22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы

Method offers simple way to maximize use of costly noble metals
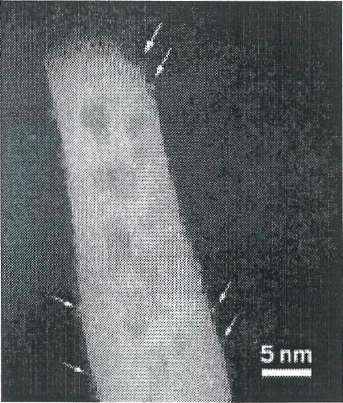 Pt atoms (arrows) on the edges of this ceria nanorod remain isolated even after the material has catalyzed CO oxidation reactions.
Pt atoms (arrows) on the edges of this ceria nanorod remain isolated even after the material has catalyzed CO oxidation reactions.
In a feat that could be described as taking the bull by the horns, researchers have exploited a process that normally destroys supported metal catalysts to make stable ones consisting of isolated platinum atoms on a solid support (Science 2016, DOI: 10.1126/science.aaf88oo).
The counterintuitive strategy may offer a practical way to maximize the use of platinum and other expensive noble metals by producing stable single-atom catalysts.
Tiny particles of platinum can transform petrochemicals, clean up engine emissions, and catalyze other reactions. But when exposed to high temperatures and oxidizing conditions, the particles agglomerate, forming larger particles. Known as Ostwald ripening, the process reduces catalytic activity by burying the majority of the atoms in the growing particles' interiors, where they can't catalyze reactions.
Rather than avoiding those conditions, a team led by University of New Mexico chemical engineer Abhaya K. Datye exploited them, sacrificing an alumina-supported platinum nanoparticle catalyst to make a catalyst consisting of isolated platinum atoms on ceria. Datye described the work in China at a conference on single-atom catalysis at the Dalian Institute of Chemical Physics.
From earlier work, Datye and coworkers knew that hot oxidizing conditions turn platinum nanoparticle catalysts into larger, less active platinum clumps by converting the metal to PtO2, which is volatile and desorbs from nanoparticle surfaces. Datye reasoned that this supply of mobile atoms could allow his team to produce single-atom catalysts. "The challenge is coming up with a way to trap those atoms individually," he said.
Datye and coworkers wondered if ceria might serve as this atom trap because other researchers had shown that small amounts of the oxide prevented nanoparticle sintering or fusing.
They mixed an alumina-supported platinum-lanthanum catalyst with various forms of ceria and heated the mixtures to 800 °C in air for a week. After the treatment, X-ray diffraction and other methods showed the complete absence of platinum nanoparticles. Electron microscopy revealed that platinum had migrated from the alumina and become trapped as isolated atoms on ceria nanorods and octahdera along lattice features known as step edges. In the absence of ceria, the harsh treatment formed large platinum crystals on alumina.
The team showed that the single-atom catalysts were effective at CO oxidation—a key reaction in engine-emissions cleanup—and that the platinum atoms remained isolated during the reaction.
"I'm very impressed with this work," said Northwestern University's Peter C. Stair, a catalysis specialist who attended the conference. Coming up with a way to stabilize isolated, catalytically active metal atoms on solid supports has practical relevance for industrial catalysis, he added. "It points to strategies for making catalysts that remain stable for years." Exactly how platinum is bound to the ceria surface remains an open question, Stair said. But that question applies equally well to most efforts to prepare single-atom catalysts.
Hybrid silica-MoS2 material could be used in optoelectronics, catalysis, and sensing
Sheets of molybdenum disulfide just a few atoms thick show promise as semiconductors and light-emitting materials for electronic and optical devices. But there is no easy, cheap process to make the nanometers-thin sheets. Now, researchers report a one-step method that grows MoS2nanosheets using the silica shells of marine diatoms as a scaffold (Chem. Mater. 2016, DOI: 10.1021/acs.chemmater.6b01738).
