
Золото-катализируемая внутримолекулярная гетероциклизация С-2-алкин-3-оксопроизводных нативных тритерпеновых кислот в синтезе нового класса фуранотритерпеноидов
Разработан прямой и атом-экономичный подход к синтезу нового класса [3,2-b] фуран конденсированных пентациклических тритерпеноидов с использованием золото-катализируемой 5-экзо-диг-гетероциклизации 2-алкинильных производных биологически активных природных пентациклических тритерпеновых кислот. Функционально толерантные условия каталитической реакции позволили вовлечь в гетероциклизацию тритерпены лупанового, урсанового и олеанового ряда, содержащие при С-2 позиции кольца А терминальные или внутренние тройные связи, замещенные функциями, чувствительными к действию “жестких” кислот и оснований. Внутримолекулярная циклизация 4-пентин-1-он фрагмента в кольце А терпена, по-видимому, реализуется через каталитический цикл, который инициируется координацией Au(I)-катализатора с ацетиленовой связью. Образование комплекса золота промотирует нуклеофильную атаку атомом кислорода на активированную тройную связь. В результате образуется золото-содержащий цвиттерионный интермедиат, протодеаурирование которого приводит к высвобождению катализатора и образованию целевого соединения.
к.х.н. А.Ю. Спивак, к.х.н. Р.Р. Губайдуллин
Институт
нефтехимии и катализа РАН, г. Уфа
Первые примеры синтеза ациклических и циклических аминопероксидов каталитической реакцией галогензамещенных анилинов с 1,1-бис-(гидроперокси)циклоалканами
Разработан эффективный метод синтеза ациклических и циклических азадипероксидов реакцией 1,1-бис(гидроперокси)циклоалканов с формальдегидом и первичными ариламинами с участием Sm-содержащих катализаторов (SmCl3·6H2O, Sm(NO3)3·6H2O, SmCl3/γ-Al2O3, Sm(NO3)3/γ-Al2O3). Обнаружено, что добавление раствора ароматического амина в ТГФ при температуре 15-20С в течение 2-3 минут к смеси 1,1-бис(гидроперокси)циклоалкана и формальдегида в присутствии каталитических количеств Sm(NO3)3·6H2O (5 мол.%) приводит к образованию аминодипероксидов. Направление реакции зависит от положения заместителя (F,Cl) в фенильном кольце в исходных ариламинах. При взаимодействии орто-арил(хлорфенил, фторфенил)аминов с формальдегидом и 1,1-бис-(гидроперокси)циклоалканами образуются ациклические 1,1-бис-[N-(пероксиметил)-N-ариламино]циклоалканы с выходами 65-75%. Реакция пара-арил(хлорфенил, фторфенил)аминов с 1,1-бис-(гидроперокси)циклоалканами приводит к циклическим тетраоксазаспироалканам с выходами 75-85%. В разработанных условиях трехкомпонентная конденсация с участием мета-арил(хлорфенил, фторфенил)аминов приводит к смеси ациклических и циклических аминопероксидов в соотношении 1 : 1. Ациклические диаминодипероксиды обладают антималярийной, противораковой, антигельминтной активностью.
к.х.н. Н.Н. Махмудиярова, асп. Г.М. Киямутдинова,
д.х.н., проф. А.Г. Ибрагимов, чл.-корр. РАН У.М. Джемилев
Институт нефтехимии и катализа РАН, г. Уфа
Первые примеры каталитического синтеза 2,7-бис-адамантилзамещенных 4,9-диметил-2,3а,5а,7,8а,10а-гексазапергидропиренов
Разработан метод синтеза ранее не описанных 2,7-диадамантилзамещенных 4,9-диметил-2,3а,5а,7,8а,10а-гексазапергидропиренов каталитической циклоконденсацией адамантиламинов с формальдегидом и 2,6-диметил-1,4,5,8-тетраазадекалинами с использованием в качестве катализатора в данной реакции гранулированного цеолита Y высокой степени кристалличности и фазовой чистоты в H-форме. Установлено, что с участием указанного катализатора 10 масс. % цеолита Y однореакторная циклоконденсация адамантиламинов (адамантил-1-амин, адамантил-2-амин, ремантадин, 3,5-диметил-адамантил-1-амин, 1-гидрокси-адамантил-3-амин) с формальдегидом и 2,6-диметил-1,4,5,8-тетраазадекалинами в условиях (растворитель MeOH–H2O, 20°С, 3 ч) проходит с селективным образованием 2,7-диадамантилзамещенных 4,9-диметил-2,3а,5а,7,8а,10а-гексазапергидропиренов с выходами 55-70%. Полученные полиазапергидропирены представляют исключительный интерес в качестве потенциальных биологически активных прекурсоров, перспективных для разработки современных лекарственных препаратов, в частности, обладающих высокой противомикробной активностью.
к.х.н. Е.Б. Рахимова, асп. В.Ю. Кирсанов,
д.х.н., проф. А.Г. Ибрагимов, чл.-корр. РАН У.М. Джемилев
Институт
нефтехимии и катализа РАН, г. Уфа
Новый универсальный метод синтеза гем-N,O-гетероциклов взаимодействием двухатомных фенолов с бис(метоксиметил)аминами с участием катализаторов на основе d- и f-элементов
Разработан эффективный метод синтеза гем-N,O-гетероциклов циклоконденсацией пирокатехина, резорцина или гидрохинона с N,N-бис(метоксиметил)-N-ариламинами с участием катализаторов на основе d- и f-элементов. Реакция N,N-бис(метоксиметил)-N-ариламинов с пирокатехином в условиях [Sm(NO3)3·6H2O (5 мол %), растворитель EtOAc, 20С, 5 ч] проходит селективно с образованием 7,16,25-триарил-7,8,16,17,25,26-гексагидро-6H,15H,24H-трибензо[f,m,t] [1,5,8,12, 15,19]-гексаокса-[3,10,17]-триазациклохеникозинов с выходами около 90%. Заместители в м- или п- положении в ароматическом кольце N,N-бис(метоксиметил)-N-ариламинов способствуют циклоаминометилированию пирокатехина по схеме [1+2]-гетероциклизации с получением 3,8-диарил-2,3,4,7,8,9-гексагидробензо[1,3]-оксазино[5,6-h][1,3]-бензоксазинов с выходами 75-85%. Циклоконденсация резорцина с N,N-бис(метоксиметил)-N-арил(о-, м-, п-хлорфенил)аминами в условиях [5 мол. % Sm(NO3)3·6H2O, EtOAc, 20С, 5 ч] приводит к 3,9-бис(арил)-3,4,9,10-тетрагидро-2Н,8Н-[1,3]оксазино[6,5-f][1,3]бензоксазинам с выходами 60-70%. Реакция N,N-бис(метоксиметил)-N-ариламинов с гидрохиноном проходит с селективным образованием 2,9-бис(арил)-1,2,3,8,9,10-гексагидро[1,3]оксазино[5,6-f][1,3]-бензоксазинов с выходами до 70%. Бензоксазины обладают гербицидной, бактерицидной, фунгицидной, а также противовоспалительной и противоопухолевой активностью.
к.х.н. Н.Н. Махмудиярова, асп. Г.М. Киямутдинова,
д.х.н., проф. А.Г. Ибрагимов, чл.-корр. РАН У.М. Джемилев
Институт нефтехимии и катализа РАН, г. Уфа
Разработка новых методов синтеза N-арил-4-(5-нитрофурана-2-ил)пиримидин-5-аминов
Разработаны новые методы синтеза N-арил-4-(5-нитрофурана-2-ил)пиримидин-5-аминов, содержащих метильные, метокси- и нитро-группы в C(2), C(4), С(3), С(5) положениях арильного заместителя в пиримидиновом цикле, основанные на комбинации реакции нуклеофильного ароматического замещения водорода и последующей реакции кросс-сочетания по Бухвальду-Хартвигу с различными замещенными анилинами. Проведена оптимизация условий реакции Бухвальда-Хартвига в присутствии различных каталитических систем на основе солей палладия. Показано, что соединения, содержащие метильные и метокси-группы в N-арильном заместителе, независимо от положения заместителей в цикле, обладают широким спектром антибактериальной активности в отношении кокковых инфекций и низкой цитотоксичностью. Указанные соединения могут быть рекомендованы для дальнейших испытаний с целью создания на их основе лекарственных препаратов для лечения заболеваний мочеполовой системы, вызванных гонококками и/или золотистым стафилококком, а также гнойно-воспалительных инфекционных заболеваний кожи и слизистых оболочек, вызванных стафилококками и стрептококками.
академик РАН В.Н. Чарушин, к.х.н. Е.В. Вербицкий,
С.А. Баскакова, к.х.н. Г.Л. Русинов
Институт органического синтеза им. И.Я. Постовского УрО РАН, г. Екатеринбург
Новые органо-неорганические гибридные композиты на основе целлюлозы рисовой шелухи
Разработан метод получения новых органо-неорганических гибридных композитов на основе целлюлозы рисовой шелухи, содержащей до 40% биогенного SiO2. Установлено, что такие Ц/SiO2 биогибридные композиты, в отличие от синтетических аналогов, обладают каталазной активностью и могут служить ингибиторами химических и биохимических радикальных процессов.

Микрофотография природного композита целлюлоза/SiO2био
академик О.Н. Чупахин, д.х.н. Л.А. Петров, к.т.н.
А.Б. Шишмаков
Институт органического синтеза им. И.Я.
