31 января 2015 года исполнилось 75 лет действительному члену Российской академии наук, декану Химического факультета Московского государственного университета имени М.В. Ломоносова, профессору Валерию Васильевичу Лунину.
31 января 2015 года исполнилось 75 лет действительному члену Российской академии наук, декану Химического факультета Московского государственного университета имени М.В. Ломоносова, профессору Валерию Васильевичу Лунину.
Академик В.В. Лунин – известный ученый в области гетерогенного катализа и физической химии поверхности, талантливый педагог и организатор научной и образовательной деятельности, автор более 800 публикаций, в том числе ряда монографий, 70 авторских свидетельств и патентов.
Широкое признание в нашей стране и за рубежом получили фундаментальные исследования физико-химических и каталитических свойств нового класса гетерогенных катализаторов на основе интерметаллических соединений и их гидридов. В.В. Луниным открыто и изучено явление ускорения структурных и фазовых превращений в полиметаллических системах под влиянием водорода гидридных фаз. Под его руководством созданы принципиально новые катализаторы различных реакций промышленного органического синтеза, нефтехимии и нефтепереработки, в том числе углубленной переработки тяжелых нефтяных остатков, синтеза метанола и высших спиртов из СО и Н2, процессов экологической защиты водной и воздушной среды, разложения и утилизации хлорсодержащих органических отходов, новый гетерогенный, не содержащий платину катализатор окисления аммиака (совместно с ИК СО РАН и ГИАП).
Цикл работ В.В. Лунина «Новые гетерогенные катализаторы на основе интерметаллических соединений и их гидридов» отмечен в 1995 г. премией имени А.А. Баландина Президиума РАН.
В 1999 г. В.В. Лунин удостоен Премии Правительства РФ в области науки и техники за разработку и промышленную реализацию технологии двухступенчатого окисления аммиака в производстве азотной кислоты на основе сотового оксидного катализатора.
В.В. Луниным предложен оригинальный способ создания тонких ферромагнитных пленок на поверхности немагнитных соединений.
За работу «Полиядерные соединения: молекулярные магнетики и катализ» В.В. Лунину присуждена Государственная премия РФ в области науки и техники за 2003 г.
Значительный фундаментальный и практический интерес представляет цикл работ по воздействию нетрадиционных видов энергии на физико-химические и каталитические свойства твердого тела. Ведутся исследования по использованию сверхкритических сред для синтеза наноматериалов с нетрадиционными свойствами, в том числе сложных оксидов, перспективных катализаторов окисления; работы по химии озона, в том числе по его применению в очистке сточных вод, гетерогенно-каталитических процессах, делигнификации лигнинсодержащих материалов. Разработан и внедрен на ряде отечественных и зарубежных производств новый, устойчивый в содержащих влагу средах катализатор разложения и удаления остаточных количеств озона.
Цикл работ В.В. Лунина «Исследование физико-химических основ синтеза озона, разработка и широкое внедрение принципиально новых лечебных технологий с использованием озона» в 2006 году отмечен Премией Правительства Российской Федерации в области науки и техники. В 2009 году В.В. Лунину присуждена Премия имени В.Н. Ипатьева Президиума РАН за работу «Физико-химические основы промышленной технологии производства водостойких катализаторов очистки газов от озона».
В 2010 году В.В. Лунин с сотрудниками удостоен Ломоносовской Премии Ученого Совета МГУ за работу «Эффекты синергизма в промышленных процессах гидрирования и гидродехлорирования».
Более двадцати лет Валерий Васильевич возглавляет химический факультет МГУ, который является признанным лидером в химическом образовании и науке как в России, так и на мировом уровне. Под руководством В.В. Лунина сложилась активная научная школа. Он подготовил 7 докторов и более 80 кандидатов наук.
Профессор кафедры физической химии с 1987 г., В.В. Лунин читает курсы лекций «Теоретические основы приготовления катализаторов» и «Химия каталитических процессов». Регулярно выступает с лекциями в университетах Германии, Италии, Японии, США, Франции и Китае. Избран Почетным доктором Архангельского государственного технического университета, Башкирского государственного университета, Санкт-Петербургского государственного университета, Казанского (Приволжского) Федерального университета, а также почетным членом Академии наук Республики Татарстан.
В.В. Лунин – автор учебника «Основы физической химии. Теория и задачи», базового школьного учебника по химии для 8-11 классов, ряда учебно-методических изданий. Под его руководством ежегодно проводится Международная Менделеевская Олимпиада, в которой участвуют школьники России, стран СНГ и Балтии, Болгарии, Румынии, Македонии, Венгрии, Турции, Саудовской Аравии, Кувейта. За крупный вклад в развитие образования и педагогическую деятельность В.В. Лунин награжден нагрудным знаком «Почетный работник высшего профессионального образования РФ», в 1997 г. удостоен Ломоносовской Премии за педагогическую деятельность, в 1998 г. получил Премию Президента РФ в области образования за разработку концепции «Новые подходы к взаимодействию средней и высшей школы в области химического образования, Премию Правительства Москвы за проведение эффективной работы по взаимодействию с общеобразовательными учреждениями (2001 г.). Лауреат конкурса «Грант Москвы» в области наук и технологий, в сфере образования. Лауреат Главной премии МАИК «Наука. Интерпериодика» за лучшую публикацию 2001 г. в издаваемых ею журналах (2002 г.). Лауреат Премии «Фонда содействия отечественной науке» в номинации «Выдающиеся ученые России».
В.В. Лунин ведет огромную научно-организационную работу. C 1992 г. он – заведующий кафедрой физической химии, председатель Ученого Совета Химического факультета МГУ, член нескольких специализированных Ученых Советов, главный редактор “Вестника Московского университета. Серия химия” и “Журнала физической химии”, с 2006 г. – главный редактор журнала «Сверхкритические флюиды. Теория и практика», член редколлегий ряда научных журналов; заместитель академика-секретаря Отделения Общей и технической химии РАН, Председатель Научного совета РАН по химии и технологии твердого ископаемого топлива, член ряда Научных советов РАН, председатель и член ряда специализированных Советов ВАК. Заместитель председателя Центрального комитета по проведению предметных олимпиад школьников Минобрнауки РФ. С 1992 г. – председатель Учебно-методического объединения Университетов России по химии, Сопредседатель и Президент Оргкомитета Международных химических олимпиад в Москве в 1996 и 2007 гг. Член Президиума РХО им. Д.И.Менделеева. Член Попечительского Совета Ассоциации выпускников МГУ в Германии.
В 2005 году В.В.Лунин избран почетным иностранным членом Общества Лейбница “Societas Leibnitiana Berolinensis ” (Германия) за многолетнее плодотворное научное сотрудничество между химфаком МГУ и университетами Германии.
В 2008 г. награжден Юбилейной медалью к 175-летию со дня рождения Дмитрия Ивановича Менделеева «За активную работу по изучению и пропаганде наследия великого русского ученого Д.И.Менделеева».
В 1990-1993 гг. – народный депутат РФ.
За многолетнюю активную научную, педагогическую и общественную деятельность Валерий Васильевич Лунин отмечен многочисленными наградами, среди них орден Почета (2000 г.), орден «За заслуги перед отечеством IV степени» (2005 г.), орден «За заслуги перед химической индустрией России» I степени (2010 г.), орден Дружбы (2011 г.).
Научный совет по катализу ОХНМ РАН и редакция Каталитического бюллетеня сердечно поздравляют Валерия Васильевича с юбилеем, желают ему крепкого здоровья и новых творческих достижений!

Отчет о научно-организационной деятельности в 2014 году
Секретариат Научного совета по катализу ОХНМ РАН (НСК) предлагает Вашему вниманию сводный отчет о деятельности Совета и научных исследованиях в области катализа, выполненных научными коллективами под руководством членов Научного совета по катализу.
Отчет состоит из трех разделов:
Тексты отчетов, полученные от членов НСК и научно-исследовательских коллективов, практически не подвергнуты корректировке.
В 2014 году в рамках научно-организационной деятельности Научного совета по катализу ОХНМ РАН (НСК) были выполнены следующие мероприятия.
1. Выпущены четыре ежеквартальных сборника «Каталитический бюллетень», содержащие оперативную информацию о важнейших результатах фундаментальных и прикладных исследований в области катализа в России и за рубежом, материалы, посвященные деятельности выдающихся отечественных и зарубежных исследователей в области катализа; дается перечень предстоящих конференций, краткие отчеты о проведенных конференциях, рабочих совещаниях и другие материалы.
2. Под эгидой Научного совета по катализу и при активном участии его членов организованы и проведены следующие конференции:
Изданы материалы проведенных конференций.
Секретариат НСК ведет переписку и текущую работу с членами Научного совета по катализу ОХНМ РАН.
Продолжается сотрудничество с организациями Академий наук РФ и стран СНГ, Министерствами РФ, институтами разных ведомств и другими организациями России, дальнего и ближнего зарубежья по различным вопросам научной, научно-организационной, учебно-преподавательской и общественной деятельности в области катализа.
Катализ хиральными кислотами Льюиса
Осуществлен асимметрический вариант реакции Фриделя-Крафтса на примере реакции индола с арилиденмалонатами с помощью иммобилизованного на полимерную матрицу катализатора состава iPr-Box/Cu(OTf)2; катализатор сохраняет активность в 5 циклах, продукты реакции получены с выходами до 99% и с энантиомерными избытками до 92% ее, а после перекристаллизации энантиомерная чистота была увеличена до 99% ее.
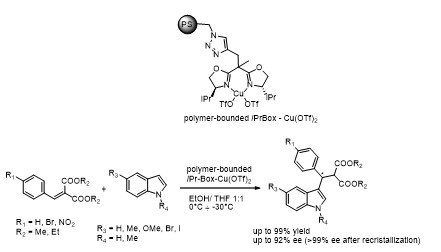
академик И.П. Белецкая
Институт физической химии и электрохимии
им. А.Н. Фрумкина РАН, г. Москва
Определены оптимальные условия формирования Fe- и Co-содержащих наноразмерных катализаторов Фишера-Тропша, полученных in situ в реакционной среде в условиях трехфазного процесса в сларри-реакторе. Варьировались такие параметры синтеза как природа дисперсионной среды, природа дисперсной фазы, природа и концентрация прекурсора железа, а также природа и концентрация ПАВ. Выявлены зависимости размера частиц эмульсий от природы и концентрации оксидных промоторов. Установлены оптимальные условия восстановительного термолиза эмульсии in situ в сларри-реакторе для получения стабильных наноразмерных Fe- и Co-содержащих суспензий.
академик С.Н. Хаджиев, к.х.н. М.В. Куликова
Институт нефтехимического синтеза
им. А.В. Топчиева РАН, г. Москва
Получены силикоалюмофосфатные катализаторы для синтеза низших олефинов из метанола с повышенной стабильностью
работы во времени. Эта цель была достигнута за счет модифицирования внешней поверхности
катализаторов либо покрытием поверхности кристаллов SAPO аморфным оксидом кремния (до 2 масс. % по кремнию), либо методом рекристаллизации, создающим на поверхности силикоалюмофосфата слой мезопористой фазы. Данный
подход к модифицированию катализаторов позволил покрыть/дезактивировать поверхностные кислотные центры, ответственные за олигомеризацию низших олефинов. Опыты по конверсии метанола в
низшие олефины показали, что модифицирование позволяет увеличить время стабильной работы катализатора в 2 раза, а также повысить селективность по олефинам С2-С4
за счет снижения вклада продуктов С5+.
академик С.Н. Хаджиев, д.х.н. И.И. Иванова
Институт нефтехимического синтеза
им. А.В. Топчиева РАН, г. Москва
Для малогабаритных электростанций нового поколения в ИНХС РАН (руководитель работы д.х.н. М.В. Цодиков) совместно с ИСМАН РАН (к.т.н. В.И. Уваров) разработаны пористые мембранно-каталитические конвертеры, проявляющие повышенную активность в паровом и углекислотном риформинге метана или продуктов ферментации биомассы (в том числе без их разделения на индивидуальные компоненты). Конверторы апробированы для работы с топливными элементами любого типа (Институт высокотемпературной электрохимии УРО РАН, руководитель д.х.н. Зайков Ю.П.). Пористые мембранно-каталитические системы, внутренние каналы которых модифицированы наноразмерными катализаторами, позволяют переработать органические субстраты в синтез-газ и водород при малом времени контакта – 0,05-0,1 с, благодаря чему достигается рекордная производительность по синтез-газу (на уровне 85000 л/ч дм3мембр). На модельной лабораторной установке, где конвертор интегрирован с высокотемпературным твердооксидным ТЭ, были достигнуты высокие показатели по выработке электроэнергии (I – 300 мА/см2, W – 150 мВт/см2). Один конвертер с габаритами 14×1,5 см способен обеспечить первичным топливом (синтез-газ) до 10 ТЭ мощностью до 100 Вт. В развитие этого направления разработаны оригинальные гибридные конвертеры, способные обеспечить очищенным и ультрачистым водородом ТЭ, работающие при средних (120-150°C) и низких (20-30°C) температурах. Для среднетемпературных ТЭ содержание СО в водороде не должно превышать 2%. В этом случае во внутреннем объеме гибридного конвертера размещают гранулированный катализатор парового риформинга СО. Для низкотемпературных ТЭ разработан и испытан оригинальный конвертер, который во внутреннем объеме содержит мембрану в виде трубки, изготовленную из палладий-содержащего сплава. В этом конвертере наряду с синтез-газом состава Н2/СО – 2,7 из реакционной зоны выделяется до 52% ультрачистого водорода.
д.х.н. М.В. Цодиков, к.х.н. А.С. Федотов, Д.О. Антонов,
к.т.н. В.И. Уваров, д.х.н. В.Н. Корчак, академик И.И. Моисеев
Институт
нефтехимического синтеза им. А.В. Топчиева РАН, г. Москва
Институт структурной макрокинетики и проблем материаловедения РАН, г. Черноголовка
Институт общей и неорганической химии
им. Н.С. Курнакова РАН, г. Москва
Одним из приоритетных направлений современной химической энергетики является разработка высокоэффективных процессов переработки возобновляемого сырья в ценные мономеры и топливные компоненты.