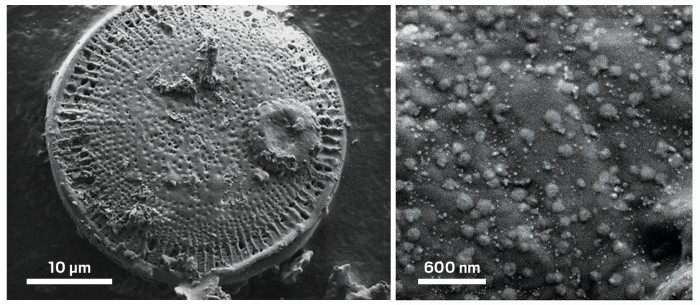
Scanning electron micrographs show a hybrid material in which nanosheets of molybdenum disulfide decorate the silica surface of a fossilized diatom.
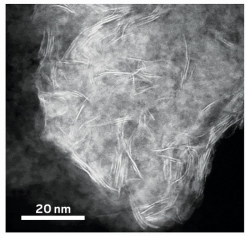
A close-up of a molybdenum disulfide flake shows that it is made of several nanosheets, the edges of which are visible as bright lines.
The technique yields a hybrid material consisting of silica particles tens of micrometers wide speckled with MoS2 flakes. The material’s blend of photonic and semiconducting properties and high surface area could make it useful for catalysis, sensing, energy storage, and optoelectronics, the researchers say.
MoS2 nanosheets are typically made using mechanical exfoliation or chemical vapor deposition. Exfoliation, which involves peeling off ultrathin layers from bulk crystals, is slow and gives limited amounts of material, while chemical vapor deposition requires expensive materials and tools.
Paul O’Brien, Sarah J. Haigh, and David J. Lewis at the University of Manchester used porous diatom shells as a scaffold to grow MoS2 nanosheets. The silica in the shells is a passive insulator material, making it an ideal scaffold for other functional materials. What’s more, the periodic arrangement of the pores helps the particles trap light and gives them a high surface area. Scientists have tried to recruit diatoms for nanotechnology, photonics, and drug delivery applications, and have coated them with materials like titanium and tin dioxide. “Nature has already preformed the shell as a nanoscaffold for us to use,” O’Brien says. “It’scheap as chips and dug out of the ground by the ton.”
To make the hybrid material, the team mixed a powder of fossilized diatom shells, known as diatomaceous earth, into a tetrahydrofuran solution containing an organometallic precursor to MoS2, which coats the particles. After filtering out the solvent, the researchers dried the powder and heated it at 450 °C, converting the precursor into crystalline MoS2 flakes that dot the silica surface. The flakes have an average diameter of about 132 nm and range in thickness from one to six atomic layers.
There are thousands of diatom species. Combining diatoms of particular architectures with other two-dimensional metal sulfides could give materials with precisely tailored properties, the researchers say. For now, they are using the method to make materials for supercapacitor electrodes.The counterintuitive strategy may offer a practical way to maximize the use of platinum and other expensive noble metals by producing stable single-atom catalysts.
Chemical processes often need to be coaxed into action with heat. Catalysts can reduce the amount of heat required to drive a reaction, enabling an ordinarily high-temperature reaction to run at lower temperatures.
At the American Chemical Society national meeting in Philadelphia on Monday, Francisco Zaera of the University of California, Riverside, described an extreme low-temperature example: a gold catalyst that drives carbon monoxide oxidation at 150 °C below zero.
The finding broadens basic understanding of low-temperature chemistry and may lead to applications in space exploration.
Catalytic reactions that run at cryogenic temperatures are quite rare. One example is the esoteric interconversion of spin isomers of dihydrogen.
Reactions involving gold catalysts used to be rare. But in recent years, researchers have discovered several reactions mediated by nanosized gold particles. Those reactions, including CO oxidation, tend to run close to room temperature.