Постовского УрО РАН, г. Екатеринбург
Каталитические свойства Li-содержащих алюмомагниевых слоистых двойных гидроксидов
Алюмомагниевые слоистые двойные гидроксиды (MgAl-СДГ), обладая оснóвными свойствами, являются перспективными катализаторами органических реакций, а также предшественниками носителей некислотного типа для каталитических систем на основе благородных металлов (для реакций превращения углеводородов). Усилить оснóвные свойства СДГ можно введением в их состав щелочного металла, например, лития. Впервые методом механохимической активации получены однофазные трёхкомпонентные MgLiAl-СДГ с разным соотношением (Mg+Li)/Al и Li/(Li+Mg) при одинаковом содержании Al. На основании метода рентгенофазового анализа доказана слоистая структура получаемых соединений и отсутствие каких-либо побочных фаз в их составе. Полученные при прокаливании соответствующих СДГ MgAlLi-смешанные оксиды обладают развитой (порядка 200 м2/г) удельной поверхностью и унимодальным распределением пор по размерам. Анализ изотерм адсорбции СО2, а также ТПД профилей СО2 показал повышенную (по сравнению с MgAl-содержащими аналогами) основность MgLiAl-содержащих образцов. Это свойство отразилось на каталитических свойствах Li-содержащих СДГ в реакции альдольной конденсации фурфурола с ацетоном. Обнаружено, что катализатор на основе Li-содержащих СДГ, обладающий большим количеством оснóвных центров различной силы (количество десорбированного СО2 составляет 340 мкмоль/г), обеспечивает существенно бóльшую (более чем в три раза) скорость превращения фурфурола (по сравнению с Mg-содержащими образцами) с селективным (95%) образованием 4-(2-фурил)-3-бутен-2-она.
чл.-корр. РАН В.А. Лихолобов, к.х.н. О.Б. Бельская,
к.х.н. Р.М. Мироненко,
к.х.н. Л.Н. Степанова, к.х.н. А.В.
Василевич
Институт проблем переработки углеводородов СО РАН,
г. Омск
Использование автоклавных технологий для растворения оксидных матриц катализаторов; разработка нового семейства гетерогенных кислотных катализаторов на основе диоксида циркония
Совместно с ИХХТ СО РАН (на базе лаборатории Химии платиновых металлов и катализаторов ИНиГ СФУ) проводятся работы по использованию автоклавных технологий для растворения оксидных матриц катализаторов и вскрытия различных соединений, химических фаз цветных и благородных металлов, входящих в состав катализаторов, с целью разработки методики контроля их структурно-химических изменений в процессе эксплуатации. Установлена эффективность данного метода при селективном выделении отдельных исследуемых фаз для повышения точности методов рентгеновской диффрактометрии. Предложена методика оценки размеров частиц платины в образцах катализатора риформинга, в том числе отработанного.
Продолжаются исследования по разработке катализаторов для изомеризации н-алканов С4-С6 на основе диоксида циркония, модифицированного редкоземельными металлами, а также методов их синтеза путем легирования основной фазы анионными промоторами и катионами двух- и трехвалентных металлов. Разработка нового семейства гетерогенных кислотных катализаторов на основе диоксида циркония является важным достижением науки в области катализа процессов нефтепереработки за последнее десятилетие, поскольку такие системы позволяют осуществлять изомеризацию н-парафинов при низкой температуре с высоким выходом высокоразветвленных изомеров.
д.т.н., проф. В.П. Твердохлебов, к.х.н., доцент Ф.А.
Бурюкин
Институт нефти и газа Сибирского федерального университета, г. Красноярск
Каталитическая активность анионных комплексов палладия в реакции Мицороки-Хека с использованием ангидридов ароматических кислот
Путем применения набора разработанных методов, базирующихся на измерениях дифференциальной селективности в сложных каталитических реакциях, предложены новые подходы к осуществлению операндо кинетических исследований. Показано, что применение кинетических методов в операндо исследованиях позволяет получать однозначную информацию о роли детектируемых интермедиатов в каталитической реакции (активный или неактивный в катализе). Полученные в ходе изучения реакции Мицороки-Хека с использованием ангидридов ароматических кислот в качестве арилирующих реагентов данные о закономерностях дифференциальной селективности позволили получить первые прямые доказательства каталитической активности анионных комплексов палладия. При этом все результаты были получены в реальных каталитических условиях без использования каких-либо модельных экспериментов.
д.х.н., проф. А.Ф. Шмидт, к.х.н. А.А. Курохтина,
асп. Е.В. Ларина, асп. Е.В. Ярош, асп. Н.А. Лагода
Иркутский
государственный университет, г. Иркутск
Исследование фазового состава Pd-P катализатора для гидрирования непредельных углеводородов
На основе совокупности данных электронной микроскопии высокого разрешения в сочетании с электронной дифракцией, рентгеновским микроанализом и РФА получены новые данные о фазовом составе Pd-P катализатора, высокоактивного в гидрировании непредельных углеводородов. Показано, что наночастицы, образующиеся в системе Pd(acac)2–0.3P–Н2 при 80С, представляют собой фосфиды палладия Pd5P2, Pd3P0.8 и кристаллиты палладия (ОКР 2 нм). Предложена схема низкотемпературного способа формирования Pd-P катализатора из Pd(acac)2 и элементного фосфора в водороде. Она включает редокс-процесс между Pd(acac)2 и белым фосфором при комнатной температуре с образованием PdP2, последующее взаимодействие PdP2 с атомами Pd(0) и переход в обогащенные палладием фосфиды. Из-за различия в скоростях взаимодействия фосфидов палладия с Pd(0) до обогащенных палладием фосфидов и восстановления части Pd(acac)2 водородом происходит сегрегация кластеров палладия на частицах фосфидов палладия. Термостатирование при высокой температуре (400С) вызывает изменение фазового состава Pd-P катализаторов с образованием фосфида Pd6P. Методом фазовых траекторий установлена природа носителей каталитической активности для Pd–P-наночастиц, содержащих как кластеры Pd(0), так и фосфиды палладия. Показано, что в мягких условиях гидрирование изомеров нитрохлорбензола протекает на кластерах Pd(0), а зависимость дифференциальной селективности Pd–P-катализаторов в гидрировании о- и м-НХБ от соотношения P/Pd связана с дисперсностью кластеров Pd(0).
д.х.н., проф. Ф.К. Шмидт
Иркутский
государственный университет, г. Иркутск
Механизм катализа присоединения кетонов к ацетилену в присутствии супероснований
В Иркутском институте химии им. А.Е. Фаворского СО РАН (совместно с Иркутским государственным университетом) экспериментальными и квантовохимическими методами (CBS-Q//B3) изучен механизм катализа нуклеофильного присоединения кетонов к ацетиленам под действием суперосновной системы КОН/ДМСО (на примере присоединения кетонов к алкоксиметилацетиленам). Реакция протекает стереоселективно с образованием моноаддуктов Z-конфигурации и диаддуктов Е-конфигурации. Установлено, что процесс включает образование алленовых интермедиатов – продуктов прототропной изомеризации исходных ацетиленов. Наряду с модельной реакцией присоединения ацетона к пропину и аллену проанализирован механизм реакций присоединения ацетона и ацетофенона к метилпропаргиловому и метилаллениловому эфирам. Показано, что лимитирующая стадия реакции присоединения исходных кетонов к ацетиленовым и алленовым системам протекает с энергией активации, типичной для винилирования кетонов. Это контрастирует с энергетическими параметрами реакции присоединения промежуточных α-карбанионов по терминальному положению метоксиаллена, для которой характерен более низкий активационный барьер. Полученные результаты хорошо предсказывают состав и стереохимию продуктов реакции и доказывают участие в реакции алленовых интермедиатов.
академик Б.А. Трофимов, д.х.н., проф. Н.М.
Витковская, д.х.н., проф. В.Б. Кобычев,
д.х.н. Е.Ю. Шмидт,
к.х.н. В.Б. Орел, асп. А.С. Бобков
Иркутский
институт химии им. А.Е. Фаворского СО РАН, г. Иркутск
Исследование механизма селективного гидрирования алкинов до алкенов на наночастицах меди, нанесенных на SiO2
Проведено исследование механизма селективного гидрирования алкинов до алкенов на перспективных катализаторах на основе наночастиц меди, нанесенных на SiO2, в частности, для оценки возможности парного пути присоединения водорода для этих катализаторов и определения влияния фосфинового лиганда на механизм реакции, селективность гидрирования и вклад парного пути реакции. Селективное гидрирование алкинов в алкены используется в промышленности для удаления алкинов из потоков олефинов для предотвращения отравления катализаторов полимеризации. Эта реакция также важна в органической химии для стереоселективного синтеза Z-олефинов. Выполненные исследования представляют актуальность также в плане поиска гетерогенных катализаторов, способных эффективно присоединять молекулярный водород к непредельным субстратам парным образом, что имеет принципиально важное значение для создания новых высокочувствительных методов на основе ЯМР и МРТ для исследования механизмов каталитических реакций и работы модельных реакторов, а также для медицинской диагностики.