В 2014 г. совместно ИНХС РАН и ИОНХ РАН разработан катализатор селективного превращения рапсового масла в узкие алкан-олефиновые фракции. На основе гетерометаллического Pt-Sn-содержащего комплекса разработан катализатор, содержащий биметаллические активные компоненты на поверхности γ-Al2O3. Установлено, что при соотношении активных компонентов Sn/Pt = 5 триглицериды жирных кислот рапсового масла селективно превращаются в узкие фракции углеводородов С3 и С18. Показано, что глицериновый фрагмент более чем на 30% превращается в пропан-пропиленовую фракцию, а углеводородные фрагменты жирных кислот более чем на 90% – в алкан-олефиновую фракцию С18. Кислород триглицерида жирных кислот селективно восстанавливается в воду, в результате чего выход «тупиковых» С1 и С2 в этом процессе не превышает 0,7%. В составе фракции углеводородов С18 содержится до 22% ценных линейных олефинов. Изучение структуры активных компонентов методом ПЭМ ВР показало, что при соотношении активных компонентов Sn/Pt = 5 размеры активных металлсодержащих компонентов на поверхности носителя ≤ 1 нм. При столь малом размере активных компонентов катализатора взаимодействие ТГЖК с активными центрами протекает лишь по кислородным атомам молекулы липида, приводя к селективной деоксигенации и практически не затрагивая углеводородные фрагменты молекулы. Таким образом, полученный результат позволяет минимизировать потери углеводородной компоненты исходной биомассы при ее переработке в ценные компоненты топлив и мономеры.
д.х.н. М.В. Цодиков, к.х.н. А.В. Чистяков, П.А. Жарова,
к.х.н. С.С. Шаповалов, д.х.н. А.А. Пасынский, к.х.н. В.Ю. Мурзин,
чл.-корр. РАН А.Е. Гехман, академик И.И. Моисеев
Институт нефтехимического синтеза им. А.В. Топчиева РАН,
Институт общей и неорганической химии им. Н.С. Курнакова РАН, г. Москва
Разработана и введена в эксплуатацию высокочувствительная установка, обеспечивающая in situ контроль топохимических превращений при синтезе катализаторов, содержащих наночастицы ферромагнитных металлов. Установка выполнена на базе вибрационного магнитометра и обеспечивает рабочие температуры в зоне реакции от 300 до 870 К при пропускании сквозь реакционную зону газов (смеси газов) со скоростью до 150 см3/мин. Приготовлены высокоактивные катализаторы синтеза Фишера-Тропша на основе Fe-содержащих композитных материалов. Разработан аппаратурный комплекс, позволяющий проводить исследования механизмов синтеза цеолитных катализаторов с помощью ЯМР под давлением и в агрессивных средах, установлены общие принципы и конкретные методические подходы метода ЯМР ВМУ in situ для исследования гидротермального синтеза и пост-синтетического модифицирования цеолитных и композитных катализаторов на их основе. Разработанный подход лег в основу проекта РНФ, направленного на создание научных основ рационального дизайна катализаторов важнейших процессов нефтегазового комплекса, включающих крекинг, алкилирование, олигомеризацию, дегидратацию и конденсацию углеводородов и спиртов.
академик В.В. Лунин, Н.Н. Кузнецова
Московский государственный университет имени М.В. Ломоносова,
Химический факультет, г. Москва
В рамках соглашения с РНФ 14-33-00018 методом пиролиза древесных опилок, пропитанных нитратом палладия, получены катализаторы Pd/C, содержащие на поверхности, по данным рентгеновской фотоэлектронной спектроскопии, только восстановленные наноразмерные частицы палладия (2-4 нм), нанесенные на активированный уголь, сохраняющий элементы пористой структуры древесины. Применение дополнительной обработки опилок перед пиролизом позволило увеличить удельную поверхность получаемых систем с 7 м2/г (без обработки) до 230 м2/г (обработка водой в гидротермальных условиях). Полученные каталитические системы проявили высокую активность в реакции гидродехлорирования хлорбензола в паровой фазе в интервале температур 150-350°C.
В рамках проекта РФФИ 13-03-00613 с использованием цетилтриметиламмонийбромида или древесных опилок в качестве темплата получены системы CuO-CeZrO2, проявляющие заметную активность в окислении СО в широком температурном диапазоне (100-450°C) и имеющие развитую пористую структуру, обусловленную темплатом. Методами рентгенофазового анализа и рентгеновской фотоэлектронной спектроскопии показано, что оксид меди присутствует в виде отдельной фазы, а другие компоненты образуют сложный оксид.
академик В.В. Лунин, д.х.н. Е.С. Локтева,
к.х.н. Е.В. Голубина, К.И. Маслаков
Московский государственный университет имени М.В. Ломоносова,
Химический факультет, г. Москва
Предложен новый метод приготовления катализаторов синтеза Фишера-Тропша, заключающийся в нанесении металлического кобальта на носитель в момент восстановления из солей раствором NaBH4.
Гидроксикарбонилированием стирола получены фенилпропионовые кислоты с выходом 97,5% при соотношении Pd : стирол = 1 : 200 и температуре 100°C. Селективность по целевой 2-фенилпро-пионовой кислоте 98,8%. Впервые обнаружена высокая каталитическая активность комплексов HRh(CO)(PPh3)3, RhCl(CO)(PPh3)2 в реакции восстановительного карбонилирования иодобензола.
чл.-корр. РАН А.Л. Лапидус
Институт органической химии им. Н.Д. Зелинского, г. Москва
Установлено, что при нанесении K2[Ru4(CO)13], K2[Os3(CO)11], K2[Fe2(CO)8] и KRe(CO5) на уголь «Сибунит» с последующим термическим разложением нанесённого карбонилметаллата в токе водорода или аргона образуются системы, способные катализировать реакции перераспределения изотопов водорода в метане, этилене и ацетилене, а также дейтероводородный обмен между метаном и D2. В случае этилена и ацетилена изотопный обмен на наиболее активных катализаторах протекает с высокой скоростью уже при комнатной температуре, а в случае метана – начиная со 150°C. На основании данных ГР-спектров сделан вывод, что активность вышеуказанных железных катализаторов в H/D обмене обусловлена железосодержащими аморфными сплавами не установленной пока природы, образующимися при термическом разложении нанесённого K2[Fe2(CO)8]. α-Железо в ходе распада K2[Fe2(CO)8] в токе H2 или Ar практически не образуется. Таким образом, впервые продемонстрирована возможность использования систем на основе нанесённых карбонилметаллатов калия для активации C–H-связей углеводородов.
д.х.н. В.Б. Шур, С.М. Юнусов, З. Руммель, М. Херман, Е.С. Калюжная
Институт элементоорганических соединений им. А.Н. Несмеянова РАН, г. Москва
Институт модификации поверхности им. Лейбница,
Лейпциг, Германия
Проведена серия работ по моделированию структуры, свойств ферментов и катализируемых ими химических превращений многоуровневыми методами квантовой механики/молекулярной механики (КМ/ММ). Характеризован полный каталитический цикл гидролиза пенициллина G ферментом пенициллинацилазой; предложены оригинальные способы ингибирования матриксных металлопротеиназ in situ; объяснена роль канцерогенных точечных мутаций в белке Ras при гидролизе гуанозинтрифосфата белковым комплексом Ras-GAP.
чл.-корр. РАН С.Д. Варфоломеев, Немухин А.В., Григоренко Б.Л.
Институт биохимической физики им. Н.М. Эмануэля РАН, г. Москва
В рамках проекта «Биокаталитическое получение органических кислот из непищевого сырья как мономеров для органического синтеза биопластиков» разработана серия оригинальных высокоактивных биокатализаторов (7 шт.) в виде иммобилизованных в криогель поливинилового спирта клеток микроорганизмов для получения органических кислот (янтарной, яблочной, фумаровой, аспарагиновой) из разнообразного возобновляемого углеродосодержащего сырья, способных функционировать длительное время (до 2000 ч) в различных исходных по характеристикам средах с максимальным выходом целевого продукта до 90% от теоретически возможного уровня. Впервые установлена эффективность использования в качестве сырья гидролизатов целлюлозосодержащей биомассы, биомассы фототрофных микроорганизмов и макроводорослей и глицерина (отхода производства биодизеля) для получения органических кислот с использованием разработанных биокатализаторов. По широте спектра образцов возобновляемого сырья, конвертируемых в фумаровую и янтарную кислоты, разработанные биокатализаторы мировых аналогов не имеют.
чл.-корр. РАН С.Д. Варфоломеев
Институт биохимической физики им. Н.М. Эмануэля РАН,
Кафедра химической энзимологии Химического факультета МГУ им. М.В. Ломоносова, г. Москва
В рамках Программы фундаментальных исследований Президиума РАН «Энергетические аспекты глубокой переработки ископаемого и возобновляемого углеродсодержащего сырья» разработан метод синтеза легкого аналога биодизеля, состоящего из этиловых эфиров уксусной, пропионовой, масляной кислот, получаемых в результате биокаталитической переработки углеводистых отходов растительного происхождения. Проведены испытания эфиров в составе топливных композиций, зарегистрирован значительный октанповышающий эффект. Исследуется возможность применения сверхкритической флюидной технологии к синтезу биодизеля и его легкого аналога на основе низших жирных кислот, продуцируемых при биокаталитической переработке растительного сырья. Имеющийся задел позволяет прогнозировать большую экономичность указанных процессов за счет сокращения их длительности, снижения расхода реагентов, отказа от использования катализатора.
чл.-корр. РАН С.Д. Варфоломеев, В.Б. Вольева, И.С. Белостоцкая, Н.Л. Комиссарова, А.В. Малкова
Институт биохимической физики им. Н.М. Эмануэля РАН, г. Москва
Проведено исследование кинетики биомиметического окисления алканов в системах на основе порфириновых комплексов металлов и бензальдегида. Установлено, что в процессе окисления алканов, катализируемом порфиринами железа и марганца, в исследованных системах параллельно образуются спирт, кетон и гидропероксид. Обнаружено влияние лигандного окружения металла на соотношение молекулярного и радикального маршрутов окисления алканов в этих биомиметических системах. Полученные результаты являются важным шагом на пути создания теоретической базы для разработки методов селективного окисления алканов.
чл.-корр. РАН С.Д. Варфоломеев, Е.И. Карасевич
Институт биохимической физики им. Н.М. Эмануэля РАН, г. Москва
Методом ГЖХ (с привлечением компьютерных программ обработки экспериментальных данных Mathcad and Graph2Digit) установлен механизм катализа комплексами {LiSt•DMF(HMPA)} в селективном окислении этилбензола молекулярным кислородом в α-фенилэтилгидропероксид (ФЭГ) (селективность SФЭГ = 90-93%, конверсия в ФЭГ C = 20-23%), являющийся полупродуктом многотоннажного производства мономеров: пропиленоксида и стирола. Рост параметров SФЭГ и C при катализе комплексами {LiSt•DMF(HMPA)} по сравнению с катализом LiSt и некатализированным окислением этилбензола связан с изменением механизма образования побочных продуктов – ацетофенона (АФ) и метилфенилкарбинола (МФК): последовательное (из ФЭГ) → параллельное ФЭГ, и торможением гетеролиза ФЭГ. Впервые с помощью метода АСМ продемонстрирована возможность формирования супрамолекулярных наноструктур на основе комплексов Ni(acac)2•ГМФА•PhOH(«А») за счёт межмолекулярных H-связей, что позволяет объяснить стабильность катализатора («А») в процессе селективного окисления этилбензола в ФЭГ, катализируемом «А».
чл.-корр. РАН С.Д. Варфоломеев, Л.И. Матиенко, Л.А. Мосолова
Институт биохимической физики им. Н.М. Эмануэля РАН, г. Москва
Продолжаются исследования и разработки новых катализаторов глубокой гидроочистки нефтяных фракций и остатков. Разработаны составы и способы синтеза Co(Ni)Mo и NiW катализаторов на основе широкопористого Al2O3, полученного с использованием мезоструктурированного гидроксида алюминия. Катализаторы позволяют провести глубокую гидроочистку тяжелого вакуумного газойля до остаточного содержания серы менее 500 ppm при 360°C, давлении водорода 5 МПа, объемной скорости подачи сырья 1.0 ч–1 и Н2/сырье 800 нл/л.
к.х.н. П.А. Никульшин, П.П. Минаев, Ал.А. Пимерзин, к.х.н. А.В. Можаев,
д.х.н. А.А. Пимерзин
Самарский государственный технический университет, г. Самара
Продолжаются исследования способов направленного формирования активной фазы сульфидных катализаторов гидроочистки. Исследовано влияние состава NiWS катализаторов, природы и текстурных характеристик носителя на каталитические свойства в реакциях гидрообессеривания, гидрирования и гидродеазотирования. Удельные активности катализаторов возрастают с увеличением содержания Ni в составе частиц NiWS фазы и их дисперсности. Показано, что частота оборотов в изученных реакциях растет с уменьшением средней длины частиц активной фазы и степени промотирования ребер кристаллитов WS2.
к.х.н. П.А. Никульшин, П.П. Минаев, к.х.н. А.В. Можаев, М.С. Куликова, д.х.н. А.А. Пимерзин
Самарский государственный технический университет, г. Самара
Ведутся исследования и разработки новых сульфидных катализаторов совместной гидроочистки нефтяных фракций и растительного (возобновляемого) сырья с целью возможного расширения сырьевой базы нефтепереработки. В зависимости от природы носителя частота оборотов для СоМо центров катализаторов снижается в ряду: SiO2 > Al2O3 > ZrO2 ≈ TiO2. Дальнейшее совершенствование катализаторов гидродеоксигенации может развиваться путем снижения длины частиц активной фазы, увеличивая содержание активных центров и оптимизируя степень декорирования промотором кристаллитов MoS2.
