22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы

В.Н. Пармон. С наступающим Новым годом
Г.М. Жидомиров, В.И. Авдеев, Л.Я. Старцева. VI Российская конференция "Механизмы каталитических реакций"
В.А. Собянин. Теория - практике. По следам VI Российской конференции "Механизмы каталитических реакций"
Л.Б. Юдина. Крупное событие в каталитическом мире
Л.Б. Юдина. Чтобы ориентироваться в современных реалиях
За рубежом.
EFCATS Awards

Вот и закончился 2002 год, год, оказавшийся очень важным для химиков-каталитиков России.
Так, после двенадцатилетнего перерыва мы впервые провели очередную общероссийскую конференцию из цикла "Механизмы каталитических реакций" (с международным участием). Традиционно именно эта конференция была и, надеемся, будет оставаться основным смотром сил отечественных химиков. Конференция уходящего года показала, что ряды наши, увы, существенно поредели. Но зато сколько произошло столь долгожданных встреч старых друзей-каталитиков бывшего СССР, а теперь стран СНГ. сколько новых молодых, энергичных и заинтересованных лиц мы увидели здесь! Уточнились не только главные действующие лица отечественного катализа на ближайшие годы, но и их нынешнее пространственное расположение.
В 2002 году впервые молодые ученые самостоятельно организовали и с успехом провели свою собственную молодежную конференцию-школу по катализу. Какое счастье было наблюдать это событие старшему поколению российских каталитиков!
В 2002 году укрепились позиции катализа и в верхнем эшелоне российской власти. В числе наиболее приоритетных направлений развития науки, освященных подписью президента России, безусловно, был специально отмечен и катализ.
Будем надеяться, что эти позиции удастся не упустить в наступившем 2003 году.
В России нам предстоит освоить технику переподготовки специалистов в области промышленного катализа, и очень важным в этом отношении будут январские курсы повышения квалификации по катализу, которые состоятся в Подмосковье.
Вновь возобновляются Российско-Американские семинары по катализу.
В конце лета нам предстоит очередной общеевропейский смотр каталитиков - конгресс EUROPACAT-6.
А сколько предстоит сделать каждому из нас на своем рабочем месте!
Поэтому давайте пожелаем друг другу в наступившем году оптимизма, больших успехов, радости в труде и жизни и, конечно же, здоровья.
Аннотационный отчет
1-5 Октября 2002 г. в Москве состоялась VI Российская конференция "Механизмы каталитических реакций". Предыдущие конференции проходили в Москве (1974, 1979, 1986, 1990 гг.) и в Новосибирске (1982 г.).
Конференция была организована Институтом катализа им. Г.К. Борескова СО РАН (Новосибирск) совместно с Московским государственным университетом им. М.В. Ломоносова (Москва) и поддержана Министерством промышленности, науки и технологий России,Научным советом по катализу ООТХ РАН (Москва), Российским фондом фундаментальных исследований, International Association for the Promotion of Cooperation with Scientists from the New Independent States of the former Soviet Union (INTAS) и Shimadzu GMBH, Germany.

Финансовую поддержку конференции оказали: ЗАО "Уралтехногенмет", Кировград, SASOL Germany GmbH, The Dow Chemical Company, Bayer AG, UOP LLC, Engelhard, ICI, Haldor Topsoe A/S.
Конференция "Механизмы каталитических реакций" проходила с участием иностранных ученых, которые прочитали часть пленарных и ключевых лекций, а также выступали со многими устными и стендовыми докладами в соавторстве с российскими исследователями.
Примечательно то, что многие доклады российских ученых, по существу, подводили итоги многолетних систематических исследований в определенной области, что, по-видимому, отчасти связано с длительным 10-ти летним перерывом в проведении крупных отечественных конференций по катализу.

В каталитическом форуме приняли участие 275 ученых, в т.ч. 224 участника из России, 15 - из стран ближнего зарубежья (Казахстана, Украины, Белоруссии и Армении) и 36 - из стран дальнего зарубежья (Германии, Франции, Польши, Финляндии, США, Италии, Японии, Австрии, Венгрии, Израиля, Норвегии и Великобритании).
Рабочими языками конференции были русский и английский.