С применением параводорода выполнено исследование селективного гидрирования 1-бутина и 2-бутина на нанесенных наночастицах меди (Cu/SiO2). Гидрирование 1-бутина на катализаторе Cu/SiO2 приводит к образованию 1-бутена с селективностью 97%. Поверхностная модификация этого катализатора трициклогексилфосфином (PCy3) увеличивает селективность по отношению к 1-бутенам до ~100%, хотя и за счет некоторого снижения каталитической активности. Аналогичные тенденции наблюдались при гидрировании 2-бутина. Эффекты индуцированной параводородом поляризации, наблюдаемые в реакциях гидрирования с использованием катализаторов на основе меди, получены впервые и демонстрируют принципиальную возможность парного присоединения водорода к субстрату такими катализаторами. Вклад парного присоединения водорода к 1-бутину составляет 0,2-0,6% для Cu/SiO2 и 2,7% для Cy3P-Cu/SiO2.
д.х.н., проф. И.В. Коптюг, к.х.н. К.В. Ковтунов,
асп. А.В. Сальников, асп. Д.Б. Буруева
Международный
томографический центр СО РАН, г. Новосибирск
Наноразмерные частицы металлов, выполняющие функции физико-химических нанороботов
Показано, что дисперсные наноразмерные частицы металлов обладают набором физических и химических свойств (функций). При определенных неравновесных условиях они выступают в роли активных полифункциональных образований, взаимодействующих с окружающей средой. В этой роли они выполняют ряд функций и могут инициировать одновременное протекание нескольких процессов разной природы и управлять ими в определенной последовательности. Показано, что такие полифункциональные наноразмерные частицы могут управлять нанотехнологиями и выполнять функции физико-химических нанороботов.
чл.-корр. РАН Р.А. Буянов
Институт катализа
им. Г.К. Борескова СО РАН, г. Новосибирск
Исследование снижения необратимой дезактивации никелевого катализатора путем введения добавок молибдена и фосфора
При разработке основ процесса каталитического гидрооблагораживания продуктов термической деструкции биомассы с целью получения ценных химических соединений была исследована первая стадия мягкой гидрообработки пиролизной жидкости (бионефти) при температурах < 250°C в проточном и статическом режимах в присутствии катализаторов с содержанием Ni более 40 мас. %. Наиболее значимым результатом является то, что совместное введение добавок молибдена и фосфора в катализатор на основе Ni позволило существенно снизить необратимую дезактивацию катализатора в результате спекания и растворения в кислой реакционной среде. Опытная партия модифицированного катализатора была испытана на пилотной установке в компании BTG (Нидерланды). Ресурсные испытания показали, что модифицированный катализатор не снижает своей активности в течение 1000 часов.
д.х.н. В.А. Яковлев, к.х.н. М.В. Алексеева, к.х.н.
С.А. Хромова,
к.х.н. А.А. Смирнов, Д.Ю. Ермаков
Институт
катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Окисление низших алкенов: эпоксидирование или алильное окисление?
Предполагается, что низкая селективность эпоксидирования пропена вызвана легкостью отрыва аллильного водорода (Налл) от молекулы С3Н6. Использование электрофильной формы Оα в составе комплекса (FeIII−O−)α на FeZSM-5 позволило показать, что первичное окисление пропена идет без участия Налл, приводя к пропеноксиду как единственному продукту. Негативная роль Налл может заключаться в ускорении протекания вторичных превращений эпоксида, ведущих к снижению селективности реакции. Полученные результаты открывают новые возможности для поиска катализаторов для эпоксидирования пропилена и других олефинов.
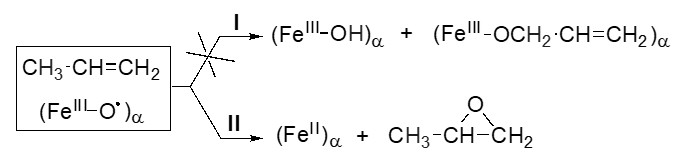
д.х.н., проф. Г.И. Панов, к.х.н. Е.В. Староконь,
к.х.н. М.В. Парфенов,
к.х.н. С.Е. Малыхин, к.х.н. Л.В.
Пирютко, д.х.н., проф. Г.М. Жидомиров, д.х.н. А.С. Харитонов
Институт
катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Исследование композитной загрузки катализатора гидрирования и катализатора дегидратации в реакции деоксигенации диизопропилкетона
Разработка искусственных мультикатализаторных систем – одно из новых стратегических направлений развития современного катализа. Ярким примером влияния друг на друга процессов, протекающих на разных катализаторах, является интеграция в одном реакционном пространстве катализаторов гидрирования и дегидратации. Впервые обнаружено, что по сравнению с катализатором гидрирования скорость превращения 2,4-диметил-3-пентанона на композитной загрузке увеличивается в 8 раз, а период стабильной работы – в ~2 раза, при этом вместо спирта с селективностью до 90% образуется разветвленный парафин 2,4-диметилпентан. Композитная загрузка может быть эффективной заменой бифункциональных катализаторов в процессах переработки растительного сырья.
д.х.н. А.С. Харитонов, к.х.н. Д.П. Иванов,
к.х.н.
Л.В. Пирютко, д.т.н., проф. А.С. Носков
Институт
катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Асимметрическая автоамплификация как новый нелинейный эффект в асимметрическом катализе
При изучении реакции окислительного кинетического разделения рацемических вторичных спиртов в присутствии хиральных биомиметических комплексов марганца обнаружен новый нелинейный эффект в асимметрическом катализе – асимметрическая автоамплификация – явление, родственное асимметрическому автокатализу и асимметрической автоиндукции. Суть явления заключается в следующем: оба энантиомера вторичного спирта способны образовывать аддукт с хиральным катализатором, давая таким образом пару диастереомерных каталитически активных центров, демонстрирующих различный уровень стереоизбирательности в отношении энантиомеров вторичного спирта. В ходе реакции более реакционноспособный энантиомер постепенно расходуется, что приводит к преобладанию каталитически активных аддуктов с менее реакционноспособным энантиомером и, как следствие, к прогрессивному росту наблюдаемого фактора селективности кинетического разделения. Предложена схема реакции кинетического разделения с асимметрической автоамплификацией, имеющая аналитическое решение.
д.х.н., проф. РАН К.П. Брыляков
Институт
катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Новый подход к синтезу ароматических органофторборанов – кислот Льюиса с регулируемой кислотностью
Разработан удобный подход к получению ароматических и фторароматических дифторборанов (ArBF2) путем взаимодействия соответствующих трифторборатов калия (ArBF3K) с ионными жидкостями, содержащими свободный растворенный хлорид алюминия. Показано, что образующиеся органодифторбораны устойчивы в растворителях различной природы (алканы, арены, хлорорганические соединения). Все органодифторбораны обладают льюисовой кислотностью, которая зависит от природы и количества заместителей в ароматическом кольце. Данный факт позволяет получать новые кислоты Льюиса с требуемыми свойствами и, как следствие, управлять селективностью кислотно-катализируемых процессов. В ходе работы была продемонстрирована высокая селективность новых каталитических систем в реакциях алкилирования полиароматических субстратов, содержащих несколько реакционных центров с различными электронными свойствами.
д.х.н., проф. РАН Н.Ю. Адонин, к.х.н. С. А.
Приходько, асп. М. М. Шмаков
Институт
катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Использование индекса опасности для определения эффективности фотокаталитической очистки воздуха от паров летучих органических соединений
Процесс фотокаталитической очистки воздуха, загрязнённого летучими органическими соединениями, был исследован с гигиенической точки зрения путем оценки двух индексов, описывающих опасность смеси из газовых компонентов в воздухе: максимальный коэффициент опасности и общий индекс опасности. На примере различных субстратов (ацетон, спирты, углеводороды и гетероатомные соединения) показано, что очистка воздуха методом фотокаталитического окисления (ФКО) позволяет значительно снизить его опасность при длительном УФ облучении.
Для таких загрязнителей, как n-спирты, ацетонитрил, диэтилсульфид и диметилметилфософонат, которые производят газообразные промежуточные вещества при фотокаталитическом окислении, индексы опасности могут увеличиваться во время процесса ФКО и в течение некоторого времени даже быть выше, чем у исходного загрязнителя. Показано, что за увеличение индексов опасности в ходе процесса ответственны С1–С3 карбонильные соединения (такие как муравьиная кислота, ацетальдегид, пропаналь) и неорганические соединения (такие как синильная кислота, двуокись серы) из-за их высоких индивидуальных показателей опасности. Для таких загрязнителей, как ацетон, метанол, циклогексан и бензол, которые окисляются без образования газообразных промежуточных веществ, показатели опасности снижаются по мере снижения концентрации первоначального загрязнителя.
Монооксид углерода является побочным продуктом в ходе фотокаталитического окисления большинства вредных веществ и даёт наибольший вклад в опасность газовой смеси после процесса ФКО. Показано, что модифицирование TiO2 благородными металлами позволяет подавить накопление СО, что значительно повышает показатели эффективности и качества очистки воздуха методом ФКО.
д.х.н., проф. РАН Д.В. Козлов, к.х.н. Д.С. Селищев,
к.х.н. М.Н. Люлюкин
Институт
катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Новые бифункциональные рутениевые катализаторы для высокотемпературного гидролиза целлюлозы в сорбитол
Разработаны новые бифункциональные катализаторы на основе наночастиц рутения, закрепленных на поверхности кислотных носителей, содержащих 25%ГПК, на Nb2O5 и ZrO2: 3%Ru-ГПК/Nb2O5 и 3%Ru-ГПК/ZrO2. Доказано, что носители, прокаленные при 450-650°С, содержат оксидные структуры WO3, образовавшиеся при высокотемпературной обработке ГПК. Носители демонстрируют высокую стабильность в гидротермальной среде (180°С) и при нанесении рутения. Все катализаторы испытаны в высокотемпературном гидролизе-восстановлении целлюлозы в сорбитол. Катализаторы 3%Ru-ГПК/ZrO2 показали более высокую активность по сравнению с 3%Ru-ГПК/Nb2O5. В присутствии 3%Ru-ГПК/ZrO2 получены высокие выходы сорбитола, 41-49% (селективность 79-84%), в чистой воде.
