22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы

Organic Synthesis: Site-selective reaction provides a general path to wide range of aryl amines
 By combining two red-hot areas of organic
chemistry—photoredox catalysis and C–H activation—chemists have discovered a method for transforming
aryl C–H bonds into C–N bonds.
By combining two red-hot areas of organic
chemistry—photoredox catalysis and C–H activation—chemists have discovered a method for transforming
aryl C–H bonds into C–N bonds.
The site-selective amination, which links arenes to aromatic nitrogen heterocycles or ammonia, offers chemists an easy way to make derivatives of aromatic compounds. Such a transformation could be a boon for medicinal chemists and agrochemical makers.
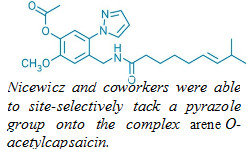
Aromatic nitrogen heterocycles turn up in many biologically active compounds, explains David A. Nicewicz, a chemistry professor at the University of North Carolina, Chapel Hill, who spearheaded the research. The new reaction lets chemists make many different kinds of such molecules using one easy procedure that tolerates numerous functional groups (Science 2015, DOI: 10.1126/science.aac9895). “We think that this will be very valuable to medicinal chemists who are looking to make different derivatives of a lead compound,” Nicewicz says.
This isn’t the only way to transform aryl C–H bonds into C–N bonds, but the reaction offers several advantages over previously reported methods. Unlike in metal-catalyzed aminations, there’s no need to add or remove templating groups to the arene. Also, the arene, which can often be time-consuming to prepare, doesn’t need to be used in excess, as it does in arene aminations that use hypervalent iodine reagents.
The new amination reaction also has the advantage of being site-specific, selectively tacking the nitrogen onto the position para to electron-donating groups on the arene in most cases. The transformation also can be performed late in a synthetic sequence, allowing researchers to add amines onto complex arenes.
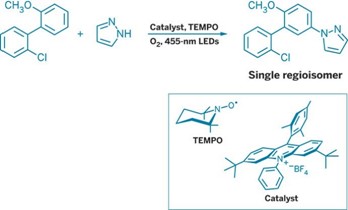
To run the reaction, chemists simply mix an arene with either an aromatic nitrogen heterocycle, such as imidazole or pyrazole, or an ammonia equivalent, such as ammonium carbamate, in the presence of an acridinium catalyst and the cocatalyst 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO). Then the chemists simply irradiate the system with blue light in the presence of oxygen.
The addition of the TEMPO cocatalyst—an idea of coauthor Nathan A. Romero—turned out to be key to getting the reaction to work well, Nicewicz tells C&EN. The chemists think the TEMPO is abstracting a hydrogen atom at a critical stage to form the aromatized product. “TEMPO is an oxygen-centered stable radical,” Nicewicz explains. “Oftentimes, it absolutely stops radical reactions dead in their tracks. But here it turns out to be the catalyst.” Although it’s not the first example of TEMPO behaving this way, he says, “it’s not the first thing you’d grab off the shelf as a cocatalyst for a radical-type reaction.”
 Tehshik Yoon, an expert in photoredox catalysis at the University of Wisconsin,
Madison, says, “This is a really great result, both from a
synthetic perspective and a fundamental photocatalytic perspective.”
Tehshik Yoon, an expert in photoredox catalysis at the University of Wisconsin,
Madison, says, “This is a really great result, both from a
synthetic perspective and a fundamental photocatalytic perspective.”
He is particularly interested in how the reagents combine so productively, noting that there are many reasons why this reaction shouldn’t work. The methodology “offers an elegant, nonobvious design for a really powerful catalyst system,” he says. “It makes you reimagine what might be possible in a photocatalytic system.”
Although there are eight
aromatic C–H bonds in this arene substrate, the reaction
transforms just one of them.
Minuscule metal clusters consisting of just a few atoms of two types of metals can catalyze chemical reactions with extraordinary selectivity if the clusters are supported on a solid and kept isolated from one another.
Those findings, presented Sunday at the American Chemical Society national meeting in Boston, suggest ways to prepare highly effective industrial catalysts from tiny amounts of costly metals.
To prepare the catalytic clusters, Franklin (Feng) Tao of the University of Kansas, Lawrence, and coworkers first synthesized cobalt oxide nanorods, which served as the solid support. Then by using a precipitation method, they deposited Rh3+ species on the nanorod surfaces and followed that with oxidation and reduction steps. High-resolution microscopy and other analytical methods confirmed that the synthesis yielded clusters of RhCo3 uniformly dispersed on the rods.