Помимо общих пленарных и ключевых заседаний работа конференции проходила по трем параллельным тематическим секциям:
Программа Мемориальной секции "Механизм катализа на мембранах", посвященная 80-летию со дня рождения академика Владимира Михайловича Грязнова, включала пленарные лекции В.Д. Ягодовского "Научная деятельность академика В.М. Грязнова" (Российский университет дружбы народов, Москва) и А. Basile "Мембранные реактора риформинга метана окисью углерода" (University of Calabria, Rende, Italy), а также ключевую лекцию В.А. Собянина "Окислительная конверсия метана в электрохимическом реакторе с твердым кислородпроводящим электролитом" (Институт катализа им. Г.К. Борескова СО РАН, Новосибирск) и 4 устных сообщения, характеризующих мембранные катализаторы и касающихся работы мембранных реакторов.
На конференции были представлены 11 пленарных и 14 ключевых лекций, 105 устных докладов, также около 160 стендовых докладов, посвященных практически всем разделам гомогенного и гетерогенного катализа.
Конференция открылась пленарной лекцией проф. А. Белла (Калифорнийский университет, США), посвященной применению теории функционала плотности (DFT) для определения путей каталитических реакций. Проф. А. Белл широко известен своими экспериментальными исследованиями механизмов окислительно-восстановительных реакций на металлических и оксидных катализаторах, и этот его выбор тематики лекции наглядно свидетельствует о растущем интересе ведущих мировых каталитиков к возможностям квантовохимических расчетов каталитических систем. Лекция содержала результаты расчетов молекулярных механизмов (определение ключевых интермедиатов, переходных состояний, реакционных путей и активационных барьеров) реакции окисления бензола в фенол закисью азота на железо-содержащих высококремнеземистых цеолитных катализаторах, а также окислительного карбонилирования метанола в диметил-карбонат и окисления метанола в формальдегид на оксидно-ванадиевых нанесенных катализаторах.
Первая из этих реакций сейчас привлекает активное внимание в каталитической литературе после фундаментальных исследований данного процесса в работах под руководством д.х.н. Г.И. Панова (Институт катализа СО РАН, Новосибирск), доведенного до промышленной стадии в сотрудничестве с фирмой Солюша (США). В пленарной лекции Г.И. Панов обратил внимание на большие перспективы использования закиси азота как окислителя в каталитических окислительных реакциях. Молекула закиси азота может выступать как донор моноатомного кислорода, который стабилизируется на активном центре (АЦ) гетерогенного катализатора с формированием очень эффективного окисляющего интермедиата. Последнее было продемонстрировано на примере высокоселективных реакций гидроксилирования ароматических соединений на катализаторе Fe/ZSM-5. Значительная часть пленарной лекции была посвящена новому направлению, развиваемому в лаборатории Г.И. Панова - селективному жидкофазному окислению алкенов закисью азота без катализаторов, ведущему к ценным карбонильным соединениям с селективностью ~ 100%.
Необычные маршруты гидропероксидного окисления, катализируемого пероксокомплексами переходных металлов, были рассмотрены в лекции д.х.н. А.Е. Гехмана (Институт общей и неорганической химии РАН, Москва). Сейчас уже найден целый ряд систем, в которых наблюдается передача синглетного дикислорода как интермедиата активации гидроперекиси на молекулу ненасыщенного субстрата. Выявлены реакции, где пероксогруппа выступает в качестве одно- и трехэлектронного окислителя.
Различные проблемы редокс - катализа были одной из главных тем в материалах конференции. В лекции чл.-корр. РАН В.А. Лихолобова (Омский филиал Института катализа СО РАН, Омск) было показано, что использование смеси газов Н2 и О2 для окисления углеводородов является перспективной альтернативой традиционного метода окисления молекулярным кислородом. Эффективными катализаторами Н2/О2 окисления могут быть металлы VIII группы (Pd, Pt), желательно также, чтобы катализаторы содержали дополнительные компоненты, стабилизирующие необходимое окислительное состояние металла. Были приведены примеры таких каталитических систем на основе гетерополисоединений и обсуждена их активность в окислении алканов, циклогексана, бензола и алкенов.