д.х.н., проф. РАН О.П. Таран, д.х.н. М.Н. Тимофеева,
к.х.н. Н.В. Громов, инж. Т.И. Медведева
Институт
катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Разработка и усовершенствование промышленных катализаторов и технологий
Отечественная технология приготовления микросферического катализатора окислительного хлорирования этилена в производстве винилхлорида
Разработан эффективный отечественный катализатор и промышленная технология его приготовления для стадии окислительного хлорирования этилена в дихлорэтан при производстве винилхлорида с целью замены импортного катализатора в реакторах промышленных установок. Новая технология включает стадии приготовления микросферического алюмооксидного носителя и закрепления на его поверхности каталитически активных компонентов. Новый катализатор по своим эксплуатационным характеристикам не уступает известным зарубежным аналогам и получен с использованием дешевого и доступного отечественного сырья и реагентов. В настоящее время в ООО «Ишимбайский специализированный химический завод катализаторов» (г. Ишимбай) наработано 10 тонн катализатора для загрузки в промышленный реактор производства винилхлорида в ОАО «Башкирская Содовая Компания» (г. Стерлитамак).
чл.-корр. РАН У.М. Джемилев, д.х.н., проф. Б.И.
Кутепов
Институт нефтехимии и катализа РАН, г. Уфа
Создание гетерогенных катализаторов на основе наноструктурированного силикалита титана для синтеза эпоксисоединений
Успешно завершен проект «Разработка нанокаталитической технологии получения эпоксисоединений из биоспиртов для производства полимерных функциональных материалов». Промышленные эпоксиды (оксид пропилена, эпихлоргидрин и глицидол) являются важными продуктами основного и тонкого органического синтеза. Цель работы – разработка малоотходных технологий получения высоковостребованных эпоксисоединений методом нанокаталитического жидкофазного эпоксидирования пропилена и его производных пероксидом водорода на силикалите титана с характеристиками продуктов, соответствующими требованиям производства полимерных функциональных материалов. Разработана серия гетерогенных катализаторов на основе наноструктурированного силикалита титана для синтеза эпоксисоединений. Проведены исследования по влиянию технологических параметров на показатели процессов получения оксида пропилена, эпихлоргидрина и глицидола. Подобраны оптимальные условия. На основании детальных кинетических исследований разработаны кинетические модели процессов. Показатели процессов существенно превышают характеристики действующих технологий: селективность по целевым эпоксидам 90-95%, конверсия олефина 95-99%, конверсия пероксида водорода 99-100%.
д.х.н., проф. В.Р. Флид, д.х.н., проф. Л.Г. Брук,
к.х.н. Ж.Ю. Пастухова
Московский технологический университет,
г. Москва
д.т.н. М.Р. Флид
НИИЦ «Синтез», г.Москва
д.т.н., проф. С.М. Данов, д.т.н., проф. А.В. Сулимов
А.В.
Дзержинский политехнический институт
Нижегородского государственного технического университета имени Р.Е. Алексеева,
г. Дзержинск
Катализатор переработки попутных нефтяных газов
Методом гидротермальной кристаллизации синтезирован галлоалюмосиликат структурного типа цеолита MFI и проведена его предварительная активация воздухом при температуре 550-800С (6 ч) и водяным паром при температуре 360-480С (3 ч). Установлено, что под действием высокотемпературной и термопаровой обработок происходит перераспределение кислотных центров галлоалюмосиликата по силе и концентрации. Показано, что для получения наиболее эффективного катализатора ароматизации пропана его термопаровую обработку следует проводить в интервале температур 400-440°С, а высокотемпературную обработку при 600С. Термопаровая обработка галлоалюмосиликата приводит к снижению в два раза количества образующегося на нем кокса, что позволяет существенно увеличить продолжительность стабильной работы катализатора. Проведение обработки катализатора при температуре 600С повышает его активность в процессе конверсии пропана – выход и селективность образования ароматических углеводородов достигают соответственно 52,9 и 55,7%, тогда как в случае прокаливания образца при других температурах эти показатели на 5-20% ниже. Использование достаточно простых и дешевых способов активации галлийсодержащего цеолита – предварительных высокотемпературной и термопаровой обработок – позволяет целенаправленно изменять его свойства и, тем самым, повышать эффективность процесса переработки газообразных углеводородов в ценные химические продукты.
д.х.н. А.В. Восмериков
Институт химии нефти СО РАН, г. Томск
Наработка реакторных порошков сверхвысокомолекулярного полиэтилена и получение композитов из латексов Нафиона с SO3H-кислотными центрами
Выполнена наработка 1,2 кг реакторных порошков сверхвысокомолекулярного полиэтилена особой морфологии и наноструктуры, способных к твердофазной переработке в сверхпрочные и сверхмодульные волокна по договору с Фондом перспективных исследований, шифр «Полимер А1». Технология разработана Санкт-Петербургским филиалом Института катализа им. Г.К. Борескова СО РАН, защищена четырьмя патентами РФ. Наработанные образцы переданы ФПИ.
Совместно с головным Институтом катализа выполнена разработка получения композитов из латексов Нафиона с SO3H-кислотными центрами, изучено их строение и каталитическая активность.
Получен патент РФ, опубликованы статьи в журналах ДАН и Кинетика и катализ, сделаны три доклада на всероссийских конференциях.
чл.-корр. РАН С.С. Иванчев
Санкт-Петербургский филиал Института катализа
им. Г.К. Борескова СО РАН, г. Санкт-Петербург
Новый процесс комплексной переработки древесины лиственницы
Разработан новый процесс комплексной переработки древесины лиственницы, основанный на интеграции методов экстракции и каталитической пероксидной делигнификации древесной биомассы. Осуществлен подбор условий одностадийной экстракции древесины лиственницы водно-этанольной смесью, обеспечивающих одновременное выделение с высоким выходом ценных биологически активных веществ – дигидрокверцетина (ДКВ) и арабиногалактана (АГ).
Предложено осуществлять гетерогенно-каталитическое фракционирование древесины лиственницы, из которой предварительно были выделены ДКВ и АГ, на целлюлозу и низкомолекулярные органические кислоты в среде уксусная кислота–вода–катализатор TiO2. С целью оптимизации процесса пероксидного фракционирования экстрагированной древесины лиственницы изучено влияние температуры и состава реакционной среды на динамику извлечения лигнина из древесины.
В результате выполненных кинетических исследований установлено, что процесс каталитической пероксидной делигнификации в интервале температур 70–100°С описывается уравнением первого порядка. Достаточно высокая энергия активации (88 кДж/моль) указывает на отсутствие значительного вклада внешне-диффузионных ограничений при используемых условиях процесса делигнификации. Математическая оптимизация процесса пероксидной делигнификации экстрагированной древесины лиственницы позволила установить следующие оптимальные условия процесса: температура 100°С, концентрации Н2О2 6 % вес., СН3СООН 25 % вес., гидромодуль 15. В этих условиях с выходом около 44 % вес. получена качественная целлюлоза, содержащая менее 1% вес. остаточного лигнина.
Методами ИКС, РФА и СЭМ установлено, что структура целлюлозы, полученной пероксидной делигнификацией экстрагированной древесины лиственницы в оптимальных условиях процесса, соответствует структуре промышленной микрокристаллической целлюлозы (МКЦ). В результате выполненного исследования оптимизированы условия процесса комплексной переработки древесины лиственницы с получением ДГВ, АГ и МКЦ.
д.х.н., проф. Б.Н. Кузнецов, к.х.н. Н.В. Гарынцева,
к.т.н. И.Г. Судакова
Институт химии и химической технологии
СО РАН, г. Красноярск
Воздействие щелочных и органических абсорбентов СО2 на прочностные и каталитические свойства промышленных никелевых катализаторов метанирования
Проведены исследования физико-химических, физико-механических характеристик и каталитической активности промышленных никелевых катализаторов НИАП-07-01 (НКМ-1) и НИАП-07-07 (НКМ-7) после воздействия на них щелочных (“Карсол”, “Бейнфилд”) и органических (МЭА и МДЭА) абсорбентов.
Обнаружено, что воздействие щелочных абсорбентов приводит к разрушению никель-алюминиевого катализатора НИАП-07-01 (НКМ-1). Установлено, что воздействие органических абсорбентов приводит к снижению механической прочности катализатора НИАП-07-01 (НКМ-1), которая, тем не менее, остается на достаточно высоком уровне, и не оказывает влияния на каталитическую активность. Показано, что воздействие щелочных и органических абсорбентов не оказывает влияния на основные свойства никелевого цементсодержащего катализатора НИАП-07-07 (НКМ-7). Для увеличения времени эксплуатации метанаторов в агрегатах по производству синтетического аммиака рекомендовано применять инновационный никелевый цементсодержащий катализатор марки НИАП-07-07 (НКМ-7), который может изготавливаться в виде таблеток, колец или экструдатов.
д.х.н., проф. Е.З. Голосман, к.т.н., доцент В.Н.