к.х.н. П.А. Никульшин, В.А. Сальников, к.х.н. А.В. Можаев, А.Н. Варакин, д.х.н. А.А. Пимерзин
Самарский государственный технический университет, г. Самара
Магнитные наночастицы могут использоваться в каталитических системах для магнитного отделения катализаторов от реакционной среды. Один из известных методов синтеза монодисперсных частиц оксида железа или феррита кобальта, которые стабильны на воздухе, это высокотемпературное разложение кислородсодержащих соединений железа и кобальта, таких как ацетилацетонаты, ацетаты или олеаты в растворах, содержащих ПАВ. За отчетный период разработаны способы формирования магнитных наночасти в полимерных матрицах, а именно в дендримерах и сверхсшитом полистироле. Узкое распределение магнитных наночастиц по размерам обеспечивается благодаря разделению по температурам зародышеобразования и роста наночастиц. В зависимости от реакционных условий можно варьировать размер, форму и кристаллическую структуру таких наночастиц, а следовательно, их магнитные свойства. Разработаны методики синтеза наночастиц каталитически активных металлов, таких как рутений, платина, палладий, иммобилизованных на магнитные частицы. Магнитноотделяемые катализаторы протестированы в реакциях селективного гидрирования и окисления.
Разработаны новые подходы для получения материалов, содержащих одновременно магнитные и каталитические частицы. При этом обеспечивается высокая магнитная восприимчивость таких материалов. С другой стороны, это не сказывается отрицательно на свойствах каталитических частиц, а наоборот, усиливает каталитическую активность и селективность.
Магнитные свойства разрабатываемых каталитических систем позволят быстрое и количественное отделение катализаторов от реакционных растворов, обеспечивая многократное использование катализаторов, приводящее к удешевлению процессов и соответствующих продуктов, существенным энергосбережениям благодаря исключению стадии фильтрации и улучшению качества целевых продуктов.
д.х.н., проф. Э.М. Сульман
Тверской государственный технический университет, Институт нано- и биотехнологий, г. Тверь
С целью разработки селективных гетерогенно-каталитических способов получения пиридина и его метилпроизводных – важных промежуточных соединений для синтеза фармацевтических препаратов, гербицидов, ингибиторов коррозии металлов, ускорителей вулканизации каучука, традиционных лигандов при химической сборке координационных соединений – исследован в проточном реакторе синтез пиридинов с участием этанола, формальдегида и аммиака в присутствии цеолитных катализаторов различного структурного типа: H-Beta (структурный тип BEA), H-ZSM-12 (MTW), H-ZSM-5 (MFI). Установлено, что наиболее активным в синтезе пиридинов является цеолит H-Beta, на котором конверсия этанола достигает 60-70% (300-400°C, 2 ч–1). Традиционно используемый для синтеза пиридинов пентасил H-ZSM-5 в изученных условиях проявляет меньшую активность (конверсия спирта 30-40%). Обнаружено, что основными продуктами реакции на цеолитах H-Beta и H-ZSM-5 являются пиридин и метилпиридины, на цеолите H-ZSM-12 дополнительно образуется до 20% диметилпиридинов. Селективность образования пиридина максимальна (49%) при 200°C, 2 ч–1. Показано, что повышение температуры, уменьшение объемной скорости и концентрации формальдегида в сырьевой смеси приводит к повышению в составе продуктов реакции количества метилпиридинов и «тяжёлых» соединений.
чл.-корр.
РАН У.М. Джемилев, д.х.н. Н.Г. Григорьева, д.х.н. Б.И. Кутепов
Институт нефтехимии и катализа РАН, г. Уфа
В развитие проводимых исследований по синтезу новых N,S-макрогетероциклов, перспективных в качестве биологически активных соединений, сорбентов благородных и редких металлов, разработан эффективный метод синтеза 3-арил-1,5,3-дитиазацикланов и 6-арил-1,11-диокса-4,8-дитиа-6-азациклотридеканов циклоаминометилированием α,ω-дитиолов (бутан-1,4-, пентан-1,5-, гексан-1,6-дитиолы, 3,6-диоксаоктан-1,8-дитиол) с помощью N,N-бис(метоксиметил)-N-ариламинов с участием Sm- и Сu-содержащих катализаторов. Установлено, что при взаимодействии N,N-бис(метоксиметил)-N-фениламина с эквимольным количеством бутан-1,4-дитиола в условиях (~ 20°C, EtOAc, 6 ч) с использованием 5 мол % катализатора Sm(NO3)3/γ-Al2O3 образуется 3-фенил-1,5,3-дитиазонан с выходом ~90%. В разработанных условиях [5 мол % Sm(NO3)3/γ-Al2O3, 20°C, 6 ч] в реакцию циклоаминометилирования с бутан-1,4-дитиолом были вовлечены N,N-бис(метоксиметил)-N-арил(м-метилфенил-, м-метоксифенил-, п-хлорфенил-, м-бромфенил- и п-бромфенил)амины с получением соответствующих 3-арил-1,5,3-дитиазонанов с выходами 85-90%. С участием гетерогенного катализатора Sm(NO3)3/γ-Al2O3 осуществлен селективный синтез 3-арил-1,5,3-дитиазеканов и 3-арил-1,5,3-дитиазациклоундеканов с выходами 75-80%. Катализируемое CuCl циклоаминометилирование α,ω-алкандитиолов и 3,6-диокса-1,8-октандитиола с помощью N,N-бис(метоксиметил)-N-ариламинов проходит по типу [1+1] циклоконденсации и позволяет осуществить одностадийный синтез перспективных N-арилзамещенных 1,5,3-дитиазамакрогетероциклов различной структуры с высокими выходами.
чл.-корр. РАН У.М. Джемилев, к.х.н. Н.Н. Махмудиярова, асп. Л.В. Мударисова, д.х.н., проф. А.Г. Ибрагимов
Институт нефтехимии и катализа РАН, г. Уфа
Разработаны новые, малостадийные методы синтеза алмазоподобных углеводородов – диамантана и триамантана. Сокращение числа стадий синтеза достигнуто благодаря использованию новых С14H20 и С18H24 углеводородов – прекурсоров, представляющих легкодоступный димер норборнадиена экзо-экзо-гексацикло[9.2.1.02,10.03,8.04,6.05,9]тетрадецена-12 и его [4+2]-аддукта с бутадиеном.
Установлено, что эффективными катализаторами скелетной изомеризации указанных углеводородов в диамантан и триамантан являются неорганические ионные жидкости [AlCl3]n·[R3N]m·HCl, которые позволяют провести процесс в мягких условиях (40-50°C, 8 ч) и с высоким выходом, 82 и 80%, соответственно.
Доступность исходных мономеров, высокие выходы целевых каркасных соединений, простота осуществления реакций делают эти методы исключительно перспективными для синтеза уникальных мономеров для специальных полимерных материалов, прекурсоров для получения противовирусных лекарственных препаратов.
д.х.н., проф. Р.И. Хуснутдинов, к.х.н. Р.И. Аминов, чл.-корр.РАН У.М. Джемилев
Институт нефтехимии и катализа РАН, г. Уфа
В развитие проводимых в лаборатории фундаментальных исследований по разработке эффективных методов введения в молекулы углеродных кластеров функциональных групп, базирующихся на выдвинутой нами ранее идее о возможности использования фуллеренов в качестве олефиновой компоненты в реакции Кулинковича, впервые показано, что нитрилы вступают в реакцию с С60-фуллереном в присутствии небольшого избытка EtMgBr и катализатора Ti(Oi-Pr)4 (5 мол.%) c селективным образованием ранее не описанных фуллеротетрагидропиридинов с выходами 15-40%. Замена нитрилов на алкилиденцианоацетаты в данной реакции позволяет направить указанную реакцию в сторону формирования ранее труднодоступных индивидуальных фуллероаминоциклопропанов, что создает перспективы для разработки на основе упомянутых выше производных фуллеренов промышленно важных материалов и веществ с полезными свойствами.
чл.-корр. РАН У.М. Джемилев, асп. З.Р. Шакирова, к.х.н. А.Р. Туктаров
Институт нефтехимии и катализа РАН, г. Уфа
Разработан бестемплатный способ золь-гель синтеза мезопористых каталитически активных алюмосиликатов с использованием тетраэтилортосиликата или его олигомерных эфиров и водно-спиртового раствора нитрата алюминия. Способ позволяет синтезировать мезопористые материалы с весьма узким распределением пор, однородной структурой и высокой кислотностью поверхности. Показано, что синтезированные алюмосиликаты являются эффективными катализаторами кислотно-основного типа, перспективными для применения в таких промышленно значимых реакциях, как олигомеризация различных олефинов и алкилирование фенолов олефинами.
чл.-корр. РАН У.М. Джемилев, д.х.н. Н.Г. Григорьева, асп. М.Р. Аглиуллин, д.х.н. Б.И. Кутепов
Институт нефтехимии и катализа РАН, г. Уфа
Синтезированы новые гибридные органо-неорганические материалы – нанокристаллические оксидные вольфрамовые бронзы на углеродных поверхностях в виде гексагональных и тетрагональных кристаллических структур. Полученные материалы проявляют на порядок большую каталазную активность по сравнению с порошковым материалом.
Результаты работы опубликованы в следующей статье: Петров Л.А., Шишмаков А.Б., Вакарин С.В., Семерикова О.Л., Меляева А.А., Микушина Ю.В., Зайков Ю.П., Чупахин О.Н. Поведение наноразмерных оксидных вольфрамовых бронз, полученных высокотемпературным электролизом в модельных процессах обессеривания нефтепродуктов // Журнал неорганической химии. 2014. Т. 59. № 1. С. 72–75.
академик О.Н. Чупахин, д.х.н. Л.А. Петров, к.т.н. А.В. Шишмаков
Институт органического синтеза им. И.Я. Постовского УрОРАН, г. Екатеринбург
С использованием методологии прямого нуклеофильного замещения водорода в азааренах (SNH реакции) получены новые продукты некатализируемого переходными металлами С-С кросс-сочетания азинов с хиральными производными ферроценов. Синтезированные оптически активные ферроценилазины в виде комплексов с палладием показали высокую эффективность как катализаторы асимметрического синтеза в аллильном алкилировании олефинов по Тсуджи-Тросту (ее 97-99%).
академик О.Н. Чупахин, к.х.н. И.А. Утепова, к.х.н. А.А. Мусихина, асп. П.О. Серебренникова
Институт органического синтеза им. И.Я. Постовского УрОРАН
Уральский федеральный университет имени первого Президента России Б.Н. Ельцина, г. Екатеринбург
Проведено варьирование содержания двух- и трехвалентного модифицирующего компонента (Zn, Ga) в составе слоистых двойных гидроксидов (предшественников полиметаллического оксидного носителя некислотного типа для платиновых катализаторов). Установлено, что введение Zn и Ga в состав носителя обеспечивает более высокие значения конверсии пропана (при селективности 99%) при использовании катализаторов 0.3%Pt/Mg(Zn)AlOx и 0.3%Pt/MgAl(Ga)Ox по сравнению с платиной, нанесенной на алюмомагниевый носитель. Для получения оптимальных значений каталитических характеристик доля модификатора в составе двух- или трехвалентных катионов составляет 0.1-0.3. Результаты важны для нефтехимической промышленности в области производства олефинов.
чл.-корр. РАН В.А. Лихолобов, к.х.н. А.В. Лавренов
Институт проблем переработки углеводородов СО РАН, г. Омск
Установлено влияние типа углеродного носителя на формирование, состояние и каталитические свойства металлических центров катализаторов аквафазного гидрирования фурфурола. Было показано, что природа носителя (наноглобулярный углерод (НГУ) и углеродные нанотрубки (УНТ)) влияет на размер частиц и зарядовое состояние нанесенного палладия и его активность в реакции гидрирования фурфурола. Катализатор Pd/НГУ проявил наибольшую селективность в образовании фурфурилового спирта (99%) в мягких условиях реакции (50°C, 5 атм). В то же время образец Pd/УНТ оказался неактивным в тех же условиях, однако способствовал восстановлению фуранового кольца в более жестких условиях (90°C, 20 атм). Результаты важны для разработки отечественных технологий переработки растительной биомассы в компоненты моторных топлив.
чл.-корр. РАН В.А. Лихолобов, к.х.н. А.В. Лавренов
Институт проблем переработки углеводородов СО РАН, г. Омск
Методом твердофазного синтеза с использованием высококремнеземного цеолита с силикатным модулем 40 и различных соединений Мо получены каталитические системы, исследованы их физико-химические и каталитические свойства в процессе неокислительной конверсии метана в ароматические углеводороды. Для приготовления катализаторов использовались нанопорошок (НП) Мо (К-1), оксид (К-2) и карбид (К-3) молибдена, молибденовый ангидрид (К-4). Содержание Мо в катализаторах составляло 4,0 % мас.
Введение Мо в цеолит приводит к уменьшению его удельной поверхности и объема пор независимо от соединения Мо. Наиболее заметное снижение удельной поверхности наблюдается при использовании молибденового ангидрида, что связано с блокировкой каналов цеолита частицами более крупного размера. Характер изменения кислотности цеолита зависит от природы используемого источника Мо, при этом во всех случаях наблюдается заметное снижение концентрации бренстедовских кислотных центров.
Катализатор с добавкой Мо2С проявил наименьшую активность в процессе конверсии метана, а наиболее высокую активность показал катализатор, содержащий НП Мо. Распределение Мо на поверхности и в каналах цеолита в этом катализаторе отличается от систем, полученных с использованием других соединений Мо. При прокаливании Мо-содержащего цеолита происходит распределение Мо на его внешней поверхности, при этом часть Мо мигрирует в каналы. В случае использования НП Мо вероятность проникновения Мо в каналы цеолита в процессе прокаливания выше, чем при использовании МоО3 и Мо2С. Частицы оксида Мо, расположенные на поверхности цеолита, легко восстанавливаются метаном в ходе реакции до Мо2С, а соединения Мо в каналах цеолита восстанавливаются медленнее, и могут оставаться частично окисленными, что благоприятно сказывается на активности катализатора в процессе ароматизации метана.