Важную роль редокс-системы играют в экологическом катализе, одними из наиболее ярких примеров являются процессы селективного восстановления оксидов азота углеводородами либо аммиаком в присутствии кислорода. Исследованию этих каталитических процессов посвящена большая литература, несмотря на это многие вопросы остаются дискуссионными. В лекции д.х.н. В.А. Садыкова (Институт катализа СО РАН, Новосибирск) был подведен определенный итог систематическим исследованиям первого из этих процессов и показано, что прочно связанные поверхностные нитраты играют роль ключевых интермедиатов, а их трансформация при взаимодействии с углеводородами и кислородом является лимитирующей стадией реакции.
Лекция акад. И.И. Моисеева (Институт обшей и неорганической химии РАН, Москва) была посвящена каталитическим реакциям восстановительной дегидратации спиртов с формированием конденсированных структур. Был приведен ряд примеров таких реакций, продуктами которых являются углеводороды с увеличенным, по меньшей мере, в два раза числом углеродных атомов по сравнению с исходными спиртами.
Обзор исследований окислительной димеризации метана на оксидных катализаторах был представлен в лекции к.х.н. М.Ю. Синева (Институт химической физики РАН, Москва). Разработана кинетическая схема, основанная на представлении о гетерогенно-гомогенном протекании процесса, которую не удается формально разделить на химические микростадии на поверхности и в объеме газовой фазы. Предложенная модель позволила объяснить ряд эффектов, наблюдающихся при каталитической трансформации метана. На этой основе предлагается концепция проведения процессов в комбинированных каталитических слоях.
В лекции д.х.н. А.Я. Розовского (Институт нефтехимического синтеза РАН, Москва) на основе собственных и литературных данных была предложена согласованная схема реакций одноуглеродных молекул на медьсодержащих катализаторах: синтез метанола, синтез Фишера-Тропша, синтез диметилового эфира, синтез метилформиата, реакции водяного пара. В заключение была рассмотрена общая схема переработки природного газа в моторные топлива.
Механизмы синтеза Фишера-Тропша на разных металлических катализаторах были обсуждены в лекции проф. Х. Шульца (Университет Карлсруе, Германия). Было отмечено различие механизмов синтеза на кобальте (никеле, рутении) и железе. В случае кобальта каталитическую активность проявляет сам металл, тогда как хорошо восстановленное железо не активно и требуется формирование некоторых карбидных структур и графитизированного углерода, что и происходит при диссоциативной адсорбции СО. Сейчас уже можно, по-видимому, считать достаточно надежно установленным, что ключевым мономером в росте углеводородной цепи является фрагмент СН2.
В лекции д.х.н. О.Н. Темкина (Московская государственная академия тонкой химической технологии) были рассмотрены механизмы окислительного карбонилирования спиртов и алкинов в растворах Pd(II) и Pd(I). Удалось определить структуру ряда каталитически активных комплексов палладия, а также синтезировать новые.
Некоторые вопросы Циглер-Наттовского катализа полимеризации ненасыщенных соединений были обсуждены в лекции акад. Ю.Б. Монакова (Институт органической химии Уфимского научного центра РАН), при этом главное внимание было уделено исследованиям роли сокатализатора.
Лекция д.х.н. Б.Н. Кузнецова (Институт химии и химической технологии СО РАН, Красноярск) характеризует современное состояние исследований механизмов катализа в процессах превращений растительных полимеров. Рассмотрены современные представления о механизме каталитических реакций деполимеризации растительных карбогидридов и лигнина растительной биомассы, карбонизации растительных полимеров в углеродные материалы и др.
Возможное влияние электрофизических явлений (возникновение двойного электрического слоя на границе раздела фаз) на механизмы гетерогенного катализа было предметом обсуждения в лекции д.х.н. А.В. Путилова (Департамент развития технологий, Министерство науки, промышленности и технологий России, Москва). Были приведены примеры наблюдения таких эффектов в каталитических реакциях окисления, разложения метана и гидрирования бензола.
Концептуальная лекция о природе Бренстедовского катализа в растворах была прочитана акад. В.Б. Казанским (Институт органической химии РАН, Москва). Рассмотрена роль сольватационных взаимодействий в кислотно-каталитических превращениях олефинов в серной кислоте и показано, что основным фактором, определяющим силу сверхкислот, является слабая сольватация протонов.
Ряд лекций и докладов был посвящен отдельным типам катализаторов. В лекции чл.-корр. РАН А.Л. Лапидуса были обсуждены механизмы каталитического действия цеолитов системы пентасила в превращениях низкомолекулярных углеводородов (олефинов, парафинов). Были рассмотрены как декатионированные, так и металлсодержащие цеолиты и сформулированы принципы подбора эффективных катализаторов превращения олефинов С2-С4 в высокооктановое моторное топливо.