Then the Kansas team evaluated the clusters’ knack for catalyzing the reduction of NO in the presence of CO to form N2 and CO2. This reaction takes place in some automobiles’ catalytic converters to clean up the vehicles’ emissions. At a symposium organized by the Division of Catalysis Science & Technology, Tao reported that the RhCo3 clusters were highly active and 100% selective in converting NO to N2 at temperatures as low as 110°C. The clusters also outperformed rhodium-cobalt alloy nanoparticles: Depending on how the particles were supported, they were either inactive as catalysts below 250°C or they converted NO to an unwanted mixture of N2 and N2O.
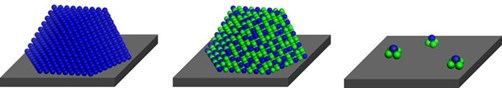
Isolating tiny clusters
(right) of two types of metals (green and blue) can boost catalytic
prowess compared with single-metal (left) and alloy nanoparticles
made from the same metals (center).
Using quantum calculations, the group showed that the difference between Rh-Co clusters and nanoparticles arises from the unique cationic nature of the clusters and the stronger bonds they form with reaction intermediates.
Hong Yang, a professor of chemical engineering at the University of Illinois, Urbana-Champaign, remarks that there has been an exciting development in catalysis in recent years in which researchers have shown that individual atoms can function as active catalytic sites. Tao and colleagues have expanded this line of research, he says, from a single atom to a single RhCo3 cluster, “which shows exceptional selectivity in reducing NO exclusively to N2.”
Grinding a crystalline metal-organic framework (MOF) material in a ball mill converts it into an amorphous structure and then into other crystal morphologies, according to research presented Tuesday at the American Chemical Society national meeting in Boston. The observation suggests researchers could use mechanochemical synthesis as a route to new MOFs.
Tomislav Friščić, a chemistry professor at Canada’s McGill University, reported the work in a symposium sponsored by the Division of Inorganic Chemistry. “We have almost direct proof of nucleation and crystal growth taking place during milling, which is very counterintuitive,” Friščić said.
MOFs are porous materials investigated for use in gas storage, catalysis, separation, and sensing. They are typically prepared from a metal salt and an organic ligand, using organic solvents in a process that often involves high temperature and bases. In contrast, mechanochemical synthesis replaces thermal with mechanical energy and can start from an easily obtainable metal oxide, eliminate the need for bases, and sharply reduce or eliminate solvents.
Friščić and colleagues, including Ivan Halasz of Croatia’s Ruđer Bošković Institute and Trong-On Do of Canada’s University of Laval, explored mechanochemical synthesis of a commercially relevant zeolitic MOF called ZIF-8 by grinding ZnO and 2-methylimidazole (HMeIm) using steel balls in a poly(methyl methacrylate) milling jar (Nat. Commun. 2015, DOI: 10.1038/ncomms7662). The clear milling jar allowed them to monitor the material using an X-ray beam at the European Synchrotron Radiation Facility, in Grenoble, France. They added small amounts of acetic acid or water to the milling jar to catalyze the reaction.
While milling, they initially observed a diffraction pattern that they had expected: ZIF-8, Zn(MeIm)2, in the “sodalite” topology. The diffraction pattern, however, disappeared with further milling, indicating conversion to amorphous material.
Then another diffraction pattern appeared. “That was fully unexpected,” Friščić said. They eventually determined that the new diffraction pattern reflected a new ZIF-8 polymorph not previously observed. The team named it katsenite, after McGill postdoctoral fellow Athanassios D. Katsenis. Ground further, katsenite turns into a third, previously known diamondoid polymorph.
Mechanically synthesizing MOFs in a safer, cleaner way is a great advance, comments Omar Farha of Northwestern University. But seeing new topologies arise from amorphous material after grinding is “spectacular.”
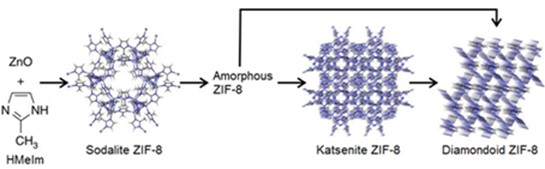
Mechanochemical milling of ZnO with HMeIm first creates the sodalite topology of the ZIF-8 MOF, which then transforms into an amorphous material before reforming into katsenite and diamondoid ZIF-8.
Honors: Annual awards recognize chemical innovations that prevent pollution and promote sustainability
Chemical plants are often vilified for pumping out toxic pollutants from their smokestacks and discharging tainted water from pipes. Environmental laws have gone a long way to curb those problems, but as an added incentive, the Environmental Protection Agency in collaboration with the White House began the Presidential Green Chemistry Challenge Awards in 1996.