д.х.н. А.В. Восмериков
Институт химии нефти СО РАН, г. Томск
Эффективность гетерогенно-гомогенного процесса окислительной конденсации метана (ОКМ) определяется структурными дефектами оксидных систем, способными инициировать образование СН3-радикалов при окислении метана. Изучено влияние дефектов структуры перовскита и феррошпинели сложного состава на их каталитические свойства в процессе ОКМ.
Показано, что каталитическое действие системы SrxGd1-xCoO3-δ (0.5 < x < 0.9) в реакциях ОКМ иглубокого окисления метана существенно зависит от характера расположения катионов Sr2+/Gd3+ по А-позициям структуры перовскита. Системы с беспорядочным расположением катионов Sr2+/Gd3+ характеризуются случайным распределением кислородных вакансий и увеличенной подвижностью кислорода, что благоприятно для реакции глубокого окисления метана до СО2. Впервые установлено, что упорядочение катионов Sr2+/Gd3+ приводит к локализации кислородных вакансий, снижению общей активности и количества слабосвязанного кислорода, что обеспечивает более высокую селективность по С2-продуктам в реакции ОКМ (Chemical Communication. 2014, V. 50, N. 46, p. 6112-6115).
При сопоставлении каталитических свойств, фазового состава и катионного распределения железа по кристаллографическим позициям железосодержащих фаз в стационарном состоянии ферросфер с содержанием Fe2O3 76-97 мас. % установлено, что при содержании Fe2O3 89 мас. % наблюдается изменение маршрута окисления метана. Для ферросфер с содержанием Fe2O3 < 88.8 мас. % основным маршрутом превращения метана является глубокое окисление. При содержании Fe2O3 ≥ 89 мас. % резко увеличивается выход C2-углеводородов на фоне низкого вклада глубокого окисления. Впервые показано, что выход C2-углеводородов коррелирует с количеством дефектов в структуре феррошпинели, которые представляют собой ионы железа с тетраэдрическим катионом Ca2+ и октаэдрической катионной вакансией среди ближайших соседей (Кинетика и катализ, в печати).
д.х.н., проф. А.Г. Аншиц
Институт химии и химической технологии СО РАН, г. Красноярск
Под действием супероснований типа гидроксид (алкоксид) щелочного металла/диметилсульфоксид две молекулы ацетилена и две молекулы кетона диастереоселективно самоорганизуются в 7-метилен-6,8-диоксабицикло[3.2.1]октаны и 3-ацил-2-циклопентен-1-олы.
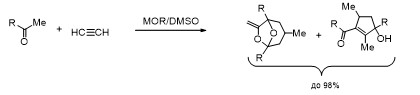
M = Li, Na, K, Cs; R = H, Alk
Соотношение структурных изомеров контролируется концентрацией катализатора и структурой субстрата. Эти каскадные сборки двух перспективных синтетических строительных блоков и прекурсоров лекарств являются уникальными реакциями образования одновременно трех C-C- и двух C-O- или четырех C-C-связей из простых реагентов в одном каталитическом процессе.
академик Б.А. Трофимов
Иркутский институт химии СО РАН, г. Иркутск
Предложено семейство кинетических методов установления механизмов сложных каталитических процессов, характеризующихся существенной нестационарностью активного катализатора, базирующихся не на традиционных измерениях каталитической активности, а на измерениях дифференциальной селективности реакций с искусственно создаваемой многомаршрутностью. Преимущества предложенных методов обусловлены независимостью дифференциальной селективности от количества активного катализатора при условии, что продукты реакции образуются на одном и том же катализаторе, что делает интерпретацию экспериментальных данных существенно проще. Условие образования продуктов на одном и том же катализаторе гарантированно выполняется при создании искусственной многомаршрутности (метод конкурирующих реакций), возникающей при введении в реакцию дополнительных субстратов. Предложен метод исследования дифференциальной селективности по изотопомерам одного субстрата, не требующий никакой модификации условий проведения реакции. Показано, что визуализация кинетических данных путем построения фазовых траекторий образования продуктов реакции позволяет оценить изменения дифференциальной селективности катализатора без использования математических описаний, базирующихся на конкретных гипотезах механизма. Полученные результаты демонстрируют, что измерения дифференциальной селективности позволяют получать информацию о природе активного катализатора, устанавливать скоростьопределяющие стадии каталитического цикла, а также оценивать степень обратимости его стадий. Кроме того, уравнения дифференциальной селективности имеют более простую форму в сравнении с уравнениями скорости, что является еще одним преимуществом исследований дифференциальной селективности. Еще одним преимуществом исследований дифференциальной селективности является возможность изучения реакций, проводимых в условиях высоких соотношений субстрат/катализатор, то есть в реальных каталитических условиях без использования модельных стехиометрических реакций, которые могут приводить к неправильным выводам.
д.х.н. А.Ф. Шмидт
Иркутский государственный университет, г. Иркутск
Установлен механизм формирования и природа каталитически активных частиц в димеризации алкенов на основе фосфиновых комплексов никеля в различных степенях окисления в сочетании с эфиратом трифторида бора. Определены каталитические характеристики индивидуальных комплексов Ni(PPh3)2(C2H4) и Ni(PPh3)nCl (n = 2,3) и систем на их основе в сочетании с кислотами Бренстеда и Льюиса в процессах олигомеризации этилена и пропилена. Обнаружена связь между временем хранения раствора BF3OEt2 и каталитическими свойствами никелевых систем в превращении низших алкенов. Показано, что наблюдаемое возрастание частоты и числа оборотов обусловлено увеличением концентрации кислот Бренстеда за счет необратимых превращений BF3OEt2 в результате взаимодействия с примесями воды в растворителе. Обнаружено резкое повышение длительности функционирования каталитических систем Ni(PPh3)2(C2H4)-BF3OEt2 при формировании их в присутствии субстратов. Комплексом физических методов изучены взаимодействия никелевых прекурсоров с эфиратом трифторида бора и установлены основные реакции формирования каталитически активных частиц. Показано, что в результате взаимодействия Ni(PPh3)2(C2H4) с эфиратом трифторида бора образуются комплексы Ni(II) с активной связью Ni-C, которые инициируют процесс ди- и олигомеризации по гидридному механизму. При использовании в качестве прекурсоров фосфиновых комплексов никеля(I) образование в присутствии субстрата и сокатализатора комплексов Ni(II) с активной связью Ni-C является результатом связывания части фосфиновых лигандов трифторидом бора в аддукты и диспропорционирования комплексов Ni(I) до комплексов Ni(0) и Ni(II). Сложность исследования процессов формирования и функционирования данных металлокомплексных катализаторов обусловлена тем, что при рассмотрении свойств систем, состоящих из комплексов никеля в различных степенях окисления (Ni(0), Ni(I), Ni(II)) и алюминий- и борсодержащих компонентов, следует учитывать как кислотно-основные, так и окислительно-восстановительные свойства последних. Алюминийалкилгалогениды и галогениды бора способны окислять фосфиновые комплексы Ni(0). Кроме того, Al- и B-содержащие сокатализаторы необходимо рассматривать не только как кислоты Льюиса, но и как исходные вещества при формировании кислот Бренстеда, которые могут образоваться из-за наличия примесей воды в условиях реального каталитического процесса. Решение данных проблем представляет не только теоретический, но и практический интерес для создания рациональной стратегии поиска новых высокоэффективных катализаторов и оптимизации существующих каталитических систем.
д.х.н. Ф.К. Шмидт, д.х.н. А.Ф. Шмидт
Иркутский государственный университет, г. Иркутск
Разработан способ получения N,N-диэтилокта-2,7-диен-1-амина, используемого в качестве полупродукта для синтеза ПАВ, антиоксидантов резины, антикоррозионных добавок. Способ осуществляют путем каталитической теломеризации бутадиена (БД) с диэтиламином в присутствии катализатора на основе катионных комплексов палладия (II), характеризующимся тем, что в качестве катионных комплексов палладия используют комплексы общей формулы [(acac)Pd(L)2]BF4 (где acac – ацетилацетонатный лиганд, L = PPh3, PiPr3, PnBu3, P(p-Tol)3 или (L)2 = дифосфиновые лиганды бис(дифенилфосфино)метан, dppm, бис(дифенилфосфино)пропан, dppp, бис(дифенилфосфино)бутан, dppb, бис(дифенилфосфино) ферроцен, dppf. Предложенный способ позволяет с высокой эффективностью получать N,N-диэтилокта-2,7-диен-1-амин с селективностью 99.9% от общей смеси продуктов реакции и выходом целевого продукта до TOFav = 1940 ч–1 и TON = 17,480 мольБД/мольPd.
д.х.н. Ф.К. Шмидт, д.х.н. А.Ф. Шмидт
Иркутский государственный университет, г. Иркутск
Впервые проведен синтез новой серии каталитических прекурсоров — катионных ацетилацетонатных комплексов палладия состава [(acac)Pd(N^N)]BF4 , где N^N – α-дииминовые лиганды. В качестве дииминовых лигандов были использованы бис[N-(фенил)имино]аценафтен, бис[N-(2,6-диметилфенил)имино]аценафтен, бис[N-(2,6-диизопропилфенил)имино]аценафтен, N,N-(этандиилиден)бис(2,6-диметиланилин), N,N-(этандиилиден)бис(2,6-диизопропиланилин), N,N-(2,3-бутандиилиден)бис(анилин), N,N-(1,2-дифенилэтан-1,2-диилиден)бис(2,6-диметиланилин), N,N-(1,2-дифенилэтан-1,2-диилиден)бис(2,6-диизопропиланилин). Каталитические системы на основе комплексов палладия и никеля с α-дииминовыми лигандами, так называемые катализаторы Брукхарта, хорошо известны в практике современного лабораторного и промышленного гомогенного катализа олиго- и полимеризации низших олефинов. Преимуществом данных каталитических систем является их относительная устойчивость по отношению к полярным функциональным группам в мономере, а также возможность получения «суперразветвленных» полиолефинов. Основные преимущества нового класса разработанных палладиевых катализаторов на основе катионных комплексов палладия, обуславливающие их конкурентноспособность: высокая технологичность и относительно низкая себестоимость синтеза прекурсоров, низкая чувствительность к присутствию в реакционной среде следовых количеств H2O и O2.
д.х.н. Ф.К. Шмидт, д.х.н. А.Ф. Шмидт
Иркутский государственный университет, г. Иркутск
Актуальность исследований обусловлена важностью развития методов МРТ и их приложений как в фундаментальных физико-химических исследованиях (включая химическую технологию и материаловедение), так и в современных биомедиицнских исследованиях и практической медицине. Одним из главных недостатков ЯМР и МРТ является относительно невысокая чувствительность. Поэтому для осуществления качественного прорыва в таких приложениях крайне актуальным и востребованным является развитие методов усиления сигнала с использованием принципов гиперполяризации ядерных спинов. Особо остро проблема чувствительности стоит для газов из-за существенно более низкой концентрации молекул по сравнению с жидкостями.
Осуществлено гетерогенное каталитическое гидрирование пропилена параводородом на катализаторе Rh/TiO2, что позволило получить поляризацию ядерных спинов пропана на уровне 1,3%, соответствующую усилению сигнала ЯМР 1Н около 810 раз. На основе этого в работе впервые получены трехмерные МРТ изображения протон-содержащих газов с высоким пространственным разрешением (625×625×625 мкм3) и коротким временем регистрации изображения (< 20 c). При этом за счет усиления сигнала на основе использования новейших методов гиперполяризации получаемое изображение по качеству не уступает МРТ изображению, полученному по сигналу воды, несмотря на значительно меньшую концентрацию протонов в газе по сравнению с жидкостью. Использование процесса гетерогенного каталитического гидрирования пропилена параводородом позволяет реализовать простой и дешевый способ получения гиперполяризованных газообразных контрастных агентов для МРТ.
Все основные результаты получены впервые. Они открывают новые возможности как в материаловедении и химической технологии для визуализации газов в пустотах и каналах (например, в пористых материалах и в реакторах), так и в биомедицинских приложениях при использовании гиперполяризованных протон-содержащих газов в качестве контрастных агентов для функциональной визуализации легких как альтернатива более сложным и дорогостоящим исследованиям на основе использования благородных газов (129Хе, 3Не).
Исследования выполнены сотрудниками МТЦ СО РАН, ИК им. Г.К. Борескова СО РАН и НГУ совместно с партнерами из Института томографических исследований Университета Вандербильта, США (Vanderbilt University Institute of Imaging Science, Nashville, TN, USA).
Результаты работы опубликованы в следующей статье: K.V. Kovtunov, D.A. Barskiy, A.M. Coffey, M.L. Truong, O.G. Salnikov, A.K. Khudorozhkov, E.A. Inozemtseva, I.P. Prosvirin, V.I. Bukhtiyarov, K.W. Waddell, E.Y. Chekmenev, I.V. Koptyug. High-resolution 3D proton MRI of hyperpolarized gas enabled by parahydrogen and Rh/TiO2 heterogeneous catalyst, Chem. Eur. J., 20, 11636-11639 (2014).
д.х.н. И.В. Коптюг, к.х.н. К.В. Ковтунов, Д.А. Барский, А.В. Сальников, к.х.н. И.П.Просвирин, чл.-корр. РАН В.И. Бухтияров
Международный томографический центр СО РАН,
Институт катализа им. Г.К. Борескова СО РАН,
Новосибирский государственный университет, г. Новосибирск
Целью выполненных исследований является разработка новых методов усиления сигнала ЯМР за счет использования параводорода для создания нового высокочувствительного инструментария ЯМР и МРТ и его применения для исследования механизмов каталитических реакций и для изучения процессов в функционирующих реакторах, а также для биомедицинских приложений.