Новые результаты по установлению структуры АЦ в Ti- и Fe-силикалитах были представлены в лекции проф. А. Зеккины (Университет Турина, Италия). В отличие от титана железо легко покидает решеточные позиции и формирует внерешеточные структуры различных типов, которые могут стабилизироваться дефектами цеолитной матрицы.
В лекции д.х.н. Л.М. Кустова (Институт органической химии, РАН, Москва) были рассмотрены физико-химические и каталитические свойства суперкислотных систем на основе оксидов металлов, промотированных анионными добавкам (сульфатированный оксид циркония, массивные и нанесенные гетерополикислоты и т.д.), проведено их сравнение с цеолитными катализаторами и жидкими суперкислотами.
Современные методы синтеза, исследования структуры и каталитических свойств нанокластеров палладия, платины, никеля и кобальта были представлены в лекции д.х.н. М.Н. Варгафтика (Институт общей и неорганической химии РАН, Москва).
В исследованиях кинетики и механизма каталитических реакций, по-прежнему, привлекает внимание изучение критических явлений в гетерогенном катализе. Так, в докладе д.х.н. В.А. Городецкого (Институт катализа СО РАН, Новосибирск) были представлены результаты по наблюдению химических волн в реакциях окислительного катализа на платиновых металлах.
Ряд докладов включал теоретический анализ различных кинетических режимов. В докладе к.х.н. В.С. Музыкантова (Институт катализа СО РАН, Новосибирск) был предложен новый подход в интерпретации данных изотопного обмена кислорода в оксидных катализаторх, исключающий временную координату.
Обсуждению автоколебательных режимов в кинетике гетерогенных каталитических реакций посвящена лекция
к.х.н. М.М. Слинько. Особое внимание было уделено возможности получения дополнительной информации о механизмах реакций из наблюдения осцилляционной кинетики.
Исследованиям редокс-катализа была посвящена отдельная секция конференции. Были представлены результаты исследования широкого круга процессов и различных катализаторов; среди них были как новые, так и традиционно изучаемые системы, в последнем случае интерес был связан либо с новыми результатами исследований, либо с перспективными модификациями известных катализаторов. Так, в докладе д.ф-м.н. Д.И. Кочубея (Институт катализа СО РАН, Новосибирск) были приведены результаты изучения влияния добавок кобальта на активность катализаторов гидрообессеривания типа MoS2/Al2O3 и сделано заключение, что активные центры процесса могут локализоваться на деформированной базальной плоскости катализатора.
В докладе д.х.н. О.В. Крылова (Институт химической физики РАН, Москва) проведен детальный анализ механизма действия нанесенных никелевых катализаторов, модифицированных гетерополисоединениями (ГПС) вольфрамового ряда. Была установлена общая структура АЦ в виде трехслойного катализатора никель-ГПС-носитель. Это позволило получить новый тип катализатора с активностью, приближающейся к платиновым катализаторам.
В докладе д.х.н. З.Р. Исмагилова (Институт катализа СО РАН, Новосибирск) был дан обзор многолетних исследований окисления спиртов и аминов на оксидных катализаторах (Ti2O, V2O5, Cr2O3, MnO2, Fe2O3, Co3O4, NiO, CuO, ZnO), на основе которых определена природа радикалов и их роль в формировании АЦ на поверхности каталитических гранул.
Природа АЦ на поверхности серебряных катализаторов в парциальном окислении одноатомных спиртов и этиленгликоля анализировалась в докладе д.х.н. Л.Н. Куриной (Томский государственный университет, Томск).
Реакции на сульфидных катализаторах гидропереработки нефтяных фракций были предметом рассмотрения в докладе д.х.н. А.Н. Старцева (Институт катализа СО РАН, Новосибирск). Рассмотрен и обоснован концертный механизм реакции гидрообессеривания нефтяных фракций на биметаллическом АЦ. Была представлена оригинальная концепция механизма формирования и действия АЦ этих катализаторов.