Ефремов
ООО “НИАП-КАТАЛИЗАТОР”, г. Новомосковск
Эффективность цементсодержащих катализаторов в получении N-производных пиперидина
Изучен каталитический потенциал новых Cu-, Cu-Zn-, Cu-Zn-Zr-, Ni-содержащих материалов на алюмокальциевой основе в получении N-алкоксиалкилпиперидинов – промежуточных продуктов для создания лекарств с различным типом биологического действия, препаратов для защиты растений, моющих средств.
Установлено, что определенная последовательность введения отдельных компонентов рецептуры на стадии приготовления Cu(-Zn)-содержащих композиций оказывает существенное воздействие на их каталитическую активность в реакции аминирования моноэфиров 1,2-диолов пиперидином. В оптимальных условиях проведения синтеза медьалюмокальциевый образец демонстрирует удельную каталитическую активность и выход целевого продукта в 1,3-1,4 раза выше в сравнении с лучшим из медьцинкалюмокальциевых контактов.
На никельалюмокальциевых катализаторах изучено влияние побочных реакций на селективность основного процесса. Предложена обобщающая схема превращений.
Разработан новый способ получения N-(2-этоксиэтил)пиперидина с использованием промышленных никельалюмокальциевых катализаторов НКМ 4А и НКМ-7, функционирующих при температурах, которые на 20-30С ниже в сравнении с прототипом, и превосходящих его в селективности по целевому амину.
Методы приготовления и рецептуры новых катализаторов, а также условия осуществления синтеза могут стать основой промышленного процесса получения соединений с бифункциональными (аминная и эфирная) группами.
к.х.н. В.В. Белов
ГВУЗ “Украинский государственный
химико-технологический университет”, г. Днепр
д.х.н., проф. Е.З. Голосман
ООО “НИАП-КАТАЛИЗАТОР”, г.
Новомосковск
Повышение эффективности установок каталитического риформинга бензиновых фракций
Одной из важнейших задач в нефтепереработке остается повышение эффективности установок каталитического риформинга бензиновых фракций со стационарным слоем катализатора за счет увеличения межрегенерационного периода катализатора при работе в «жестком» режиме и снижения содержания ароматических углеводородов в риформате.
Группой исследователей из ООО «НПП Нефтехим» разработана новая модификация катализатора (марка REF 140), которая обеспечивает выработку риформата с октановым числом 98-99 пунктов и межрегенерационным периодом 3-4 года.
Вторая разработка, созданная в 2017 году тем же коллективом, – катализатор риформинга, который позволяет снизить концентрацию ароматических углеводородв в риформате на 4-5% без снижения октанового числа, что позволяет повысить эффективность производства автобензинов по стандартам ЕВРО-5 и обеспечить платформу для производства автобензинов по стандартам ЕВРО-6.
к.х.н. А.Н. Шакун, М.Л. Федорова, И.С. Семашова,
Т.В. Карпенко
ООО «НПП Нефтехим», г. Краснодар
Исследование ингибиторов разложения перекисных продуктов жидкой фазы тройных систем MeOH-H2O2-H2O
В рамках госконтракта проведено исследование ингибиторов разложения перекисных продуктов жидкой фазы тройных систем MeOH-H2O2-H2O (где Me – K, Na, Li). Результаты исследования использованы при разработке нового поколения регенеративных продуктов с улучшенными эксплуатационными характеристиками.
В.Г. Матвейкин, к.т.н. Ферапонтов Ю.А., Плотников
М.Ю.,
к.т.н. Дорохов Р.В., к.т.н. Рылов Ю.Б.
ОАО «Корпорация
Росхимзащита», г. Тамбов
Разработка озонового конвертера системы кондиционирования воздуха
Совместно с Инжиниринговым химико-технологическим центром (г. Томск) разработаны аванпроект и эскизный проект озонового конвертера системы кондиционирования воздуха. Объектом исследования являлись выбор носителя и катализатора разложения озона для систем кондиционирования воздуха пассажирских воздушных судов в рамках программы импортозамещения.
В.Г. Матвейкин, к.т.н. Ю.А. Ферапонтов,
к.т.н.
Л.Л. Ферапонтова, Н.В. Постернак
ОАО «Корпорация
Росхимзащита», г. Тамбов
IV Международная конференция
Катализ для переработки возобновляемого сырья:
топливо, энергия, химические продукты (CRS-4)
4-8 сентября 2017 г., Габичче Маре, Италия
http://conf.nsc.ru/en/page/CRS4
IV Международная конференция “Катализ для переработки возобновляемого сырья: топливо, энергия, химические продукты” (CRS-4) прошла 4-8 сентября 2017 года в городе Габичче Маре, расположенном на Адриатической Ривьере Италии.

Место проведения конференции было выбрано членами Международного научного комитета конференции CRS-3, прошедшей на Сицилии в 2015 году. Тогда было предложено провести следующее мероприятие в Италии и сделать его сателлитом 13-го Европейского конгресса по катализу “ЕВРОПАКАТ 2017”, который должен был пройти во Флоренции. Одним из председателей Конгресса являлся профессор Габриеле Ченти, который был сопредседателем конференции CRS-3 и внес значительный вклад в ее организацию.
Выбор района Италии для проведения конференции CRS-4 основывался на желании продолжить традицию посещения объектов комплексной и глубокой переработки растительного сырья с получением ценных химических продуктов и топлива. В провинции Эмилия-Романья на Адриатике сконцентрированы крупные заводы по производству топлива из биологических отходов, а также мусоросжигательные заводы, вырабатывающие энергию. Поэтому традиция посещения производственных объектов участниками конференции в этом районе могла быть прекрасно реализована.
Научная программа конференции CRS традиционно посвящена глобальным проблемам роли катализа в регулировании баланса энергетических ресурсов, защите окружающей среды и сокращении зависимости от ископаемых видов топлива за счет использования возобновляемых источников энергии.

В работе IV Международной конференции “Катализ для переработки возобновляемого сырья: топливо, энергия, химические продукты”, которая стала сателлитом 13-го Европейского конгресса по катализу “ЕВРОПАКАТ 2017”, приняли участие около 100 специалистов в области каталитической переработки возобновляемого сырья из 25 стран мира. Научная программа конференции включала 6 пленарных лекций, 6 ключевых лекций, 42 устных и около 25 стендовых докладов. В рамках устной сессии состоялись онлайн-презентации устных докладов участников из Южной Африки, которые лично не смогли присутствовать на конференции.
 Пленарную сессию открыла профессор Симони М. Пленц Менегетти
(Федеральный университет Алагаоса, Бразилия) с лекцией “Примеры
каталитических систем, применяемых в биотехнологиях”.
Свой доклад она посвятила роли олеохимии, и в частности, конверсии
производных целлюлозы в поддержании экологической целостности и
запасов природных ресурсов. Профессор Менегетти рассказала о процессе
переработки возобновляемого сырья с использованием нескольких
каталитических систем с применением кислот Льюиса.
Пленарную сессию открыла профессор Симони М. Пленц Менегетти
(Федеральный университет Алагаоса, Бразилия) с лекцией “Примеры
каталитических систем, применяемых в биотехнологиях”.
Свой доклад она посвятила роли олеохимии, и в частности, конверсии
производных целлюлозы в поддержании экологической целостности и
запасов природных ресурсов. Профессор Менегетти рассказала о процессе
переработки возобновляемого сырья с использованием нескольких
каталитических систем с применением кислот Льюиса.
Чл.-корр. РАН Сергей Дмитриевич Варфоломеев (Институт биохимической физики им. Н.М. Эммануэля РАН, Москва, Россия) выступил с лекцией “Химия биомассы: новые каталитические процессы деполимеризации, новые биотоплива, новые биопластики”. По мнению профессора Варфоломеева, развитие технологических процессов освоения возобновляемых источников сырья и энергии требует создания принципиально новой научной базы в области химии биомассы. На конкретных примерах различных систем С.Д. Варфоломеев продемонстрировал возможности и особенности производства принципиально новых полимерных материалов из возобновляемого сырья. Им были проиллюстрированы возможности получения полимерных экоматериалов нового поколения с использованием возобновляемых источников сырья и новых технологических подходов.
Профессор Эрик Хеерес (Университет Гронингена, Нидерланды) посвятил свое выступление валоризации твердых запасов биомассы (лигнинов и гуминов) с использованием каталитических подходов. Как и предыдущие докладчики, профессор Хеерес подчеркнул тенденцию возрастания внимания к биотехнологиям в современном обществе. Свою лекцию он посвятил вопросам биопереработки лигноцеллюлозной биомассы в биотопливо и химические биоматериалы.
Тему валоризации биомассы продолжил профессор Хосе Антонио Лопес-Санчес (Университет Ливерпуля, Великобритания), представивший доклад “Нетрадиционные каталитические пути валоризации биомассы с использованием света и микроволн”. Как справедливо было отмечено докладчиком, химия и процессы, связанные с переработкой биомассы, создают множество вызовов ученым, которые постоянно находятся в поисках более эффективных и творческих способов минимизации требуемых энергетических затрат и воздействия на окружающую среду. В своем докладе профессор Лопес-Санчес обратил внимание на проблемы развития технологий термохимической переработки, которые, с одной стороны, обладают многими преимуществами по сравнению с биопереработкой, но с другой стороны, высокие энергетические требования по-прежнему являются негативным фактором. Применение альтернативных энергетических технологий преобразования биомассы, основанных на использовании микроволнового излучения, звукохимии (сонохимии) или механохимии, открывает новые перспективы.