Актуальность исследований обусловлена важностью процессов гидрирования непредельных соединений в современной химической промышленности, которые преимущественно используют катализаторы на основе переходных металлов. Стремление снизить стоимость катализаторов и их воздействие на окружающую среду определяет интерес к созданию новых каталитических систем для процессов гидрирования на основе более распространенных и безвредных элементов. В частности, среди систем на основе элементов главных групп значительный интерес представляют фрустрированные Льюисовские пары, для которых в последнее время продемонстрирована возможность использования в процессах активации Н2 и гидрирования широкого круга непредельных соединений.
В работе впервые показана возможность наблюдения усиления сигнала ЯМР за счет эффекта индуцированной параводородом поляризации ядер (ИППЯ) при активации параводорода с использованием не содержащей металла системы, представляющей собой фрустрированную Льюисовскую пару анса-аминоборана. Продемонстрировано, что активация параводорода этим «молекулярным пинцетом» приводит к усилению сигнала ЯМР 1Н не только обменивающихся, но и других атомов водорода в структуре соединения, а также сигналов ЯМР атома 11B. Полученные результаты демонстрируют применимость метода ИППЯ для изучения механизма активации Н2 системами, не содержащими атома металла.
Все основные результаты получены впервые и являются существенным продвижением вперед в области развития методов значительного усиления сигнала ЯМР и применения этих методов для исследования механизмов важных каталитических процессов.
Исследования выполнены сотрудниками МТЦ СО РАН совместно с финскими коллегами из университета Оулу и университета Аалто (Department of Physics, University of Oulu, and Department of Materials Science and Engineering, Aalto University, Finland) и поддержаны грантом РФФИ (12-03-00403-a) и грантом Президента РФ (MK-1329.2014.3).
Основные результаты опубликованы в следующей статье: 1. V.V. Zhivonitko, V.-V. Telkki, K. Chernichenko, T.J. Repo, M. Leskela, V. Sumerin, I.V. Koptyug. Tweezers for parahydrogen: a metal-free probe of non-equilibrium nuclear spin states of H2 molecules, J. Am. Chem. Soc., 136, 598-601 (2014).
к.х.н. В.В. Живонитко, д.х.н. И.В. Коптюг
Международный томографический центр СО РАН, г. Новосибирск
Исследована кинетика полимеризации этилена в присутствии гомогенных катализаторов на основе бис-иминопиридильных комплексов Fe(II) при варьировании состава тридентатного лиганда L в бис-иминопиридильных комплексах LFeCl2, природы и состава сокатализатора (МАО, триалкилы алюминия). Найдены составы каталитических систем, позволяющих получать линейный полиэтилен с молекулярной массой от 1×104 до 2×106 г/моль и регулируемым молекулярно-массовым распределением. Методами ЯМР 1Н и 2Н изучены механизм активации и структура интермедиатов, образующихся при взаимодействии LFeCl2 с различными активаторами (МАО, триалкилы алюминия) в процессе формирования активного компонента этих систем.
С использованием полученных для гомогенных систем данных разработаны нанесенные катализаторы LFeCl2/SiO2, имеющие высокую и стабильную активность при полимеризации этилена с сокатализатором – триалкилом алюминия при реальных для технологии температурах (80-90°C) и позволяющие получать линейный полиэтилен с различной молекулярной массой. Эти результаты послужили основой для разработки оригинального нанесенного бикомпонентного катализатора для производства ПЭ с бимодальным молекулярно-массовым распределением газофазным методом. Новый нанесенный катализатор содержит в своем составе комплекс железа (LFeCl2), на котором образуется низкомолекулярный линейный полиэтилен, и соединение хрома, на котором образуется разветвленный высокомолекулярный полиэтилен. Результаты испытаний этого катализатора в НТЦ ОАО «Казаньоргсинтез» подтвердили возможность получения на этом катализаторе бимодального полиэтилена типа ПЭ-100, получаемого в настоящее время на ОАО «Казаньоргсинтез» с использованием катализатора, закупаемого по импорту.
д.х.н. В.А. Захаров, к.х.н. Н.В. Семиколенова, д.х.н. К.П. Брыляков, к.х.н. И.Е. Сошников, д.х.н. Е.П. Талзи, к.х.н. М.А. Мацько
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Работа велась по трём основным направлениям: моделирование наночастиц и их взаимодействия с молекулами, фотокаталитическое окисление дибутилсульфида на фуллеренах с возможными применениями в тонком органическом синтезе, фотокаталитическое окисление фосфорорганических загрязнителей воды.
Разработаны новые кластерные модели наночастиц диоксида титана в кристаллической модификации анатаз с релаксированными атомами. Разработанные модели и подходы открывают новые перспективы в компьютерном описании процессов адсорбции и катализа с участием твердых наночастиц.
Исследуется кинетика фотокаталитического окисления органического сульфида на фуллеренах под видимым светом. Идентифицированы основные продукты реакции, выяснено влияние концентрации начального вещества и интенсивности облучения на скорость и селективность реакции. Использованный метод может быть использован для селективного парциального окисления органических веществ таких как сульфиды и алкены с использованием солнечного или искусственного света.
Обнаружена повышенная эффективность фотокатализаторов, содержащих гетерополикислоту, адсорбированную на поверхности диоксида титана, в реакциях глубокого окисления фосфорорганических загрязнителей воды. Фотокатализаторы могут использоваться для разработки устройств тонкой очистки воды.
д.х.н. А.В. Воронцов
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
В настоящее время одним из важных аспектов современных химических процессов становится преобразование биомассы и сырья, полученного из биомассы, в ценные химические вещества и топливо; в частности, переэтерификация растительного масла позволяет получать биодизель и глицерин в качестве побочного продукта. Между тем, глицерин, содержащий три гидроксильные группы, способен претерпевать химические превращения, обеспечивая получение ценных продуктов. Известно, что дегидратация глицерина в присутствии кислых поверхностных центров приводит к образованию акролеина, а в присутствии основных – молочной кислоты.
На основе предложенного подхода, основанного на модифицировании двойных слоистых ZnAl-гидроксидов с соотношением Zn/Al ≥ 2 как путем замены межслоевого аниона NO3– на An– {An– = [H2W12O40]6–, WO42–, HPO42}, так и нанесением РW- или SiW-ГПК 12 ряда, получены композиции, представляющие собой слоистые Zn-Al-гидроксиды со структурой, подобной гидроталькиту. Термическая обработка модифицированных образцов в интервале 450-600°C приводит к формированию твердого раствора на основе ZnO, а для образцов с нанесенными РW(SiW)-ГПК появляется дополнительная фаза ГПК в высокодисперсном состоянии. На поверхности рассмотренных образцов присутствуют как бренстедовские (БКЦ), так и льюисовские (ЛКЦ) кислотные центры, причем концентрация БКЦ увеличивается при модифицировании ZnAl-гидроксидов как при замене межслоевого аниона, так и при нанесении ГПК, а ЛКЦ, наоборот, уменьшается. Показано, что основными продуктами газофазной дегидратации глицерина на указанных образцах являются акролеин и гидроксиацетон (ацетол); доля других продуктов невелика. Активность катализаторов увеличивается с повышением температуры реакции (Тр) и при Тр = 325°C конверсия глицерина в зависимости от природы катализатора изменяется в интервале 30 ÷ 99%. Максимальная селективность по ацетолу (~74%) наблюдается на немодифицированном ZnAl-образце, в то время, как по акролеину (~56%) – на модифицированных SiW/ZnAl-образцах. В свою очередь, выход акролеина коррелирует с концентрацией БКЦ, а выход ацетола – с концентрацией ЛКЦ. Полученные зависимости согласуются с литературными данными, согласно которым БКЦ являются ответственными за образование акролеина.
д.х.н. А.С. Иванова
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Традиционным промышленным способом получения изобутилена является дегидрирование изобутана в кипящем слое с использованием циркулирующего мелкосферического алюмохромового катализатора. Дегидрирование проводят чередующимися циклами дегидрирование – регенерация. Высокие температуры, попеременное пребывание катализатора в окислительной и восстановительной средах и непрерывная циркуляция определяют повышенные требования к таким эксплуатационным показателям как механическая прочность гранул и невысокая абразивность. По эксплуатационным показателям в процессе дегидрирования изобутана наиболее соответствуют микросферические алюмохромовые катализаторы (КДМ, КДМ-М), производимые в промышленном масштабе на OOO «НПК Синтез», недостатком которых является относительно невысокая селективность (93-94%) по изобутилену.
В лаборатории приготовления катализаторов совместно с лабораторией нестационарных каталитических методов очистки газов разработана технология получения усовершенствованного микросферического алюмохромового катализатора, обеспечивающего более высокую селективность (96-97%) по изобутилену при сохранении требуемых эксплуатационных характеристик. Усовершенствование микросферического алюмохромового катализатора проведено на основе алюмооксидных носителях, производимых на OOO «НПК Синтез».
д.х.н. А.С. Иванова, д.х.н. Г.А. Зенковец
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
В Cанкт-Петербургском филиале ИК СО РАН впервые разработана новая технология получения сверхвысокомолекулярного полиэтилена (СВМПЭ) с использованием особого класса моноцентровых постметаллоценовых каталитических систем, которая обеспечивает улучшенную морфологию СВМПЭ и его способность перерабатываться при температурах ниже температуры плавления (из твердой фазы) в сверхпрочные и сверхмодульные изделия (волокна, пленки и т.д.).
Перерабатываемость, высокие прочностные и модульные характеристики полученных в СПб филиале ИК СО РАН образцов реакторных порошков (РП) СВМПЭ были подтверждены в Институте синтетических полимерных материалов им. Н.С. Ениколопова РАН.
Новая технология отличается от применяемой в настоящее время в мировой практике переработки РП СВМПЭ в изделия из раствора методом гель-формования через фильеры высокой производительностью, упрощённым аппаратурным оформлением и низкими энергетическими затратами.
По разработанной технологии синтеза СВМПЭ получен патент РФ № 2459835С2 с приоритетом от 18.11.2010 г. (опубликован 27.05.2012 г.) и подана заявка №2013124441 от 24.07.2013 г. на патент РФ, которая находится на рассмотрении в Федеральном институте промышленной собственности.
чл.-корр. РАН С.С Иванчев
Санкт-Петербургский филиал Института катализа им. Г.К. Борескова СО РАН, г. Санкт-Петербург
Впервые исследовано эпоксидирование этилена поверхностным анион-радикальным α-кислородом, образующимся на цеолите FeZSM-5. Реакция С2Н4 + Оα протекает без отрыва водорода и ведет к селективному присоединению Оα по двойной связи С=С, с образованием этиленоксида в качестве первичного продукта. Модификация образца натрием приводит к значительному увеличению селективности по этиленоксиду, достигающей 80-84% при температуре реакции –60°C. Способность α-кислорода, обладающего высокой электрофильностью, вести селективное эпоксидирование может служить дополнительным аргументом в пользу теории о ключевой роли электрофильного кислорода в эпоксидировании этилена на Ag-содержащих катализаторах.
На поверхности FeZSM-5, модифицированного Na, обнаружена ранее не известная реакция – взаимодействие этиленоксида с этиленом с образованием бутаналя:
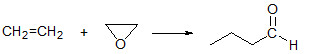
Результаты работы опубликованы в следующей статье: Eugeny V. Starokon, Mikhail V. Parfenov, Larisa V. Pirutko, Igor E. Soshnikov, Gennady I. Panov. Epoxidation of ethylene by anion radicals of α-oxygen on the surface of FeZSM-5 zeolite. Journal of Catalysis, Vol. 309, January 2014, p. 453-459.
д.х.н. А.С. Харитонов, к.х.н. Е.В. Староконь, М.В. Парфенов,
к.х.н. Л.В. Пирютко, к.х.н. И.Е. Сошников, д.х.н., проф. Г.И. Панов
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Проведена разработка второй опытной установки полного цикла с реакторами промышленного масштаба по получению синтетических жидких углеводородов из природного газа в интересах ООО «ИНФРА». Установка построена, испытана и принята в эксплуатацию в ООО «ИНФРА».
Проведены успешные исследования механизма реакций на композиционных цеолитных катализаторах синтеза Фишера-Тропша. Установлено, что карбкатионный механизм играет решающую роль в составном процессе. По результатам исследования опубликован обзор в журнале «Успехи химии».
Установлено влияние введения различных металлов (алюминий, медь, цинк, железо) в композитные цеолитсодержащие катализаторы синтеза Фишера-Тропша.
Разработаны исходные данные для строительства катализаторной фабрики в интересах ООО «ИНФРА».
д.х.н. В.З. Мордкович
Технологический институт сверхтвердых и новых углеродных материалов, г. Троицк, г. Москва
Продолжены исследования оригинального процесса и катализатора синтеза бутадиена-1,3 из возобновляемых ресурсов – этанола или смеси этанола и ацетальдегида. По технологии ОАО НИИ «Ярсинтез» выход дивинила на пропущенный этанол приблизительно в два раза выше при селективности по дивинилу на ~ 20% отн. выше, чем в классическом процессе. Основное внимание уделялось практическим вопросам реализации процесса. В настоящее время заканчивается монтаж опытной установки для подготовки данных на проектирование промышленного процесса.
Проведены работы по изучению стабильности химического и фазового состава, каталитических и физико-химических характеристик нового высокоэффективного катализатора в процессе дегидрирования пропана. Установлено, что в течение 1000 ч работы катализатора в реакции дегидрирования не происходит снижения его не происходит снижения его каталитической активности. Заключено лицензионное соглашение на процесс дегидрирования и производство катализатора.