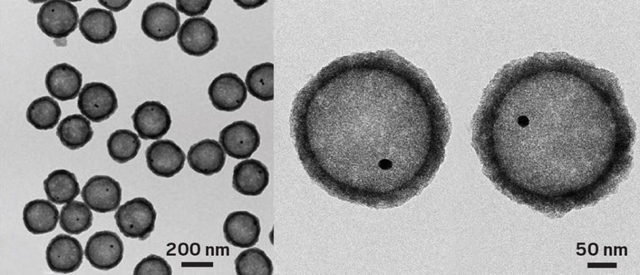
These yolk-shell particles catalyze CO oxidation at -150 °C.
The UC Riverside group’s catalysts are also nanosized, but they do their job at a frigid 120 K. Speaking at a session sponsored by the Division of Catalysis Science & Technology, Zaera explained that his group prepares the catalysts by growing gold particles roughly 15 nm in diameter and encapsulating them in a 200-nm-wide silica sphere, which they in turn coat with a 20-nm thick titania shell. The team then etches away the silica, leaving a yolk-shell structure—so named because it resembles an egg without the egg white (Surf. Sci. 2016, DOI: 10.1016/j.susc.2015.10.008).
The porous titania shell allows gases to diffuse to and from the catalytic cores and protects the gold particles from fusing together, a common problem that deactivates nanoparticle catalysts.
The amorphous titania shell contains sodium titanate and silicon impurities that prove critical to the cryogenic activity. Removing the impurities kills the catalysis. Spectroscopy measurements indicate that the mechanism governing cryogenic CO oxidation differs from the one active at room temperature.
Jeffrey G. Weissman, manager of catalyst development at Precision Combustion in North Haven, Conn., remarked that catalysts working at cryogenic temperatures could find applications in space exploration. For example, in an extended manned mission to an asteroid or Mars, astronauts may need to collect ice and other materials found on location and convert them to oxygen, water, fuel, and possibly plant growth medium. Weissman’s team has conducted research in support of such a mission and has developed cabin-air cleaning devices for the International Space Station.
“One aspect I find intriguing,” Weissman said, “is that the yolk-shell morphology provides a means to exclude certain gas species from reaching the catalyst.” For example, larger hydrocarbons or sulfur contained in a gas mixture may be physically blocked from reaching the catalyst surface, thereby preventing catalyst deactivation. Such a capability could be important in devices that clean up exhaust gas or air in confined living spaces.
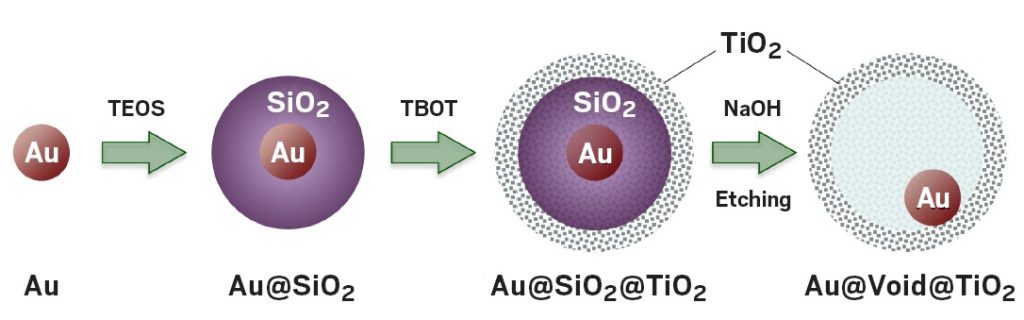
To create new cryogenic catalysts, chemists treated gold nanoparticles with tetraethyl orthosilicate (TEOS)
to form a sacrificial silicon dioxide shell, and then reacted that product with tetrabutyl titanate (TBOT).
Etching away the silicon dioxide yields a largely hollow particle with a titanium dioxide shell and tiny gold core.