This year’s awards were presented on July 13 in a ceremony at EPA headquarters in Washington, D.C. As the name suggests, the awards program challenges chemical companies to do better and recognizes their successes in developing innovative technologies with demonstrable human health and environmental benefits.
Among this year’s winners, LanzaTech took home the Greener Synthetic Pathways Award for developing a microbial fermentation process to convert carbon monoxide and carbon dioxide from steel mill and other industrial waste gas streams into fuels such as ethanol and commodity chemicals such as 2,3-butanediol.
Soltex landed the Greener Reaction Conditions Award for its fixed-bed solid-state catalyst system for manufacturing polyisobutylene, an intermediate used to make additives for lubricants and gasoline. Polyisobutylene is typically synthesized using corrosive liquid formulations of a Lewis acid catalyst such as BF3, which requires costly handling equipment and generates substantial wastewater. Soltex alleviated those problems by creating a BF3-alcohol complex affixed to solid alumina beads.
Hybrid Coating Technologies and Nanotech Industries received the Designing Greener Chemicals Award for creating polyurethane coatings and foam insulation made with cyclic carbonates and amines instead of isocyanates and polyols. Isocyanates are useful chemicals but have long raised safety and health concerns because they are irritants and potential carcinogens.
Renmatix garnered the Small Business Award for its process using supercritical water hydrolysis to deconstruct cellulosic plant material to unlock sugars that can then be used as feedstocks to make biobased fuels and chemicals. The technology offers a cleaner, faster, and more economical alternative to acids, enzymes, and solvents that are typically used to process biomass.
Algenol was given the Climate Change Award for developing genetically enhanced strains of blue-green algae (cyanobacteria) that are highly efficient at producing ethanol and biobased crude oil by feeding on carbon dioxide trapped at industrial facilities. As a bonus, the algae grow in saltwater that can be taken from the ocean rather than using up more precious freshwater resources.
Chemistry professor Eugene Y.-X. Chen of Colorado State University got the nod for the Academic Award for designing greener condensation reactions. These reactions, which fuse molecules together, typically require a metal catalyst and can generate significant waste as unneeded molecule fragments are discarded. Chen’s group developed organocatalysts for derivatizing the biobased feedstock 5-hydroxymethylfurfural and for making polyesters in metal-free, and in some cases solvent-free, processes that are 100% atom-economical.
Chen’s green condensation reactions are being applied to the preparation of unsaturated polyesters and to the derivitization of the biobased feedstock 5-hydroxymethylfurfural (HMF).
Catalysis: Four-atom copper fragments speed up greenhouse gas conversion without piling on the pressure
With the help of the right catalyst, carbon dioxide emitted by fossil-fuel power stations could be used as a chemical feedstock, rather than contributing to greenhouse gas emissions. Researchers have now found that tiny clusters of copper atoms can generate methanol from CO2 at an unusually low pressure (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b03668).
Copper is already used to catalyze industrial methanol production from syngas—a mixture of carbon monoxide, CO2, and hydrogen—at pressures of 10 to 100 atm and temperatures of a few hundred degrees C. But these conditions make the process energy-intensive and costly. “We wanted to use lower pressure to save energy,” says Larry A. Curtiss of Argonne National Laboratory.
Curtiss—working with Peter Zapol, Stefan Vajda, and colleagues—calculated that copper clusters containing four atoms would offer higher catalytic activity than larger clusters or copper surfaces. The smaller clusters have more coordination sites available to bind reaction intermediates, offering a lower-energy reaction pathway.
The team peppered a thin film of alumina with Cu4+ clusters, and then monitored reactions over this catalyst in a gas stream containing 1% CO2, 3% H2, and 96% helium at an overall pressure of 1.25 atm. As they increased the temperature to 125 ºC, the H2 reduced the copper clusters to catalytically active Cu40. Methanol production peaked at 225 ºC, giving the highest reported activity for CO2 reduction to methanol at such a low CO2 partial pressure, a pressure around one-twentieth of that in work with previous catalysts.
Although promising, it remains to be seen whether this would be a chemically—or economically—viable way of utilizing CO2 waste from power plants. For now, Curtiss and his colleagues are looking for clusters that produce longer-chain hydrocarbons from CO2, potentially offering a renewable source of liquid fuels. Preliminary calculations suggest that a Cu20 cluster might be ideal, he says.