д.т.н., проф. Г.Р. Котельников
ОАО НИИ «Ярсинтез», г. Ярославль
Каталитический риформинг является одним из базовых процессов переработки нефти. Уровень технологии этого процесса, техническая и экономическая эффективность в существенной степени зависят от свойств применяемого катализатора. Наибольшей надежностью в эксплуатации отличаются катализаторы с оптимальным содержанием Pt, Re, и Cl, нанесённые на носитель – оксид алюминия. В настоящее время в России преобладает технология со стационарным слоем катализаторов, в основном импортного производства.
В институте нефти и газа СФУ г. Красноярска изучены физико-химические свойства частично отработанного катализатора Pt-Re-Cl/Al2O3 риформинга нефтяных фракций (R-98 UOP), ОАО «Ачинский НПЗ» (компания «Роснефть»).
При исследовании физико-химических свойств платинорениевого катализатора риформинга R-98 установлено, что в ходе его эксплуатации происходило необратимое изменение структурных свойств: укрупнение кристаллитов активной формы носителя η-Al2O3, его частичное фазовое превращение в корунд. Обнаружение корунда α-Al2O3 в отработанном катализаторе служит указанием на то, что в реакторе имело место значительное повышение температуры при выжигании углеродистых отложений, что вызвало фазовый переход активной окиси алюминия η-Al2O3 в неактивную высокотемпературную модификацию корунда.
Установлено, что в ходе эксплуатации происходило уменьшение величины удельной поверхности и некоторое увеличение размера пор. При этом показатели механической прочности мало изменялись.
Важная особенность проб отработанного катализатора связана с повышенным содержанием в них примеси оксида железа, который является ядом для металлического компонента катализатора. Накопление оксида железа может быть связано с коррозией оборудования под действием агрессивной среды, содержащей пары воды и хлористые соединения.
Присутствие углерода в пробах отработанного катализатора указывает на неполное удаление коксовых отложений на стадии окислительной регенерации, что может быть вызвано недостаточной длительностью процедуры выжига или нарушениями газового потока в реакторе.
Методом ДСК показано существование различных типов углеродистых отложений на поверхности катализатора R-98. Полное удаление остаточных отложений кокса происходит при температуре 500-550°C.
На основании проведённых исследований разработаны рекомендации по увеличению эффективности и продолжительности срока службы используемого катализатора.
Подробные результаты работы опубликованы в Журнале Сибирского федерального университета. Техника и технологии, 2014, том 7, № 8, с. 919-928.
д.т.н. Н.Н. Довженко, д.х.н. В.П. Твердохлебов, П.Н. Кузнецов, А.В. Казбанова, Л.И. Кузнецова, Л.С. Тарасова
Сибирский Федеральный университет, Институт нефти и газа, г. Красноярск
Для промышленной очистки пропана от метанола, диметилового эфира в среде циркулирующего водородсодержащего газа используется дорогостоящий алюмоплатиновый катализатор. Процесс осуществляется при температурах до 370°С, которые позволяют очистить пропан не только от метанола, диметилового эфира, непредельных углеводородов, но и от органических соединений серы.
В «НИАП-КАТАЛИЗАТОР» (г. Новомосковск) на основе ряда промышленных катализаторов разработаны модифицированные образцы, которые прошли длительные испытания в процессе очистки пропана в заводской лаборатории гидрирования углеводородов. Предлагаемые катализаторы значительно дешевле используемого драгметального контакта.
Результаты исследования эксплуатационных качеств разработанных никелевых, никельмедных, никельмедьмарганцевых алюмокальциевых модифицированных катализаторов сравнивались с результатами испытаний алюмоплатинового, никелькизельгурового, никельхромового и других катализаторов. Испытания активности проводили при объемной скорости 200 – 500 ч–1, давлении 8 – 20 кгс/см2, температурах 180 – 380°С. Перед испытаниями проводилась активация катализаторов в токе водорода при различных температурах.
Проведенные исследования одного из разработанных катализаторов показали ряд существенных достоинств применительно к процессу гидроочистки пропана. Катализатор стабильно работает, не теряя каталитической активности. На этом этапе время испытаний составляло более 2 суток. На данном катализаторе происходит и гидрирование диоксида углерода. В отличие от других контактов, где содержание СО и СО2 увеличивалось, концентрация СО2 уменьшалась, а образование СО не отмечалось, что является положительным фактором для данного процесса. На синтезированных катализаторах не отмечено значительное образование тяжелых углеводородов С5 и выше. На данном катализаторе удалось значительно снизить температуру процесса.
| Марка катализатора |
Никель кизельгуровый |
Алюмо- платиновый |
Никель- хромовый |
новый контакт "НИАП-КАТАЛИЗАТОР" |
| Температура процесса | 320 – 380 °C | 260 – 280 °C | 200 – 240 °C | 150 – 190 °C |
К настоящему времени из серии разработанных контактов пока прошли испытания два катализатора. Начата отработка режима снижения температуры процесса активации, приемлемого для промышленной эксплуатации катализаторов.
д.х.н., проф. Е.З. Голосман
«НИАП-КАТАЛИЗАТОР», г. Новомосковск
Для процесса получения легких олефинов из природного газа через промежуточный синтез и пиролиз хлористого метила проводились исследования по синтезу хлористого метила методом каталитического окислительного хлорирования метана. Для эффективного съема тепла реакции и увеличения селективности по хлористому метилу в сумме хлорметанов не менее 90-92% процесс оксихлорирования метана проводится с ~ пятикратным по отношению к хлору избытком метана.
В целях увеличения единичной производительности реакторов окислительного хлорирования метана проведены исследования процесса при давлении до 10 ата.
Экспериментально показана возможность увеличения производительности реактора оксихлорирования метана за счет повышения давления с коэффициентом пропорциональности ~ Р0.85. Селективность образования метилхлорида при этом не изменяется.
Полученные результаты могут быть использованы при разработке технологии промышленного процесса получения низших олефинов из природного газа через промежуточный синтез метилхлорида.
д.х.н., проф. Ю.А. Трегер, к.х.н. В.Н. Розанов
Научно-исследовательский инженерный центр «Синтез», г. Москва
Использование высокотемпературных (240-260°C) катализаторов в процессе окислительного хлорирования этилена в псевдоожиженном слое позволяет в 1,5-2 раза повысить производительность действующих промышленных реакторов, что является составной частью увеличения мощности производств винилхлорида по сбалансированной схеме. Увеличение производительности реакторов достигается за счет увеличения Δt между слоем катализатора и системой теплосъема.
По сравнению с низкотемпературными (210-225°C) высокотемпературные катализаторы в этих условиях обладают пониженной активностью (константа скорости при 220°C ниже в 4-5 раз). Компенсация достигается путем повышения температуры процесса.
Снижение активности катализатора с одновременным повышением селективности образования целевого 1,2-дихлорэтана достигается путем ввода в катализатор наряду с хлоридом меди щелочных/щелочноземельных металлов.
В результате проведенных исследований показано, что наличие в системе MgCl2 способствует стабилизации активных комплексов на поверхности зерна и ведет к повышению эффективности использования этилена в «воздушном» процессе до 95-96%; выход продуктов глубокого окисления при 240оС не превышает 1,5-1,8%.
Использование в качестве стабилизирующей добавки хлорида лития позволяет получить схожие результаты, хотя показатели процесса при 240-250°C несколько уступают системам, содержащим хлорид магния.
Показано, что смесь катализаторов дает результаты, близкие к системам, содержащим CuCl2-MgCl2/Al2O3.
д.т.н. М.Р. Флид
Научно-исследовательский инженерный центр «Синтез», г. Москва
Для процесса получения низших олефинов из природного газа через промежуточный синтез и пиролиз хлористого метила проведены широкие поисковые исследования по подбору аналога силикоалюмофосфатного катализатора SAPO-34. Были использованы различные исходные вещества и условия синтеза, различные связующие.
Определены оптимальные физико-химические характеристики катализатора, позволяющие достичь степени превращения метилхлорида более 70% за проход при суммарной селективности образования этилена и пропилена более 80%.
Обнаружено, что селективность процесса пиролиза хлористого метила до низших олефинов значительно возрастает и достигает максимума при определенной степени зауглероживания катализатора, в то время как активность катализатора монотонно падает по мере зауглероживания. Показано, что после регенерации воздухом активность катализатора полностью восстанавливается.
д.х.н., проф. Ю.А. Трегер, к.х.н. В.Н. Розанов
Научно-исследовательский инженерный центр «Синтез», г. Москва
Разработана технология производства кобальт-фталоцианинового катализатора ИВКАЗ в жидкой форме (20%-ый водно-щелочной раствор) для процессов очистки нефтей (ДМС) и нефтепродуктов (ДМД). Произведено и поставлено российским и зарубежным заказчикам 2500 кг катализатора ИВКАЗ.
академик АН РТ, Генеральный директор, д.т.н. А.М. Мазгаров
ОАО Волжский научно-исследовательский институт углеводородного сырья, г. Казань
В процессе разработки катализатора изомеризации С7-фракции для снижения ароматических углеводородов в товарных автобензинах проведены пилотные испытания опытно-промышленной партии катализатора СИ-4. Подтверждены показатели процесса «Изомалк-4» для промышленного внедрения.
При разработке катализатора изомеризации н-бутана проведены пилотные испытания катализатора СИ-4. По результатам пилотных испытаний разработан базовый проект установки изомеризации н-С4 в компании Shandong Sincier Petrochemical Co., Ltd. (Китай).
Усовершенствована технология промышленного производства катализатора изомеризации пентан-гексановых фракций. Выпущены две промышленные партии катализатора СИ-2 для ЗАО «Роснефть-РНПК» и ОАО «Орскнефтеоргсинтез».
Генеральный директор, к.х.н. А.Н. Шакун
ОАО НПП «Нефтехим», г. Краснодар
Проводятся работы по синтезу гидрофобных цеолитов применительно к очистке атмосферы от вредных компонентов. Наиболее актуальной проблемой является, в частности, каталитическое разложение токсичных компонентов до безвредных веществ в процессе адсорбции. В этой связи в Корпорации ведутся работы по поиску новых фотокатализаторов на основе титано-силикатных цеолитов. В настоящее время осуществляются работы по синтезу нового класса сорбентов и катализаторов на основе металл-органических структур для очистки обитаемых помещений от токсичных компонентов.
д.т.н. С.Б. Путин
ОАО «Корпорация «Росхимзащита», г. Тамбов
Asymmetric Synthesis: Pairing ferrocene with an imidazolium ring system results in planar chiral catalysts
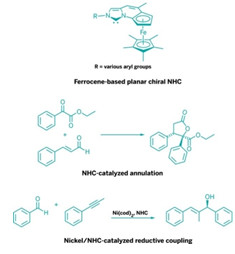 Iidazolium-based N-heterocyclic carbenes (NHCs) are a versatile set of catalyst molecules. Chemists use them as ligands in transition-metal
catalysts, and they use them alone as metal-free organocatalysts. They would also like enantioselective versions of these compounds to
prepare chiral molecules, but so far only a few successful versions have been reported.
Iidazolium-based N-heterocyclic carbenes (NHCs) are a versatile set of catalyst molecules. Chemists use them as ligands in transition-metal
catalysts, and they use them alone as metal-free organocatalysts. They would also like enantioselective versions of these compounds to
prepare chiral molecules, but so far only a few successful versions have been reported.
A research team led by Christopher T. Check and Karl A. Scheidt of Northwestern University set out to change that situation by fusing a metal sandwich complex with an NHC framework. In doing so, they created a versatile new class of NHCs with a rigid planar chiral imidazolium ring system that can be tuned to serve as a ligand or organocatalyst by altering the substituents on the imidazolium ring (Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201410118)
The Northwestern chemists first synthesized a ferrocene derivative with a cyclopentadienyl ligand on one side of the sandwich and a cyclopentapyridinyl ligand bearing a pseudoephedrine side chain on the other. After separating the resulting enantiomers, they carried out an additional reaction sequence to finalize formation of the planar chiral imidazolium ring system.
“This new molecule represents a creative translation of the notion of planar chirality to the bond-forming business end of the NHC ligand-catalyst scaffold,” says Scott J. Miller of Yale University, whose group develops catalytic methods for synthesizing stereochemically complex molecules. “In doing so, the researchers have provided new dials to turn for optimizing catalysts. It is very thought-provoking and stimulating work.”
With the ferrocene-based chiral NHC in hand, Scheidt and coworkers carried out a series of reactions. In one example, the team used the new NHC as an enantioselective organocatalyst for aryl homoenolate additions to aryl α-ketoesters. In another example, they paired the NHC with nickel for reductive coupling of phenylpropyne with aldehydes to form allylic alcohols in high regioselectivity and enantiomeric excess—among the best ever reported for this reaction, Scheidt notes. The researchers further used the new NHC to prepare and study copper and rhodium complexes.
“These NHCs are a result of really smart modular design,” comments NHC specialist Frank Glorius of the University of Münster, in Germany. “The researchers convincingly show that these NHCs are incredibly versatile, being applicable as chiral organocatalysts in their own right and as ligands in nickel-and copper-catalyzed asymmetric transformations. It would be great if this family of NHCs could quickly become commercially available.
Catalysis: Chalcogenide gel mimics nitrogen fixation and photosynthesis
 By taking cues from two biological processes, researchers have made a catalytic material that converts nitrogen to ammonia when irradiated by white light (J. Am. Chem. Soc. 2015, DOI: 10.1021/ja512491v).
The new strategy may one day help scientists achieve energy savings in various catalytic processes by capitalizing on abundant sunlight to produce valuable chemicals.
By taking cues from two biological processes, researchers have made a catalytic material that converts nitrogen to ammonia when irradiated by white light (J. Am. Chem. Soc. 2015, DOI: 10.1021/ja512491v).
The new strategy may one day help scientists achieve energy savings in various catalytic processes by capitalizing on abundant sunlight to produce valuable chemicals.
Manufacturers worldwide produce some 200 million tons of ammonia annually, mainly for use as fertilizer and for making nitrogen-containing compounds. The standard industrial process, the Haber-Bosch method, involves reacting nitrogen, which is relatively inert, with hydrogen at 400 °C and at a pressure roughly 250 times atmospheric pressure in the presence of an iron-based catalyst. It is, of course, highly energy intensive.