Традиционно, большое внимание в материалах конференции было уделено применению физических (прежде всего спектральных) методов исследования каталитических систем. Нужно отметить, что эта секция пользовалась наибольшим вниманием участников конференции, зал часто был переполнен. При обсуждении результатов физико-химических исследований каталитических процессов для повышения информативности привлекают результаты изучения модельных систем и тогда могут возникать проблемы состыковки результатов, полученных в несколько отличных условиях, получившие в литературе аббревиатуру "pressure gap" и "material gap". Эти проблемы были подробно проанализированы в лекции д.х.н. В.И. Бухтиярова (Институт катализа СО РАН, Новосибирск) на примере катализа реакции эпоксидирования этилена на металлических серебряных катализаторах.
Применение физических методов в науке о катализе составило методическую основу секции конференции, посвященной методам исследования механизма каталитических реакций. Активно привлекается метод 1Н-, 2Н-, 3С- ЯМР для исследования цеолитных катализаторов, 51V-ЯМР в изучении нанесенных ванадиевых катализаторов. Новым направлением здесь является применение ЯМР томографии для исследования явлений массопереноса и каталитических реакций, активно развиваемым в совместных работах Международного томографического центра и Института катализа СО РАН в Новосибирском научном центре. Полученные результаты и перспективы таких исследований были обсуждены в докладе к.ф-м.н. И.В. Коптюга и др.
Как всегда, очень большой спектр приложений имеет колебательная спектроскопия, как для изучения адсорбционных комплексов, так и кинетических исследований "in situ".
Интересное направление применения ИК-спектроскопии для изучения возбужденных состояний молекул было представлено в докладе д.ф-м.н. А.А. Цыганенко (Петербургский университет).
В докладе д.х.н. Е.А. Паукштиса (Институт катализа СО РАН, Новосибирск) были обсуждены новые возможности применения ИК- и оптической спектроскопии в исследовании промышленных и лабораторных катализаторов на примере использования спектрометров Shimadzu FTIR-8300 UV-Vis-4501.
Ряд докладов включал результаты исследований методами рентгено- и фото-эмиссионной и рентгеновской спектроскопии.
Сравнительно мало было представлено исследований, использующих строгие квантовохимические методы. Помимо стартовой лекции проф. А. Белла, в сообщениях к.х.н. В.И. Авдеева и к.х.н. И.И. Захарова (Институт катализа СО РАН, Новосибирск) была продемонстрирована эффективность использования методов типа DFT для анализа природы АЦ и реакционных путей на поверхности катализатора.
В рамках конференции была проведена презентация нового журнала "Катализ в промышленности".
Следует отметить большое количество работ, получивших финансовую поддержку ИНТАС, РФФИ и других международных фондов.
Конференция завершила свою работу презентацией Института катализа им. Г.К. Борескова (акад. В.Н. Пармон) и Московского государственного университета им. М.В. Ломоносова
(акад. В.В. Лунин).

На заключительном заседании конференции:
Г.М. Жидомиров, В.И. Авдеев, Л.Я. Старцева
Институт катализа им. Г.К. Борескова
СО РАН, Новосибирск
VI Российская конференция "Механизмы каталитических реакций" в определенном смысле явилась преемницей широко известных во времена СССР Всесоюзных конференций "Механизмы каталитических реакций", которые обычно проводились в Москве, первая - в 1974 г., а последняя, пятая, - в 1990 г.
По существу, эта конференция является самым крупным за последние 12 лет отечественным форумом каталитиков, на котором обсуждались фундаментальные проблемы и вопросы по всем разделам гетерогенного и гомогенного катализа. Вместе с тем, несмотря на теоретическую направленность конференции, в ряде докладов были приведены результаты, которые уже сейчас представляют серьезный интерес для промышленности и именно поэтому заслуживают особого внимания в этом сообщении. Может быть субъективно, что вполне естественно, сюда следует отнести результаты, полученные под руководством д.х.н. Г.И. Панова (Институт катализа им. Г.К. Борескова, СО РАН) в области синтеза кислородсодержащих органических соединений с использованием закиси азота, и результаты, полученные под руководством д.х.н.
Б.Н. Кузнецова (Институт химии и химической технологии, СО РАН) в области переработки возобновляемого растительного сырья. Эти результаты были представлены в пленарных лекциях Г.И. Панова и Б.Н. Кузнецова. Рассмотрим их более подробно.