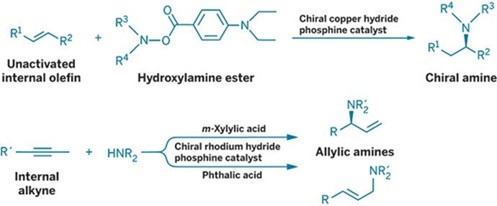
Organic Synthesis: Two groups turn underutilized internal alkenes and alkynes into chiral amines
In a showcase of metal hydride catalysis, two research groups have developed reactions to transform simple unsaturated building blocks—alkenes and alkynes—into valuable chiral amine products.
What makes these so-called hydroamination reactions noteworthy is that the multiple bonds are located at internal carbon atoms in the molecules rather than at terminal positions. Organic chemists have long considered it a challenge to easily transform the less reactive internal unsaturated bonds.
In the case of alkenes, a team led by Stephen L. Buchwald of Massachusetts Institute of Technology solved the challenge with the help of a chiral copper hydride catalyst incorporating an aromatic bidentate phosphine ligand (Science 2015, DOI: 10.1126/science.aab3753).
In the past, metal hydrides have had a low binding affinity for internal alkenes and the resulting intermediates have reacted sluggishly with nitrogen nucleophiles such as secondary amines. Buchwald’s group flipped that approach around and created a copper catalyst that forms a nucleophilic alkylcopper intermediate, which allows the team to use an electrophilic nitrogen oxidant—a hydroxylamine ester—as the amine source. As a result, chemists can now take a basic building block such as 2-butene and turn it into a range of enantiopure amines.
In the case of alkynes, Vy M. Dong and coworkers at the University of California, Irvine, have developed the first example of an enantioselective alkyne hydroamination (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b05200). The key was exploiting the ability of their catalyst, a rhodium hydride paired with a chiral bidentate aliphatic phosphine ligand, to isomerize alkynes to form an electrophilic rhodium-allyl complex. When treated with a secondary amine, the complex undergoes hydroamination to generate allylic amines.
The researchers found that the choice of an organic acid additive—m-xylylic acid versus phthalic acid—helps guide the hydroamination to selectively form chiral branched amines or linear amines, respectively. All previous alkyne hydroaminations have yielded only achiral enamines, imines, or linear allylic amines.
Between the two transformations, both research groups agree the internal alkene hydroamination ranks higher in importance. “The demonstration of enantioselective copper-catalyzed alkene hydroamination addresses one of the long-standing challenges in catalysis, namely the selective synthesis of nitrogen-carbon bonds from abundant alkene precursors and amines,” says Paul J. Chirik, a chemistry professor at Princeton University and editor-in-chief of the jounal Organometallics. “The Buchwald work also demonstrates the value of integrating theory and experiment to solve important problems in synthetic methodology.”
The new approaches should inspire further advances on the emerging theme of using hydrofunctionalization for preparing complex molecules, Dong says. For example, hydroformylation (the oxo process) to convert an olefin into an aldehyde is already the leading example of homogeneous catalysis in the chemical industry, she notes. Other metal-catalyzed hydrofuctionalizations, such as hydroamination, could follow suit. “They have enormous potential,” Dong says.

Biobased Chemistry: New method for making aviation fuel could cut greenhouse gas emissions
A California-based research team has shown that chemical building blocks derived from sugarcane can be converted in nearly 100% yield to a new family of potentially high-performing jet fuels. An environmental impact analysis of the biobased strategy suggests that it would lead to a reduction of up to 80% in greenhouse gas emissions, compared with an approach that makes the same compounds from petroleum (Proc. Natl. Acad. Sci. USA 2015, DOI: 10.1073/pnas.1508274112).
To reduce global dependence on petroleum and mitigate the harmful effects of climate change, numerous research groups have developed methods for producing biobased car and truck fuels and various types of chemicals. Stringent specifications for aviation fuels, however, have limited researchers’ success in producing those products from biological sources.
For example, aviation fuels need to be formulated from oxygen-free compounds that yield high-energy-density blends with low freezing points and low viscosity values. Those high-altitude requirements are best met with branched and cyclic hydrocarbons, which are not easy to produce from current biobased methods: Some of these approaches to making jet fuels give low yields or are energy intensive.
A new synthesis strategy developed by University of California, Berkeley, scientists Alexis T. Bell, Corinne D. Scown, F. Dean Toste, and coworkers can overcome these obstacles. The team demonstrated that condensing alkyl methyl ketones derived from Brazilian sugarcane, followed by deoxygenation, leads to high-yield cyclic products in the C12 – C45 range that can serve as jet fuels (C12 – C21) as well as automotive lubricants (C33+).
The energy density of the jet fuel compounds exceeds that of conventional jet fuels. And the compounds’ freezing points, cold-flow characteristics, and other viscosity-related properties are superior to those of biobased jet fuels made via other methods. The lubricants’ viscosity-related properties are comparable to high-quality synthetic automotive lubricants, far exceeding those of standard petroleum oils.