Представитель Института нефти IFPEN (Лион, Франция) доктор Жан-Франсуа Жоли представил лекцию “Производство топлив и химической продукции из биомассы: некоторые научные проблемы ускорения инновационно-технологического развития”. Он констатировал, что развитие технологий – это сложный процесс, который начинается с анализа технических и экономических возможностей инновационных предложений, возникающих в результате исследовательской работы, и заканчивается вводом в эксплуатацию новой промышленной установки. Далее доктор Жоли представил методологию проведения разработок в IFPEN, которая существенно отличается от существующих. Он подробно рассказал о всех стадиях и этапах этого методологического процесса, вкючая анализ инвестиционных и эксплуатационных расходов.
Завершающую лекцию пленарной сессии представил профессор Дэвид Чиарамонти (Университет Флоренции, Италия). Его лекция “Промышленный пиролиз для энергетики и получения ценных продуктов: какие возможности приоритетны?” носила частично дискуссионный характер и выявила важные вопросы для общего обсуждения. Профессор Чиарамонти представил много интересных результатов по производству и использованию биотоплива, как жидкого или газообразного, так и твердого. Особое внимание было уделено процессам пиролиза и термохимического преобразования биомассы. Будучи координатором Технологической рабочей группы Италии по производству устойчивого авиационного топлива, он подробно рассказал об этом направлении исследований. В завершении доклада были представлены макеты моделей существующих экспериментальных и пилотных установок, в частности, применяемых в авиационном секторе.

В рамках пленарной сессии был организован брифинг “Возобновляемые источники для нефтепереработки – за и против”. Координатором и председателем брифинга выступил профессор Клод Миродатос (Институт катализа и защиты окружающей среды в Лионе (IRCELYON), Франция). Мероприятие было проведено в формате панельной дискуссии. С короткими сообщениями выступили профессор Клод Миродатос (“Перспективы интеграции производства биотоплива в инфраструктуру нефтеперерабатывающих предприятий”), профессор Саша Керстен, Университет Твенте, Нидерланды (“Особенности использования растительного сырья (бионефти, растительных масел) в процессах нефтепереработки”) и профессор Вадим Анатольевич Яковлев, Институт катализа им. Г.К. Борескова СО РАН, Новосибирск, Россия (“Стабильность катализаторов гидроочистки при совместной переработке возобновляемых оксидантов и масел”). Предложенная на брифинге тема вызвала живую дискуссию, в которой приняли участие представители как академических кругов, так и промышленных компаний из Нидерландов, Канады, Германии, Франции, Австралии, России, Великобритании и других стран. Несмотря на то, что производство биотоплива расширяется во всем мире, до сих пор оно слабо интегрировано в инфраструктуру нефтеперерабатывающих заводов. Биотопливо могло бы играть более существенную роль в снижении зависимости от нефти, если бы имелись экономичные варианты его смешивания или переработки на традиционных нефтеперерабатывающих заводах. Дискуссия вызвала много вопросов: отношения между биомассой и ископаемым топливом – что с ними делать? Смешивание или совместная переработка? Какой тип совместной переработки: гидроочистка или каталитический крекинг? Речь шла и об автомобильном топливе – дизель или бензин каталитического крекинга? Прозвучало мнение, что следует рассмотреть и альтернативные методы – микроволновое излучение и ультразвуковая обработка действительно могут использоваться на биоперерабатывающих заводах.
Ключевые лекции были представлены профессором Франческо Фрустери (Институт Николо Джордано, Мессина, Италия), профессором Сашей Керстеном (Университет Твенте, Нидерланды), профессором Борисом Николаевичем Кузнецовым (Институт химии и химической технологии СО РАН, Красноярск, Россия), профессором Дмитрием Юрьевичем Мурзиным (Або Академии Университет, Турку, Финляндия), профессором Марком Вениаминовичем Цодиковым (Институт нефтехимического синтеза РАН, Москва, Россия), профессором Карен Вилсон (Университет Астона, Великобритания).
 Заключительной
и, как всегда, существенной частью программы конференции CRS-4 стала
традиционная поездка на завод по производству электроэнергии и
топлива путем глубокой переработки мусора и растительного сырья.
Участники ознакомились с особенностями технологий, применяемых на
заводе, увидели действующие реакторы и аппараты. Специалисты
предприятия выступили с презентациями перед участниками конференции.
Заключительной
и, как всегда, существенной частью программы конференции CRS-4 стала
традиционная поездка на завод по производству электроэнергии и
топлива путем глубокой переработки мусора и растительного сырья.
Участники ознакомились с особенностями технологий, применяемых на
заводе, увидели действующие реакторы и аппараты. Специалисты
предприятия выступили с презентациями перед участниками конференции.
На встрече пленарных и ключевых лекторов, председателей заседаний, членов Международного научного комитета, традиционно проходящей в рамках работы конференции, было предложено провести следующую конференцию CRS-5 в Греции. Предложение было горячо подержанно всеми участниками конференции на ее закрытии, на котором аудитории был предложен видеоролик о прошедшем форуме, подготовленный организаторами.
Труды конференции традиционно публикуются в специальном выпуске журнала “Катализ для устойчивой энергетики”.
Материал
подготовили Т.В. Замулина, С.С. Логунова, О.О. Заикина, В.А.
Яковлев
(ИК СО РАН, Новосибирск)
Sulfones expand the reach of radical cross-couplings
New reagents offer streamlined synthesis of valuable fluorinated products
This is one of more than 60 examples of radical cross-couplings using new sulfone reagents with unique reactivity.
Cross-coupling reactions, wherein a catalyst brings together molecular partners to forge a new bond, are powerful transformations that are among the most used tools in synthesis. For decades, researchers have mined this area of chemistry, developing methods for a litany of coupling partners. Now chemists have found a novel, and valuable, pairing.
A team of researchers led by Phil Baran at Scripps Research Institute, California, has introduced alkylsulfones as coupling partners for radical cross-coupling reactions, providing access to valuable fluorinated structures that would be cumbersome to make with typical alkyl coupling partners (ChemRxiv 2017, DOI: 10.26434/chemrxiv.5715106.v1). Whereas classic cross-couplings conjoin two aryl partners, this type of cross-coupling is particularly well-suited for threading together aryl and alkyl partners.
With the help of a nickel catalyst and pyridine ligand, the reaction couples arylzinc compounds with alkylsulfone reagents—in which the oxidized sulfur atom is connected to a five-membered ring loaded with nitrogens. The researchers demonstrated the scope of the reaction by reacting compounds containing a variety of substitution patterns and by synthesizing known biologically relevant molecules to highlight the shortened synthetic routes enabled by the technique.
Notably, the sulfone reagents let researchers directly install fluorine atoms at the alkyl coupling site, whereas previously chemists would have had to run difficult deoxyfluorination reactions to make the requisite fluorinated coupling partner, says Scott Denmark of the University of Illinois, Urbana-Champaign. “The unique reactivity of the N-phenyltetrazole sulfones is surpassed only by their practicality as bench stable and odorless, crystalline compounds,” he says.
The team produced a handful of the fluorinated sulfone reagents on a large scale. In a tweet, the Baran lab offered up free samples of the compounds, giving interested chemists the opportunity to try out the method before the work is published in a peer-reviewed journal.
Cathleen Crudden of Queen’s University in Ontario and Masakazu Nambo of the Institute of Transformative Bio-Molecules at Nagoya University, who collaborated with each other on a different cross-coupling reaction using phenyl sulfones, also highlight the value of the new sulfone reagents’ ability to incorporate fluorine, especially in medicinal chemistry applications. “There is no doubt that this work is going to be game-changing and illustrates that there is still much to do in the field of cross coupling,” they told C&EN.
Catalyst treatment could boost exhaust cleanup
Steam treating enhances platinum’s durability and knack for scrubbing CO from engine emissions
The catalysts that clean up automotive emissions typically consist of particles of platinum and other precious metals anchored on oxides. Because only the metal atoms at the particle surfaces come in contact with reactants and catalyze reactions, catalyst manufacturers strive to make these particles as tiny as possible.
But these supported catalysts come with trade-offs. Dispersing the precious metal as finely as possible can go too far, and the catalysts can become unstable: The metal particles diffuse, coalesce, and lose their catalytic oomph. And the catalysts are often inactive when the temperature of the exhaust is low, which is the case when today’s engines start on a cold morning and will regularly be the case with future energy-efficient engines.
A new study on automobile exhaust cleanup describes a way to bypass those problems in a catalytic two-for-one deal. Researchers have shown that a simple procedure can stabilize a platinum-based automotive catalyst and reduce the temperature at which it can thoroughly strip CO from engine exhaust (Science 2017, DOI: 10.1126/science.aao2109).
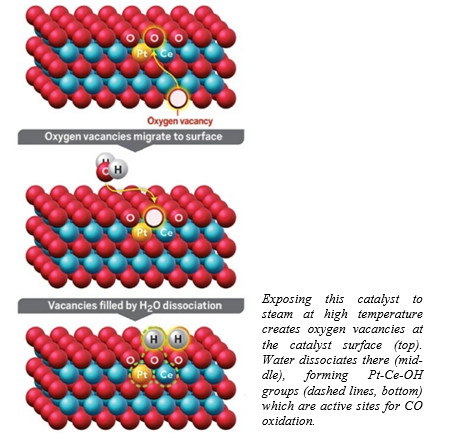
Such a treatment could help clean up emissions from future engines designed to recover energy lost in hot exhaust, which results in lower temperatures of the gas that passes through the catalytic converter.