В пленарной лекции Б.Н. Кузнецова "Современные представления о механизме действия катализаторов в процессах превращения растительных полимеров" обсуждались современные представления о механизме каталитических реакций деполимеризации растительных карбогидратов в органические соединения и деполимеризации лигнина растительной биомассы экологически чистыми реагентами с получением целлюлозы и низкомолекулярных соединений. В частности, было показано, что путем подбора катализаторов и условий осуществления процесса гидролиза растительных карбогидратов удается повысить
выход ценных соединений левулиновой кислоты, гидроксиметилфурфурола, левоглюкозенона, фурфурола и их производных. С точки зрения прикладного катализа наибольший интерес представляет завершенная авторами разработка процесса получения ванилина окислением лигносульфонатов молекулярным кислородом в присутствии медьсодержащего катализатора. По сравнению с известными промышленными процессами предложенная разработка позволяет увеличить скорость каталитического процесса почти в 10 раз, селективность по ванилину - в несколько раз и сократить на порядок продолжительность экстракционного выделения ванилина при использовании экологически безопасных экстрагентов.

Г.И. Панов в своей пленарной лекции "Новые реакции окисления в органическом синтезе" показал большие перспективы применения закиси азота в качестве окислителя углеводородов в кислородсодержащие органические соединения. В гетерогенном окислительном катализе необычные свойства закиси азота как окислителя связаны с новой формой поверхностного кислорода (a -форма), которая образуется на особых комплексах Fe в цеолитных матрицах. Для окислительного катализа a -кислород можно назвать уникальным. Он совмещает в себе два, казалось бы несовместимых, свойства: высокую реакционную способность и высокую селективность. Реакционная способность a -кислорода настолько высока, что он уже при комнатной температуре способен окислять различные углеводороды, включая метан, приводя к селективному образованию гидроксилированных продуктов.
Наиболее ярким примером использования закиси азота как окислителя является реакция окисления бензола в фенол на катализаторе Fe/ZSM -5. На этой основе Институтом катализа им. Г.К. Борескова СО РАН совместно с американской фирмой Solutia (Monsanto ) разработан новый одностадийный фенольный процесс, который успешно прошел пилотные испытания и включен фирмой в план промышленного внедрения.
В этой же лекции Г.И. Панов впервые представил новую многообещающую область применения закиси азота - некаталитическое жидкофазное окисление алкенов в карбонильные соединения:
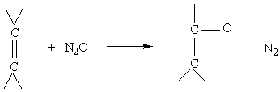
Карбонильные соединения (альдегиды и кетоны) относятся к числу важнейших продуктов органической химии. В настоящее время их получение, как правило, ведется путем сложных малоэффективных технологий. Авторы провели широкий скрининг субстратов и показали, что жидкофазное окисление закисью азота применимо для карбонилирования алкенов различных классов, включая линейные, циклические, гетероциклические алкены, а также диеновые углеводороды. Реакции протекают в области
200-250ОС с высокой селективностью, которая во многих случаях близка к 100%.
Закись азота является экологически чистым и недорогим окислителем. Стоимость активного кислорода в N 2 O в 4-5 раз ниже, чем в пероксиде водорода. Это открывает большие перспективы для создания новых технололгий синтеза ценных органических веществ с использованием N2O как в газовой, так и жидкой фазах.
В целом, работа конференции показала, что весьма активно, хотя и не столь широко, как прежде, исследования в области катализа ведутся во многих научных и образовательных учреждениях России, и наука о катализе, несмотря на кризисные годы, до сих пор в нашей стране остается в почете.
В.А. Собянин
Институт катализа им. Г.К. Борескова
СО РАН, Новосибирск
Безусловно, крупнейшим событием уходящего года
химики-каталитики называют прошедшую в Москве VI Российскую конференцию "Механизмы каталитических реакций" (с международным участием), основным организатором которой выступили Институт катализа им. Г.К. Борескова Сибирского отделения РАН и Московский государственный университет
им. М.В. Ломоносова. Это был своего рода съезд каталитиков, которые в таком составе не собирались, считай, лет десять. На каталитическом форуме было представлено 11 пленарных и
14 ключевых лекций, 105 устных докладов, около 160 стендовых докладов практически по всем разделам гомогенного и гетерогенного катализа.

Корреспондент газеты "Наука в Сибири" Людмила Юдина обратилась к двум участникам конференции, членам оргкомитета, с просьбой дать оценку (скорее эмоциональную), конгрессу (называли и так) каталитиков.