Nature also converts nitrogen to ammonia, albeit far more slowly, through a process known as nitrogen fixation. The reaction, which runs under much milder conditions, occurs in microbes containing nitrogenase enzymes. These catalysts tend to include a reactive cluster of iron, molybdenum, and sulfur.
In an effort to understand nature’s energy-efficient ways, researchers previously made synthetic analogs of these clusters. They found that a few of them can catalyze ammonia production from nitrogen under strongly reducing conditions.
A team of Northwestern University chemists, including Abhishek Banerjee and Mercouri G. Kanatzidis, has now demonstrated a potentially more useful catalyst: one that can be switched on and driven by light to mediate ammonia production at room temperature and ambient pressure. Dubbed a chalcogel, the material, which mimics some aspects of nitrogen fixation and photosynthesis, is a light-absorbing, porous, amorphous solid composed of Mo2Fe6S8 clusters linked by Sn2S6 ligands.
The team bubbled nitrogen through aqueous solutions containing the chalcogel, a proton source (pyridinium hydrochloride), and an electron donor (sodium ascorbate). They detected ammonia shortly after aiming a white light source at the gel and report that during irradiation the chemical’s concentration increased continuously.
Control tests show that solutions lacking the catalyst and those kept in the dark do not produce ammonia. The team acknowledges that the chalcogel evaluated in this study produces ammonia too slowly for industrial use but notes that the material remained stable with no loss of activity during a 72-hour test.
“This is extremely elegant work,” says University of Liverpool chemistry professor Matthew J. Rosseinsky. He notes that the study paves the way for further research projects to determine the catalyst’s active site structure and the role of the Sn2S6ligands. Rosseinsky also wonders whether there is a relationship between the electronic structure of the cluster and a wavelength dependence of the catalytic activity.
Organometallics: Team makes stable version of hard-to-come-by gold(III) catalyst
More of gold’s charms have been revealed, as chemists report a rare stable gold catalyst in the +3 oxidation state. The high-valent transition-metal catalyst offers molecule makers a shiny new tool that does chemistry distinct from that of gold catalysts in the more common +1 oxidation state.
For more than a decade, gold(I) catalysts have proven to be increasingly useful in the assembly of complex molecules. Gold(III) catalysts, on the other hand, have been harder to come by because it usually takes harsh reagents to prepare them and they tend not to be stable.
F. Dean Toste, Chung-Yeh Wu, Takahiro Horibe, and Christian Borch Jacobsen, chemists at the University of California, Berkeley, have now made a stable gold(III) catalyst by getting a gold(I) catalyst to insert into a carbon-carbon bond—a process known as oxidative addition (Nature 2015, DOI: 10.1038/nature14104).
Toste tells C&EN that the team got the idea while studying the opposite reaction, the reductive elimination of gold(III) to gold(I). Because gold prefers to be in the +1 oxidation state, that reaction is thermodynamically favored. But the Berkeley team reckoned that by adding some strain to the system, they could tip the scales in favor of the +3 oxidation state. Gold(III) turns out to behave differently from gold(I) in several different types of reactions, including Mukaiyama-Michael additions and Diels-Alder reactions. “You’ve changed the electronic nature of the metal, and therefore it’s going to have different reactivity,” Toste explains.
“This work is particularly impressive because it puts together several well-known principles of gold chemistry and employs this knowledge in combination with brilliant new ideas to come up with novel and highly versatile synthetic protocols,” comments Hubert Schmidbaur, an expert in gold chemistry at Germany’s Technical University of Munich.
Steven P. Nolan, an expert in organometallic catalysis at the University of St. Andrews, in Scotland, adds, “This contribution will provide access to up-to-now only proposed entities that will enable a vast landscape of reactivity to be developed.”
Gold catalysts in different oxidation states behave differently in this Mukaiyama-Michael addition.
Chemical Synthesis: New catalyst expands stereochemical repertoire
of alkene dichlorination
In conventional alkene anti-dichlorination (top), Cl+ and Cl− attack opposite faces of the carbon-carbon double bond. In the new syn reaction (bottom), two Cl− ions attack the same face, giving a stereoisomeric product.
In a development that could revise organic chemistry textbooks, a new catalytic version of alkene dichlorination makes the reaction more versatile. Adding Cl2 to double-bonded carbons to give saturated dichlorinated products is a fundamental reaction taught early in the organic chemistry curriculum. It mostly proceeds in just one way: via an “anti” mechanism, in which Cl+ and Cl− ions attack opposite faces of the double bond.
Now, researchers have devised the first catalytic alkene dichlorination that proceeds by the alternative “syn” route, in which two Cl− ions attack the same face of the double bond. This approach provides a direct route to stereoisomers of anti-dichlorination products.
Chemists have reported alkene syn-dichlorinations before, using antimony and molybdenum chloride reagents, but these reactions were not catalytic and their applicability was severely restricted, primarily to nonsubstituted alkene substrates. The only other way to get alkene syn-dichlorination products has been to use multiple steps.
Alexander J. Cresswell, Stanley T.-C. Eey, and synthetic organic chemist Scott E. Denmark at the University of Illinois, Urbana-Champaign, have now designed a selenium(IV) reagent that catalyzes alkene syn-dichlorinations in one step (Nat. Chem. 2015, DOI: 10.1038/nchem.2141). They report 27 examples of cyclic and acyclic dichlorinated products synthesized using the strategy, including dichlorocyclohexane.
In the reaction, Se4+ gets reduced to Se2+. According to synthetic chemist Ross Denton of the University of Nottingham, in England, the key to making the process catalytic was “identifying a suitable oxidant that would convert Se2+ back to Se4+ in the presence of the alkene and not interfere with the dichlorination process itself.” Denmark’s group did that, Denton says.
In a classic anti-dichlorination, Cl+ forms a cyclic intermediate on one side of the alkene. The ring is opened when Cl− attacks the other side. In the syn-reaction, the Se4+ reagent forms a cyclic intermediate on one side of the alkene that is opened by Cl−on the opposite side. The Se4+ reagent then gets displaced by a second Cl− attacking on the same side as the first.
“The magic comes about from a deep-seated appreciation of reaction mechanism that follows from analytical thinking about the individual steps that constitute the process,” says synthetic organic chemist Erick M. Carreira of ETH Zurich. “The simplicity and availability of catalyst and reagents ensure that the method—and more generally, the concepts—will be widely adopted.”
Synthetic organic chemist Takehiko Yoshimitsu of Osaka University says the reaction “is a major breakthrough that could provide easier access to polychlorinated compounds, such as chlorosulfolipid natural products.”
Organic Synthesis: Chemists use nickel to replace palladium in an important catalytic reaction for making aryl amines

AMMONIA AMINATION
The addition of a single aryl group to ammonia using a nickel catalyst enables the synthesis of a variety of aryl and heteroaryl amines, such as in the reaction shown.
By developing a stable nickel catalyst system, a Canadian research team has fulfilled a quest to move beyond using expensive palladium catalysts in cross-coupling reactions to make aryl amines from ammonia. The approach taken by Andrey Borzenko, Mark Stradiotto, and coworkers of Dalhousie University, in Halifax, Nova Scotia, is expected to find quick adoption in the pharmaceutical industry, where it would be used to help synthesize complex drug candidates.
Ammonia is the simplest and most abundant N–H source in chemistry—virtually all synthetic nitrogen-containing compounds originate from the inexpensive feedstock. Industrial syntheses of amines from ammonia, however, typically require heterogeneous catalysts at relatively high temperatures and pressures, resulting in modest product selectivity. Only recently have chemists devised homogeneous palladium catalysts for the task, which allow for more selective ammonia couplings under milder conditions.
The Dalhousie team’s method is the first example of nickel-catalyzed arylation of ammonia to make amines. The researchers used Ni(cyclooctadiene)2 or NiCl2(dimethoxyethane) with a ferrocenyl phosphine ligand known as JosiPhos to link up a range of substituted aryl and heteroaryl bromides, chlorides, and tosylates with ammonia to make diverse aryl and heteroaryl amines (Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201410875).
“Catalysis with nonprecious metals is one of our primary goals in the pharmaceutical industry,” says Robert A. Singer, a process chemist at Pfizer. There are a number of attributes to like about the new work, Singer notes. Nickel is much more abundant and less expensive to use than palladium. The new catalyst is also air stable and less susceptible to ammonia deactivation or ligand dissociation.
The method does require more catalyst than might be ideal, however, which could pose a challenge in purifying final products, Singer notes. And the specialty phosphorus ligand is costly, which might require a replacement. But process chemists in pharma “will find this specific work interesting in that Stradiotto’s group demonstrates that nickel-catalyzed arylations of ammonia can be competitive with palladium-catalyzed technology,” Singer says.
Organic Synthesis: New iron-based catalyst links reactants, avoids heteroatom byproducts
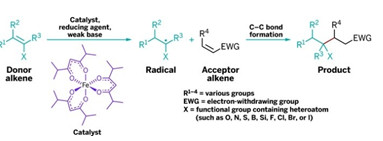
RADICAL REACTION
A new reaction uses an iron-based catalyst, a reducing agent, and a weak base to combine heteroatom-containing alkene “donors” with “acceptor” alkenes bearing electron-withdrawing groups.A new reaction uses an iron-based catalyst, a reducing agent, and a weak base to combine heteroatom-containing alkene “donors” with “acceptor” alkenes bearing electron-withdrawing groups.
Chemical groups containing oxygen, nitrogen, silicon, and other heteroatoms often react during alkene coupling reactions, generating undesirable product mixtures. But a new, iron-catalyzed radical reaction, developed by Phil S. Baran and coworkers at Scripps Research Institute, La Jolla, Calif., couples heteroatom-containing alkenes without all the messy side products (Nature 2014, DOI: 10.1038/nature14006). The heteroatom group remains unmodified while a carbon-carbon linkage forms in a highly controlled and predictable way.
About a year ago, Baran and coworkers developed a radical reaction in which an iron-based agent catalyzed the coupling of all-carbon alkene substrates (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja4117632). Although this method is useful and practical, the products it generates can also be obtained—albeit with difficulty, in some cases—from known reactions of nonalkene substrates such as alkyl halides, alcohols, and carboxylic acids. The researchers have now identified a new iron-based catalyst that extends the scope of the reaction to heteroatom-containing alkene substrates. The paper shows 60 products, “90% of which have never been made before,” Baran says.
The extended reaction uses the new catalyst, a reducing agent, and a weak base to form C–C bonds that link heteroatom-substituted olefin “donor” substrates to “acceptor” alkenes bearing electron-withdrawing groups. The catalyst removes an electron from the donor, producing a radical that then combines with the acceptor.
A variety of readily available heteroatom-containing alkene donors can be used, including enol ethers, enamides, vinyl boronates, vinyl thioethers, vinyl silanes, and vinyl halides. The reactions work at ambient pressure, and no special precautions need to be taken to exclude air and moisture. To prove just how versatile the reactions are, Baran and coworkers showed that they could be run in a variety of commercial alcoholic beverages as solvents.
“It is surprising that the substrate scope of the reaction can be extended to such a wide variety of heteroatom-substituted olefins,” comments senior synthetic chemist Jonas Brånalt of AstraZeneca R&D, in Mölndal, Sweden, who uses the Baran group’s original all-carbon alkene reaction in his lab. “The new methodology will allow synthetic chemists to access novel structures that would be either difficult or even impossible to make using traditional synthetic routes.”
The approach “has the potential to shift the retrosynthetic paradigm,” says catalytic reaction specialist Michael J. Krische of the University of Texas, Austin. “It unlocks new possibilities for the construction of C–C bonds that are otherwise difficult to achieve, and its operational simplicity—the use of an inexpensive iron catalyst in ethanol solvent—makes it especially attractive.” According to Baran, his team has some exciting follow-up plans: “Let me just say that this is the tip of the iceberg. There’s much more to come.”
Chemical Kinetics: Method reveals how pressure affects reaction rates without need for experimental data
Researchers have devised a new theoretical method for calculating the speediness of reactions whose rates depend strongly on pressure. In the past, such calculations have always needed at least a bit of empirical help, but the new technique does the job purely theoretically, with no experimental data needed.
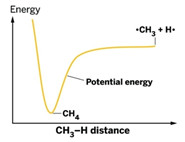 PRESSURE PROCESS
PRESSURE PROCESS
Reaction coordinate shows changes in potential energy during pressure-dependent radical dissociation of methane. Theoretical rate calculations on the system model not only these changes in energy but also changes in angular momentum (not shown).
The speed of some reactions depends mostly on temperature, and rates of those reactions could in many cases already be determined theoretically without experimental assistance. But rates of some reactions depend primarily on pressure, and calculating them has proven to be much more difficult.
The new technique could make it easier to obtain such information, especially for reactions for which experimental kinetics data are unavailable. Pressure-dependent reaction rates are important because researchers can use them to optimize reactions for industrial use, understand chemical reactions in space, and estimate environmental and other effects of combustion and atmospheric processes.
The new approach was developed by Stephen J. Klippenstein of Argonne National Laboratory, Ahren W. Jasper of Sandia National Laboratories, and coworkers (Science 2014, DOI: 10.1126/science.1260856). In each of the three reactions they studied, their theoretical rate predictions were quantitatively accurate—within about 20% of previous experimental data.
The work is “the first completely a priori, first-principles calculation of pressure-dependent reaction rates,” comments chemical kinetics modeling expert William H. Green of MIT. “All the other pressure-dependent rate calculations in the literature contain at least one adjustable parameter tuned to match experiments. Pressure-dependent reaction rates are important in almost all high-temperature situations above about 500 °C, including all of combustion and much of industrial chemistry, and also at low pressures, such as in interstellar space.”