Xinhe Bao, a catalysis research group leader at China’s Dalian Institute of Chemical Physics, remarks that the study provides “a viable strategy for sustainable production of jet fuels and lubricant oils in a sugarcane refinery.” He adds that the synthesis strategy, which is based on inexpensive, recyclable catalysts, leads to a substantial reduction in the emissions of greenhouse gases.
The University of Wisconsin’s James A. Dumesic, a biomass conversion specialist, notes that not only is the process efficient, but it’s also industrially scalable. He adds: “This work offers important guidelines to scientists for future research, as well as insight to policy-makers.”
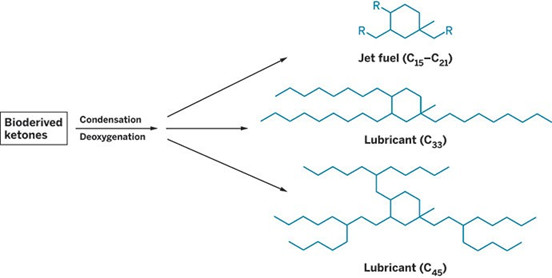
Starting with ketones derived from
sugarcane, researchers have synthesized potential jet fuels and automotive lubricants.
Computational Chemistry: Chemists conduct epic quest for stable new oxides of copper, silver, and gold
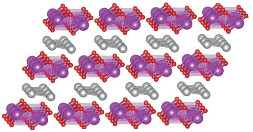
This unusual structure of AgBiO2 was
predicted by a sweeping search for stable, new, structural
variations of three-element copper, silver, and gold oxides. Layers
of BiO2 (purple
and red) separate layers of Ag (silver).
As-yet-undiscovered materials that could be the key to better electronics or solar cells may give the first glimpse of themselves to a computer. A team of theoreticians has now explored the periodic table and identified silver, copper, and gold oxides with never-before-seen structures (Chem. Mater. 2015, DOI: 10.1021/acs.chemmater.5b00716). Their search accelerates the hunt for new materials by establishing that it can be done on a much larger computational scale than pursuits of the past.
The work of finding new, stable combinations of elements is like combing a beach for sand dollars. A beachcomber might do a quick search at the tide line where sand dollars tend to lie. But to find all of them, a seeker must methodically walk the length and width of the beach.
In the past, theoretical chemists have mostly searched for new materials by starting with known crystal structures from nature, switching up the elements, and then looking for stable variations. This quick method works, but it doesn’t find structures different than ones that are already known, says Miguel A. L. Marques of Martin Luther University of Halle-Wittenberg, in Germany.
So Marques set out to show that researchers now have the computational power, codes, and algorithms to perform more thorough searches of hundreds of element combinations. Marques found ways to streamline a technique called global structural prediction and use it for new-materials discovery—the equivalent of walking the entire beach to find sand dollars.
Marques and his coworkers focused on three-element oxides of copper, gold, and silver—183 different combinations. Such oxides tend to be transparent and conducting, and therefore could be useful in touch screens and solar cells. The team wanted to screen for stable configurations and predict whether any might be promising enough for experimental chemists to synthesize and test.
To search for all possible structures of each oxide, the researchers programmed the Curie supercomputer at the Very Large Computing Center (TGCC), in France, to start with a random structure and rearrange it until it found a stable, low-energy configuration. After creating three or four structures this way, the researchers applied a technique that traverses the energy landscape from one relatively stable structure to the next, comparing structures along the way, and repeats this journey until it identifies the ground-state structure with the lowest energy.
After evaluating 21,000 low-energy structures, the computer unearthed 81 stable compositions. Of those, only 36 have been reported. Some of the structures, such as silver bismuth oxide (AgBiO2), have never been observed or predicted before.
Two structures in particular, silver scandium oxide (AgScO2) and silver yttrium oxide (AuYO2), have some predicted properties that make them potential transparent conductors, says Renaud Leturcq of the Luxembourg Institute of Science & Technology. But this is “a preliminary study that requires more precise calculation” to fully evaluate their properties, he adds.
The gold and silver in these candidates means making and using these materials would be expensive, adds M. Geoffroy Hautier of Catholic University of Louvain, in Belgium. Hautier is more excited by how the study applies global structural prediction on a much larger scale than before. “I am sure that people will start doing it more and more,” he says.
Since the study, Marques and his collaborators have compared not just hundreds of compositions at a time, but thousands. “The number we can try,” he says, “is just limited by the size of the supercomputer.”
Chemical & Engineering News