In the run-up to the new study, a team led by University of New Mexico chemical engineer Abhaya K. Datye took dispersing metal particles on an oxide support to the extreme. In 2016, the group reported that isolated platinum atoms on ceria could convert CO to CO2, a key reaction in engine-emissions cleanup. But the catalyst worked weakly.
So the team, which includes Yong Wang of the Pacific Northwest National Laboratory, searched for chemical and physical treatments that would boost the Pt-CeO2catalyst’s activity without causing it to fail quickly, a common occurrence during catalyst development.
Eventually the group found that heating the catalyst to 750 °C in steam drastically improves its ability to mediate CO oxidation. Specifically, in contrast to the untreated catalyst, which needs to be heated to roughly 210 °C to begin oxidizing CO and achieves 100% CO conversion at 320 °C, the treated catalyst begins working at just 60 °C and reaches 100% conversion at 148 °C. Furthermore, the treatment makes the catalyst durable: It showed no signs of deactivation even after 300 hours of testing.
Microscopy and spectroscopy analyses indicate that the steam enhances CO-oxidation performance by creating catalytically active sites featuring ceria-bound Pt-OH groups.
“This discovery could help advance the technology for vehicle exhaust conversion,” remarks Bruce C. Gates, a catalysis specialist at the University of California, Davis. “The authors’ catalyst characterizations provide deep insights and point the way forward.” The characterization work also raises intriguing questions for further study, he adds. For example, Gates proposes that researchers should examine the nature of the sites on ceria at which platinum bonds and determine if they are defects. He also wonders if a metal cheaper than platinum would work similarly.
A light approach to installing heavy hydrogens
Photoredox catalysis shines in a reaction that swaps out hydrogen for deuterium or tritium
To follow the fate of a drug or a drug metabolite as it wends its way through the body, scientists will often label the compound by switching out a hydrogen atom for one of its heavier isotopes—deuterium or tritium. This molecular switcheroo sounds simple, but it can actually be a lot of work, requiring chemists to resynthesize a molecule so it has an atom, usually a halogen, or unsaturated bond where the heavy hydrogen can be swapped in or added. The process can take months.
Chemists at Princeton University and Merck & Co. have now come up with a way to switch hydrogen out for deuterium or tritium in a matter of moments. The reaction uses photoredox-mediated hydrogen-atom transfer to replace the hydrogens at C–H bonds adjacent to amines with deuterium or tritium from D2O or T2O (Science 2017, DOI: 10.1126/science.aap9674).
Amines are common motifs in biologically active molecules, and the chemists, led by Princeton’s David W. C. MacMillan, show the reaction works on myriad drug molecules (examples shown). For the tritiation reactions, the chemists also figured out how to make T2O, which isn’t commercially available, during the reaction using T2 gas and platinum oxide.
The new labeling methodology could “open the door to earlier and expanded use of isotopic labeling in drug discovery, significantly enhancing our ability to study drug candidates on a deeper level and across a range of applications,” says Jennifer Lafontaine, senior director of synthesis and analytical chemistry in Pfizer’s oncology medicinal chemistry group.
These
pharmaceuticals were tritiated (indicated with a red dot) using
a
photoredox-catalyzed exchange reaction.
Ian Davies, a coauthor on the paper who recently moved from Merck to Princeton, points out that the reaction could also help chemists studying the biology of complex natural products. “You don’t have to resynthesize the natural product,” he explains. As long as chemists have isolated the molecule, this reaction can deuterate or tritiate it.
Paul Greenspan, senior director of discovery chemistry at Takeda Pharmaceuticals, says the work “provides yet another impressive example of the power and versatility of photoredox catalysis, which continues to surprise us with mild, controllable, and highly practical transformations that would have been previously unattainable.”
MacMillan’s lab has been working in photoredox catalysis for several years. He says that the idea for the isotopic labeling reaction came about when he was visiting Merck, and Davies had set up a meeting for him with the company’s isotopic labeling group. “I said, ‘Why am I meeting with these people? What’s the point?’ ” MacMillan recalls. Davies’s reply was: “I don’t know.” But he assured MacMillan that there was value to be found in the meeting between scientists who don’t often interact.
MacMillan says that only hours after the meeting chemists in his lab were running experiments to test whether photoredox chemistry could help with isotopic labeling. “By that evening we knew we were onto something pretty interesting,” he says.
Despite deuterium and tritium’s rich history in physical organic chemistry and biochemistry, the isotopes have been underused in recent decades, notes Jacob M. Hooker, a molecular imaging expert at Massachusetts General Hospital. MacMillan and Merck’s photoredox method “is likely to draw attention to and revitalize the field,” he says. “This will spark additional innovative methods that drive quantitative chemical biology and pharmacology once again.”
Chemical & Engineering News
ПРИГЛАШЕНИЯ НА КОНФЕРЕНЦИИ
|
19-20
апреля 2018 г. IVII Всероссийская конференция c международным участием “Актуальные вопросы химической технологии и защиты окружающей среды” Новочебоксарск, Россия |
mailto:himtech@bk.ru |
|
May
13-16, 2018 3rd Green & Sustainable Chemistry Conference Berlin, Germany |
https://www.elsevier.com/events/conferences/ green-and-sustainable-chemistry-conference |
|
May
15-16, 2018 11th International Conference on Bio-based Materials Maternushaus, Cologne, Germany |
http://bio-based-conference.com/ |
|
May
16-18, 2018 Designing Nanoparticle Systems for Catalysis. Faraday Discussion London, UK |
http://www.rsc.org/events/detail/25362 |
|
May
20-23, 2018 5th International School-Conference on Catalysis for Young Scientists “Catalyst Design: From Molecular to Industrial Level” Moscow, Russia |
http://conf.nsc.ru/catdesign2018 |
|
June
4-7, 2018 13th International Symposium on the Synthesis and Applications of Isotopes and Isotopically Labelled Compounds Prague, Czech Republic |
http://www.iis-prague2018.cz/ |
|
June
4-8, 2018 10th European Meeting on Solar Chemistry and Photocatalysis: Environmental Applications (SPEA10, 2018) Almeria, Spain |
http://www.spea10.com/ |
|
June
6-9, 2018 1st International Conference on Reaction Kinetics, Mechanisms and Catalysis Budapest, Hungary |
https://rkmc.akcongress.com/ |
|
June
7-10, 2018 2nd Conference “From Carbon-Rich Molecules to Carbon-Based Materials” Nassau, Bahamas |
https://www.fusion-conferences.com/ conference74.php |
|
June
17-20, 2018 2nd International Symposium on Clean Energy from Ethanol (ISCEE 2018) Krakow, Poland |
http://iscee.com.pl/conference/conference-site |
|
June
18-20, 2018 Environment, Green Technology and Engineering International Conference (EGTEIC) Caceres, Spain |
www.egteic.com |
|
June
21-22, 2018 20th International Conference on Catalysis (ICC 2018) Dubai, UAE |
https://www.waset.org/conference/ 2018/06/dubai/ICC/home |
|
June
25-27, 2018 3rd Conference “Fundamentals and Applications of Cerium Dioxide in Catalysis” Barcelona, Spain |
https://ceria2018.upc.edu/en |
|
June
25-29, 2018 IEFCATS School on Catalysis Liblice Castle, Czech Republic |
http://www.jh-inst.cas.cz/efcats.school/ |
|
26-30
июня 2018 г. III Всероссийская научная конференция с международным участием “Актуальные проблемы теории и практики гетерогенных катализаторов и адсорбентов” Костромская область, Россия |
http://isuct.ru/conference/apak-2018 |
|
June 29
– July 2, 2018 International Symposium on Power and Chemical Engineering Sofia, Bulgaria |
mailto:vbeschkov@yahoo.com |
|
July
1-6, 2018 The World Conference on Carbon (Carbon 2018) | http://www.carbon2018.org/ |
|
July
2-6, 2018 16th International IUPAC Conference on High Temperature Materials Chemistry (HTMC-XVI) Ekaterinburg, Russia |
http://htmc16.ru/ |
|
July
7-14, 2018 Post-graduate Summer School on Green Chemistry Venice, Italy |
http://www.unive.it/greenss2018 |
|
July
8-12, 2018 12th International Symposium on Scientific Bases for the Preparation of Heterogeneous Catalysts (PREPA12) Louvain-La-Neuve, Belgium |
http://www.prepa12.org/ |
|
July
8-13, 2018 21th International Symposium on Homogeneous Catalysis (ISHC-21) Amsterdam, The Netherlands |
https://www.ishc21.amsterdam/ |
|
July
8-13, 2018 27th IUPAC International Symposium on Photochemistry (PhotoIUPAC 2018) Dublin, Ireland |
http://photoiupac2018.com/ |
|
July
9-11, 2018 4th International Symposium on Catalysis for Clean Energy and Sustainable Chemistry (CCESC 2018) Bilbao, Spain |
http://www.ccesc2018.com/ |
|
July