The Argonne and Sandia scientists demonstrated the technique on hydrocarbon radical dissociation reactions, such as the pressure-dependent radical dissociation of methane. But they note that it could be used to model a wide range of other pressure-dependent reactions as well. In future work, they hope to demonstrate its applicability to complex multistep reactions, particularly those occurring in combustion processes.
Dissociating molecules in pressure-dependent reactions have two characteristic dynamic properties, total internal energy and angular momentum. The new procedure simulates pressure-dependent changes that occur in these two parameters as molecules collide and react. It then uses mathematical models to convert the resulting data into conventional chemical reaction rates, which depend on both pressure and temperature.
“Putting together the combination of calculations that are needed for this is a complicated business,” says theoretical chemistry specialist George C. Schatz of Northwestern University. “It is great to see that when this is implemented it is possible to calculate rates that accurately agree with careful measurements. This connection of theory and experiment has been long sought after.”
Polymer Science: Process marries right- and left-handed polymer chains to create a stereocomplex material with commodity potential
Taking advantage of a chiral cobalt catalyst, Geoffrey W. Coates and coworkers at Cornell University have copolymerized propylene oxide enantiomers and succinic anhydride to form poly(propylene succinate), the first member of a new class of thermoplastics (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja509440g).
A new chiral cobalt catalyst can take (S)- or (R)-propylene oxide and copolymerize it with succinic anhydride to make (S)- or (R)-poly(propylene succinate). The two polymers, when combined, form a stereocomplex material with improved thermal properties.
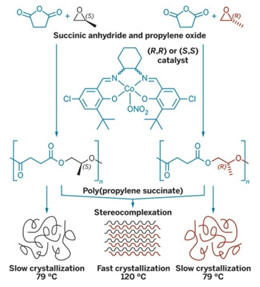
The team’s new polymer forms a semicrystalline stereocomplex, meaning it is a material made from combining right- and left-handed polymer chains. In addition to starting from commodity and biobased monomers and being inherently biodegradable, the polymer has an ability to form a stereocomplex, providing it with a melting point comparable to that of low-density polyethylene. These properties could one day make the new polymer competitive to polyethylene and isotactic polypropylene, the two most widely produced polymers in the world.
“Stereocomplexes in general spark the interest of chemists who enjoy structure,” says Kenneth B. Wagener, senior member of the George & Josephine Butler Polymer Research Laboratory at the University of Florida. “This new work is typical of the Coates group—making something new of chemistry well-known, epoxides and anhydrides in this case.”
Coates and graduate students Julie M. Longo and Angela M. DiCiccio first designed a chiral cobalt catalyst featuring N,N′-bis(salicylidene)cyclohexanediimine as a ligand. Using either the (R,R) or (S,S) version of the catalyst, the Cornell researchers copolymerized (R)- or (S)-propylene oxide with succinic anhydride to produce (R)- or (S)-poly(propylene succinate).
When right- and left-handed polymers are combined, they typically mix to form a random amorphous material or form a semicrystalline material with segregated right- and left-handed regions. The two polymer chains could also crystallize together in ways they can’t do alone, such as forming paired helices or interdigitated sheets to make a stereocomplex. This structural feature gives polymer chemists better control over the thermal properties and biodegradability of polymers. Stereocomplexes are exceedingly rare, however, with only about a dozen examples known.
When the Cornell team mixed right- and left-handed poly(propylene succinate), they found that the chains snuggled together to form a stereocomplex, the first known example for a an epoxide-anhydride copolymer. The stereocomplex has a melting point of about 120 °C, which is 40 °C higher than either of the polymers individually.
With the wide range of epoxides and cyclic anhydrides available, chemists should be able to create a broad class of new polymers, Coates notes. Currently, his team uses enantiopure propylene oxides, which are pretty expensive. “We are actively looking for a catalyst that will make the stereocomplex from racemic propylene oxide, which is considerably cheaper,” he says. Potential uses for poly(propylene succinate) include biomedical applications and large-scale packaging applications where biodegradability is needed. Cornell has patented the technology but has not yet licensed it for commercial development.
“This development bears the stamp of thorough expertise in homogeneous polymerization catalysis,” says Eric P. Wasserman, a senior research scientist at Dow Chemical. “It is relevant to the world of industrial polymers because it addresses issues with biodegradation, renewable raw materials, and the demands placed on modern plastics. In this case, that is the ability to crystallize quickly from the melt and have a melting point above 100 °C. In principle, this discovery could be a keystone of a new line of thermoplastic polymers.”
Chemical Engineering News
|
IX Международная конференция молодых ученых по химии “Менделеев-2015” Санкт-Петербург, Россия |
|
|
3rd International Conference on Advances in Applied Science and Environmental Engineering (ASEE 2015) Kuala Lumpur, Malaysia |
|
|
2015 Spring International Conference on Chemical Engineering (CEN-S) Beijing, China |
www.engii.org/scet2015 |
|
2015 Spring International Conference on Material Sciences and Technology (MST-S) Beijing, China |
|
|
World Coal-To-X 2015 Conference Beijing, China |
|
|
X Международный Конгресс “Биомасса: топливо и энергия” Москва, Россия |
http://www.biotoplivo.ru/kongress_
|
|
XII Международная конференция студентов, аспирантов и молодых ученых “Перспективы развития фундаментальных наук” Томск, Россия |
|
|
Программа повышения квалификации “Современные тенденции в получении и исследовании функциональных материалов” Томск, Россия |
|
|
IV Всероссийская конференция молодых ученых “Актуальные вопросы углехимии и химического материаловедения” Кемерово, Россия |
http://www.iccms.sbras.ru/ru/Pages/conf/conf-2015/conf_2015.aspx |
|
8th Annual World Congress of Industrial Biotechnology-2015 (IBIO-2015) Nanjing, China |
|
|
1st International Solar Fuels Conference (ISF-1) Uppsala, Sweden |
|
|
3rd International Symposium on Green Chemistry (ISGC 2015) La Rochelle, France |
|
|
E-MRS Spring Meeting Lille, France |
|
|
International Conference on Energy and Environmental Systems Engineering (EESE2015) Beijing, China |
|
|
V International Conference on Operando Spectroscopy (Operando V) Deauville, France |
|
|
12th International Conference on Chemical and Process Engineering (ICheaP12) Milan, Italy |
|
|
V Международная научно- техническая конференция “Альтернативные источники сырья и топлива” (АИСТ-2015) Минск, Беларусь |
|
|
International Conference NANOMEETING 2015 Minsk, Belarus |
|
|
X Всероссийская конференция “Химия и медицина” и Молодежная научная школа Абзаково, Уфа, Башкортостан |
|
|
2-я Всероссийская молодежная научно-техническая конференция “Инновации в материаловедении” Москва, Россия |
|
|
10-я Всероссийская конференция “Химия фтора” Томск, Россия |
|
|
17th International Conference on Materials, Methods & Technologies” Elenite Holiday Village, Bulgaria |
http://www.sciencebg.net/en/conferences/ |
|
International Symposium on Advances in Hydroprocessing of Oil Fractions (ISAHOF) Cuernavaca, Mexico |
|
|
28th European Symposium on Applied Thermodynamics (ESAT 2015) Athens, Greece |
|
|
3rd Conference on Catalysis (ICC 2015) Suzhou, China |
|
|
18th International Conference on Composite Structures (ICCS18) Lisbon, Portugal |
|
|
7-я Всероссийская цеолитная конференция с международным участием «Цеолиты и мезопористые материалы: достижения и перспективы» Звенигород, Россия |
|
|
6th International Congress on Ionic Liquids (COIL-6) Jeju, Korea |
|
|
12th International Symposium “Activation of Dioxygen and Homogeneous Catalytic Oxidation” (ADHOC 2015) Madison, Wisconsin, USA |
|
|
12th International Conference on Catalysis in Membrane Reactors (ICCMR12) Szczecin, Poland |
|
|
7th European Summer School on Electrochemical Engineering (ESSEE 2015) Leeuwarden, the Netherlands |
|
|
XIII Андриановская конференция “Кремнийорганические соединения. Синтез, свойства, применение” Москва, Россия |
|
|
18th IUPAC International Symposium on Organometallic Chemistry Directed Towards Organic Synthesis (OMCOS 18) Sitges/Barcelona, Spain |
|
|
10-я Международная школа молодых ученых и специалистов “Взаимодействие изотопов водорода с конструкционными материалами” Москва, Россия |
|
|
XII Всероссийская конференция с международным участием “Проблемы сольватации и комплексообразования в растворах” Иваново, Россия |
|
|
III Всероссийская конференция с международным участием “Топливные элементы и энергоустановки на их основе” Черноголовка, Россия |
|
|
5th European Fuel Cell Forum Lucerne, Switzerland |
|
|
XVIII международный симпозиум и школа молодых ученых “Молекулярная спектроскопия высокого разрешения” ( HighRus-2015) Томск, Россия |
|
|
9th International Summer Schools on Nanosciences & Nanotechnologies, Organic Electronics & Nanomedicine (ISSON 15) Thessaloniki, Greece |
|
|
7th International Conference on Green and Sustainable Chemistry (GSC-7) Tokyo, Japan |
|
|
V мемориальный семинар “Молекулярный дизайн катализаторов для процессов переработки углеводородов и полимеризации” Республика Алтай, Россия |
|
|
13th International Conference on Carbon Dioxide Utilization (ICCDU XIII) Singapore |
|
|
21st EuCheMs Conference on Organometallic Chemistry (EuCOMC-XXI) Bratislava, Slovakia |
|
|
2nd Russian Conference on Medicinal Chemistry and 6th Russian-Korean Conference “Current Issues of Biologically Active Compound Chemistry and Biotechnology” Novosibirsk, Russia |
|
|
12th International Conference on Nanosciences & Nanotechnologies (NN 15) Thessaloniki, Greece |
|
|
17th International Symposium on Relations between Homogeneous and Heterogeneous Catalysis (ISHHC17) Utrecht, Netherlands |
|
|
21st International Symposium on Olefin Metathesis and Related Chemistry (ISOM XXI) Graz, Austria |
|
|
9th International Symposium on Effects of Surface Heterogeneity in Adsorption and Catalysis on Solids (ISSHAC-9) Wrocław, Poland |
|
|
Catalysis: Fundamentals and Practice (Summer school) Liverpool, UK |
http://www.rsc.org/ConferencesAndEvents/ |
|
12th International Conference on Biocatalysis and Biotransformations (Biotrans 2015) Vienna, Austria |
|
|
1st International Conference on Applied Surface Science (ICASS) Shanghai, China |
|
|
45th World Chemistry Congress (IUPAC-2015) Busan, Korea |
|
|
34th International Conference on Solution Chemistry Prague, Czech Republic |
|
|
III International School-Conference on Catalysis “Applied Nanotechnology and Nanotoxicology” (ANT-2015) Kazan, Russia |
http://www.europacat2015.com/index.php/scientific-program/congress-satellites |
|
XII European Congress on Catalysis (EuropaCat XII) Kazan, Russia |
|
|
17th Conference on Materials Science and Engineering YUCOMAT 2015 Herceg Novi, Montenegro |
|
|
4th International School- Conference on Catalysis for Young Scientists “Catalyst Design. From Molecular to Industrial level” Kazan, Russia |
|
|
XI International Symposium on Heterogeneous Catalysis Varna, Bulgaria |
|
|
HYdrogen POwer – THeoretical and Engineering Solutions – International Symposium (HYPOTHESIS IX) Toledo, Spain |
|
|
3rd International Conference “Catalysis for Renewable Sources: Fuel, Energy, Chemicals” (CRS-3) Catania, Sicily, Italy |
|
|
International Summer School on Photovoltaics and New Concepts of Quantum Solar Energy Conversion (Quantsol 2015) Hirschegg, Austria |
http://www.helmholtz-berlin.de/events/
|
|
8th International Symposium on Feedstock Recycling of Polymeric Materials (ISFR) Leoben, Austria |
|
|
10-й Всероссийский симпозиум “Термодинамика и материаловедение” C.-Петербург, Россия |
|
|
Конференция “Графен: молекула и 2D кристалл” Новосибирск, Россия |
|
|
IV Symposium on Modeling of Exhaust-Gas After-Treatment (MODEGAT IV) Bad Herrenalb/Karlsruhe, Germany |
http://www.itcp.kit.edu/deutschmann/1746.php |
|
E-MRS Spring Meeting Warsaw, Poland |
|
|
VIII Научно-практическая конференция c международным участием “Сверхкритические флюиды: фундаментальные основы, технологии, инновации” Зеленоградск, Калининградская обл., Россия |
|
|
Международная конференция “Металлоорганическая и координационная химия: проблемы и достижения” (VI Разуваевские чтения) Нижний Новгород, Россия |
|
|
European Congress and Exhibition of Advanced Materials and Processes (Euromat 2015) Warsaw, Poland |
|
|
IX Международная конференция ”Химия нефти и газа” Томск, Россия |
|
|
10th European Congress of Chemical Engineering (ECCE10) Nice, France |
|
|
XXIX Научно-техническая конференция с участием иностранных ученых “Химические реактивы, реагенты и процессы малотоннажной химии” (РЕАКТИВ-2015) Новосибирск, Россия |
|
|
Международный Российско- Казахстанский Симпозиум “Углехимия и экология Кузбасса” Кемерово, Россия |
http://www.iccms.sbras.ru/ru/Pages/conf/ |
|
European Symposium on Chemical Reaction Engineering (ESCRE 2015) Munich, Germany |
|
|
5th Asian Symposium on Advanced Materials: Chemistry, Physics & Biomedicine of Functional and Novel Materials (ASAM-5) Busan, Korea |
|
|
3rd World Congress on Petrochemistry and Chemical Engineering (Petrochemistry-2015) Atlanta, USA |
|
|
4th Nano Today Conference Dubai, UAE |
|
|
|
|
|
Designing New Heterogeneous Catalysis: Faraday Discussion London, UK |
http://www.rsc.org/ConferencesAndEvents/ |
|
16th International Congress on Catalysis (ICC 16) Beijing, China |
|