Technique uses metal insertion to derivatize methylene C–H bonds catalytically and enantioselectively
The ability to activate specific C–H bonds in organic compounds so they can be converted into C–C bonds or other derivatives has been a longtime goal in synthetic organic chemistry.
Over the past few decades, chemists have learned to activate several types of C–H bonds by inserting metal atoms into them. But the ability to activate the most common class of C–H bonds, those in methylene (CH2) groups, and to convert them into chiral centers has been a largely unsolved problem.
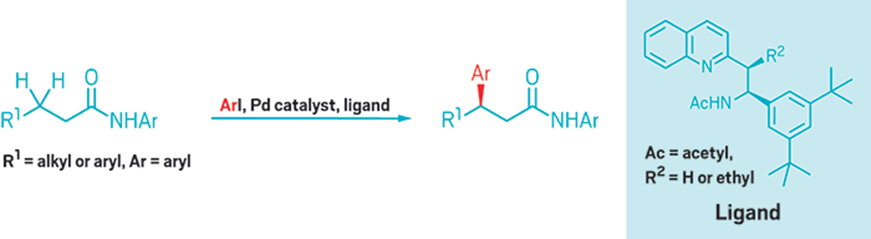
Yu and coworkers use a Pd catalyst and a ligand to activate one of two methylene
C–H bonds to form chiral aryl derivatives.
Jin-Quan Yu of Scripps Research Institute California and coworkers have now developed what could become a textbook reaction—the first that uses metal insertion to derivatize methylene C–H bonds catalytically and enantioselectively. It converts methylenes that are two carbon atoms away from amide and carboxyl groups into chiral centers (Science 2016, DOI: 10.1126/science.aaf4434).
Activating these so-called β-methylenes enantioselectively “was my first independent project, started in 2002, back in Cambridge University,” Yu says. “It took 14 years to finally achieve this goal.”
“Yu’s trailblazing group has managed to bring to reality what might have been considered seemingly impossible,” comments Erick Carreira, an expert on asymmetric synthesis at the Swiss Federal Institute of Technology (ETH), Zurich. “It represents a giant leap for the field that opens new vistas for the synthesis of optically active building blocks.”
Yu believes the approach can be extended to other classes of substrates besides those containing amides and carboxyls. A group at Bristol-Myers Squibb that he collaborates with is already using the new reaction to synthesize drug candidates.
Conjugate addition, a classical reaction that does not involve metal insertion, can also create chiral centers adjacent to carbonyl groups, but it requires that an alkene group be available or preinstalled at the β-position, and that is not always possible or easy to arrange.
In the new reaction, palladium and a simple bidentate ligand—one that coordinates with the substrate in two different ways—cause Pd to insert into one of the C–H bonds of a β-methylene. Chemists can then substitute aryl groups for the metal. The reactions have good yields and produce high ratios of products with specific chirality.
Although the ligand looks simple, engineering its structure so it worked just right took a considerable amount of effort and was key to developing the new reaction. Kendall N. Houk of the University of California, Los Angeles, and coworkers helped that effort by developing a computational model of ligand-substrate interactions.
Technique accelerates evaluation of amine-containing chiral compounds
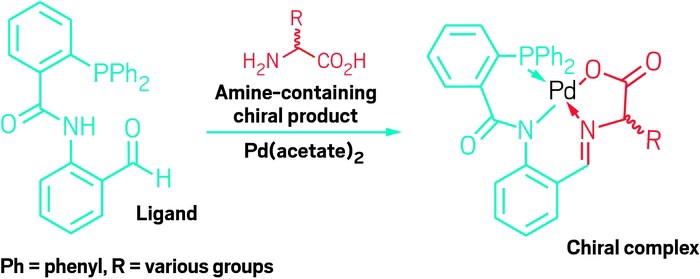
A complexation reaction forms a Schiff base with an amine, enabling measurement of yield,
enantiomeric excess, and absolute configuration in a single step.