15-18, 2018 8th International Gold Conference Paris, France |
http://www.gold2018.org/ |
|
July
22-25, 2018 8th International Symposium on Relations between Homogeneous and Heterogeneous Catalysis (ISHHC 2018) Sydney, Australia |
http://www.ishhc18.com/ |
|
July
23-25, 2018 World Congress & Expo on Chemical Engineering & Catalysis (WCECEC-2018) Osaka, Japan |
http://scientificfederation.com/catalysis-2018/ |
|
August
5-9, 2018 International Symposium on Zeolites and Microporous Crystals (ZMPC 2018) Yokohama, Japan |
http://www.jaz-online.org/ZMPC2018 |
|
August
5-10, 2018 8th Tokyo Conference on Advanced Catalytic Science and Technology (TOCAT8) Yokohama, Japan |
http://www.shokubai.org/tocat8zmpc2018 |
|
August
26-28, 2018 Nordic Symposium on Catalysis Copenhagen, Denmark |
http://www.conferencemanager.dk/NSC2018 |
|
August
26-29, 2018 XII International Symposium on Heterogeneous Catalysis Sofia, Bulgaria |
http://12symp.ic.bas.bg/ |
|
August
26-30, 2018 7th EuCheMS Chemistry Congress Liverpool, UK |
https://www.euchems2018.org/ |
|
September
9-14, 2018 8th IUPAC International Conference on Green Chemistry (8th ICGC) Bangkok, Thailand |
http://www.greeniupac2018.com/ |
|
September
13-15, 2018 2nd Edition of Global Conference on Catalysis, Chemical Engineering & Technology (CAT-2018) Rome, Italy |
https://catalysis-conferences.magnusgroup.org/ |
|
13-16
сентября 2018 г. 14-я российская конференция “Физико-химические проблемы возобновляемой энергетики” Черноголовка, Россия |
http://re2018.lssi.su/ |
|
September
16-21, 2018 13th International Conference on Solid State Chemistry (SSC 2018) Pardubice, Czech Republic |
http://www.ssc-conference.com/2018 |
|
16-27
сентября 2018 г. III Симпозиум “Современная химическая физика” Туапсе, Россия |
http://www.chemphysics.ru/ |
|
17-21
сентября 2018 г. XII Международная конференция молодых ученых по нефтехимии Звенигород, Россия |
http://conference.forenewchemistry.ras.ru/ |
|
23-26
сентября 2018 г. IV Междисциплинарный симпозиум по медицинской, органической и биологической химии и фармацевтике Новый Свет, Крым, Россия |
http://mobi-chem.org/ |
|
September
23-27, 2018 10th International Conference on Environmental Catalysis & 3rd International Symposium on Catalytic Science and Technology in Sustainable Energy and Environment Tianjin, China |
http://icec-eecat2018.ydlilab.com/ |
|
September
24-28, 2018 14th International Conference on Fundamental and Applied Aspects of Physical Chemistry “Physical Chemistry 2018” Belgrade, Serbia |
http://www.socphyschemserb.org/en/events/pc2018/ |
|
25-29
сентября 2018 г. V Международная научная школа-конференция молодых ученых “Катализ: от науки к промышленности” Томск, Россия |
http://www.catconf.tsu.ru/ |
|
1-5 октября 2018 г. X Международная конференция "Химия нефти и газа" Томск, Россия |
http://www.ipc.tsc.ru/conf/10m2018/ |
|
2-4
октября 2018 г. XXXI Международная научно-техническая конференция “Химические реактивы, реагенты и процессы малотоннажной химии” (РЕАКТИВ-2018) Минск, Республика Беларусь |
http://i29770zd.bget.ru/ |
|
7-10
октября 2018 г. VII Международный российско-казахстанский симпозиум “Углехимия и экология Кузбасса” Кемерово, Россия |
http://www.iccms.sbras.ru/ccsymp-2018 |
|
9-10
октября 2018 г. Международная научно-практическая конференция “Инновативные перспективы развития нефтепереработки и нефтехимии” Баку, Азербайджан |
http://nkpi.az/ (99412) 902-476 (99412)902-443 zmea_nkpi@box.az ipcp_lab3@mail.ru |
|
16-19
октября 2018 г. XV Российская ежегодная конференция молодых научных сотрудников и аспирантов с международным участием “Физико-химия и технология неорганических материалов” Москва, Россия |
http://m.imetran.ru/ |
|
October
21-24, 2018 15th International Conference on Micro Reaction Technology (IMPET 2018) Karlsruhe, Germany |
http://www.dechema.de/IMRET2018 |
|
November
5-9, 2018 XXIII International Conference on Chemical Reactors (CHEMREACTOR-23) Ghent, Belgium |
http://conf.nsc.ru/CR_23 |
|
November
8-9, 2018 International Conference on Mathematics in (bio)Chemical Kinetics and Engineering (MaCKiE-2018) Ghent, Belgium |
http://conf.nsc.ru/mackie2018 |
|
May 15-17, 2019 5th International Conference on Polygeneration (ICP 2019) Fukuoka, Japan |
http://therme.mech.kyushu-u.ac.jp/ICP2019/ICP2019.html |
|
June
26-30, 2019 6th European Conference on Environmental Applications of Advanced Oxidation Processes (EAAOP-6) Portoroz-Portorose, Slovenia |
http://eaaop6.ki.si/ |
|
July
5-12, 2019 50th General Assembly & 47th IUPAC World Chemistry Congress Paris, France |
https://www.iupac2019.org/ |
|
August
18-23, 2019 14th EuropaCat – European Congress on Catalysis "Catalysis without Borders" Aachen, Germany |
http://www.europacat2019.eu/ |
12 января 2018 года в Мюнхене скончался выдающийся ученый-каталитик профессор Роберт К. Грасселли (Robert K. Grasselli).
Имя Роберта Грасселли, несколько раз выдвигавшегося на Нобелевскую премию, одно из самых признанных в каталитической науке. Роберт К. Грасселли известен не только как ученый, но и как изобретатель. Он автор многочисленных патентов США, в том числе ключевого патента на так называемый процесс SOHIO по селективному окислению и окислительному аммонолизу углеводородов. Коллеги профессора Грасселли из Технического университета Мюнхена характеризуют его как неиссякаемый источник фундаментальных и прикладных знаний. Полученные им новаторские результаты по селективному окислению углеводородов и их использованию в крупномасштабных промышленных процессах сделали его одним из столпов мирового каталитического сообщества.
Роберт К. Грасселли родился в 1930 году. Изучал химию в Гарвардском университете и получил докторскую степень в Университете Case Western Reserve, США (1959 г.). Уже с 1952 года он работал в компании Standard Oil Co. (Sohio) в качестве научного сотрудника, а затем директором по катализу и физике твердого тела (Director of Catalysis and Solid State Science). В 1986 году он покинул Sohio/BP America и перешел в Управление военно-морских исследований (Office of Naval Research), где до 1989 года занимал должность директора отдела химии. С 1989 по 1995 год Роберт Грасселли – сотрудник Центральной научно-исследовательской лаборатории Принстонской Корпорации мобильных исследований и разработок (Mobil Research and Development Corporation). С 1983 по 1990 год – профессор химии в Университете Кейс Вестерн Резерв, США, а с 1996 года – профессор по химической технологии и материаловедению (Professor of Chemical Engineering and Materials Science) в Университете штата Делавэр,США.

Роберт
Грасселли делает доклад на сцене Дома Ученых Новосибирского
Академгородка
в рамках конференции, посвященной 100-летию со дня
рождения основателя
Института катализа СО РАН академика Георгия
Константиновича Борескова
Роберт К. Грасселли – автор 175 патентов и более 150 научных работ, член Национальной академии инженерных наук США (US National Academy of Engineering), член Национального Зала Славы инженерии, науки и техники США (US National Hall of Fame for Engineering, Science and Technology). Знаменитому ученому было присуждено множество наград, в том числе премия имени Александра фон Гумбольдта, премия Мерфи по промышленной и химической инженерии (E.V. Murphree Award in Industrial and Engineering Chemistry), Нефтехимическая премия (Petroleum Chemistry Award), медаль Морли, присуждаемая Американским химическим обществом. Роберт К. Грасселли удостоен почетной докторской степени нескольких европейских университетов.
Роберт Грасселли плодотворно сотрудничал с Институтом катализа СО РАН. Профессор Геннадий Иванович Панов вспоминает: “Помимо выдающихся научных достижений, Роберт Грасселли известен как большой энтузиаст по объединению мирового сообщества ученых-каталитиков. Особенно в области окислительного катализа, где он был непререкаемым авторитетом. Именно ему принадлежит основная заслуга в создании Международного конгресса по окислительному катализу (WCOC), открытие которого состоялось в 1989 году. Руководящим органом WCOC является Совет Конгресса, включающий 10-12 представителей от ведущих каталитических стран (Представители от России в разные годы: О.В. Крылов, 1989-2005 г.; Г.И. Панов, 2005-2013 г.; В.И. Бухтияров, с 2013 г. – прим. ИК СО РАН). Грасселли был неизменным членом Совета, представлявшим США, и дважды избирался его Президентом”.
“Мне приходилось неоднократно общаться с Робертом Грасселли, – продолжает Г.И. Панов, – а также вести весьма обширную переписку, как в связи с моим участием в нескольких симпозиумах, организуемых и спонсируемых им, так и особенно в связи с его приездом в Новосибирск для участия в Мемориальной Боресковской конференции в 2007 году. Его лекции всегда носили яркий и глубокий характер. Как публичные выступления, так и личные беседы неизменно оставляли впечатление, что перед вами не только выдающийся ученый, но и замечательный человек высокой культуры, умеющий быть объективным, доброжелательным и остроумным. Именно таким останется профессор Грасселли в моей памяти”.
Руководство и сотрудники Института катализа СО РАН скорбят по случаю смерти выдающегося ученого и выражают соболезнования семье и друзьям покойного.
www.catalysis.ru