A new technique makes it much easier and cheaper to determine key characteristics of amine-containing chiral compounds synthesized individually or in parallel. The technique, which measures product yield, enantiomeric excess (ee), and absolute configuration in one step, could find widespread use in organic chemistry laboratories, including in drug discovery.
Chemists typically determine product yield by isolating synthesized compounds and weighing them. They generally measure ee by analyzing reaction products with chiral high-performance liquid chromatography and absolute configuration by using nuclear magnetic resonance spectroscopy or X-ray crystallography. These techniques are time-consuming, use large amounts of solvents and reagents, and are impractical for analyzing thousands of samples synthesized in parallel.
Christian Wolf and grad student Zeus A. De los Santos at Georgetown University have now developed a complexation reaction that makes it possible to analyze the yield, ee, and absolute configuration of amine-containing chiral products in a single step, without having to isolate the compounds (J. Am. Chem. Soc. 2016, DOI: 10.1021/jacs.6b08892). In the reaction, a carbonyl-containing ligand, in the presence of palladium acetate, forms a Schiff base with amine groups in the compounds to be analyzed.
The researchers then use circular dichroism (CD) and ultraviolet (UV) spectroscopy to directly analyze as little as 1 mg of each complexation reaction mixture. The technique works because the complexes produce much stronger and higher-wavelength CD and UV signals than the original amine groups, making it possible to use CD to measure ee and absolute configuration and UV to assess product yield without substantial interference from signals associated with other molecules in the mixtures. The method can be automated for high-throughput operation, Wolf says.
“The direct analysis of crude asymmetric reaction mixtures is a long-sought goal in the synthetic community,” comments Vadim A. Soloshonok of the University of the Basque Country, whose group developed an earlier version of the complexation reaction. The new approach “has the potential to significantly speed up reaction development at reduced cost and waste production in thousands of industrial and academic laboratories,” he says. The assay has wide substrate scope because many important chiral compounds have amine groups.
Eric Anslyn of the University of Texas, Austin, an expert on ee determination and the editor who handled the Wolf group’s JACS paper, says the technique “is far easier, far more rapid, and involves less waste and effort than current methods.” It is broadly applicable and of great utility, Anslyn adds.
Simple treatment keeps natural polymer from turning into sludge
During biomass processing, tens of millions of metric tons of lignin waste are produced each year. This wasteful sludge could be avoided by simply adding formaldehyde in the early stages of the processing, chemists report. The formaldehyde treatment chemically protects the natural polymer lignin so that it can be transformed into valuable chemical feedstocks.

When chemists add formaldehyde during lignin extraction (left),
they prevent the formation of a dark brown sludge seen without it (right).
Lignin accounts for as much as 30% of plants and trees and is made up of valuable aromatic subunits. “Lignin is one of the few natural sources of aromatics, and aromatics are absolutely essential in our chemical industry,” says Jeremy S. Luterbacher, a chemist at the Swiss Federal Institute of Technology, Lausanne (EPFL), who spearheaded the work.
Despite its rich molecular makeup, lignin becomes a waste product because the process that’s used to separate it from a plant’s cellulose and hemicellulose components—which are valuable biofuel feedstocks—causes irreversible C–C bonds to form within the lignin, creating a sludge. Luterbacher’s team found that formaldehyde could chemically prevent those C–C bonds from forming at two key positions (Science 2016, DOI: 10.1126/science.aaf7810).
Formaldehyde adds a hydroxy¬methyl group at a nucleophilic position on one of lignin’s aromatic components and creates a 1,3-dioxane at a position that can become an electrophile. These two blocking strategies prevent irreversible C–C bond formation between the nucleophile and electrophile in the lignin.
So far, Luterbacher and colleagues have scaled the reaction up to 1 L. They’ve also patented the process and are currently deciding whether to move forward by starting their own company or by partnering with an existing firm.
Chemical & Engineering News