Валерий Бухтияров, молодой заместитель директора Института катализа, доктор химических наук.
В.Б. Конференцию я бы назвал долгожданной. Это был смотр лучших сил практически из всех академических институтов, где активно ведутся исследовательские работы в сфере каталитической химии! Мы долго не встречались - кризисные годы не особенно располагали к мероприятиям подобного масштаба. Но работы по разным направлениям обозначенной темы, разумеется, велись, может быть не столь широко, как прежде, но более концентрированно и по приоритетным направлениям.
Конференция позволила на всероссийском уровне обменяться последними достижениями, представить новые направления, возникшие за последние годы школы. А это интересно и корифеям, которых на конференции было немало, и молодым ученым, которые, я бы отметил, выглядели в Москве очень достойно.
Л.Ю. Какое из направлений, обсуждаемых на форуме, привлекло особое внимание?
В.Б. Могу оценить, исходя из области своих профессиональных интересов. Безусловно, развитие и использование физических методов для изучения механизмов каталитических реакций. Причем, на атомарном уровне. В режиме in situ, то есть, в условиях реальных каталитических процессов.
Интереснейшие доклады были сделаны немецкими коллегами. Любопытные результаты представили сотрудники нашего Института катализа и многие московские ученые. Тезисы всех докладов "едва поместились" в 2 томах.
Цикл пленарных докладов открывал Почетный доктор Сибирского отделения РАН, профессор Алекс Белл (University of California, Berkeley, USA).
Л.Ю. Это дань уважения большому другу СО РАН и Института катализа, или его работы имеют основополагающее значение?
В.Б. В силу той и другой причин. Профессор А. Белл широко известен своими экспериментальными исследованиями механизмов окислительно-восстановительных реакций на металлических и оксидных катализаторах. Его лекция была посвящена изучению молекулярных механизмов гетерогенного катализа с квантово-химической точки зрения.
На нашем съезде каталитиков было много ярких личностей, жарких дискуссий и интересных встреч.. О высоком уровне конференции свидетельствует и тот факт, что на заключительном заседании был заслушан и одобрен Устав Российского каталитического общества.
Илья Мишаков, аспирант Института катализа.
И.М. Впечатления самые прекрасные! Тем более, что многие тонкости наблюдал изнутри, работая в оргкомитете.
Л.Ю. За что отвечали?
И.М. Миссия у меня была не столь важная - отвечал за аппаратуру в конференц-зале. По этой причине не смог прослушать многие интересные доклады, которые шли на двух других параллельных секциях. Кстати, на конференции работали три подсекции практически по одной тематике и частенько получалось так, что тематика докладов совпадала. "Знаешь, - говорил кто-нибудь, - я сейчас бы с удовольствием пошел не на свой доклад в конференц-зал, а послушал такого-то...".
В следующий раз надо эту деталь учесть и постараться сделать так, чтобы интересы не пересекались.
Докладов интересных было множество! Можно привести немало примеров сообщений, сделанных на высоком уровне, с прекрасным подбором и оформлением слайдов, их подачей. И здесь один из лучших - норвежский ученый Джавер Перез-Рамирез (Norsk Hydro ASA, Research Centre, Porsgrunn).

Очень здорово было общаться с людьми разного уровня, обсуждать на равных серьезные проблемы. Когда я делал стендовый доклад, ко мне подходило много коллег, даже из нашего института. Наш директор, академик Валентин Николаевич Пармон, заметил - в одном институте работаете, а встречаетесь и договариваетесь в Москве.
А ведь верно, мы иногда и не знаем, что делается нового в соседней лаборатории. Потому на таких вот сборах самое главное - встречи, наведение мостов.
Л.Ю. Илья, говорят, что Вы на исходе года совершили подвиг - буквально сразу после конференции защитили кандидатскую диссертацию, еще не закончив аспирантуры. Кстати, тема Вашей диссертации?
И.М. На подвиг, это, конечно, не тянет! Но конференция была своеобразной проверкой моей работы, тема которой - "Разработка методов утилизации органических отходов".
Л.Ю. Дальнейших Вам успехов в Новом году!
"Наука в Сибири" январь, 2003
В первые дни морозного декабря в Институте катализа прошла международная конференция молодых ученых "Каталитический дизайн: от исследований на молекулярном уровне -- до практического применения". Подобного форума не проводилось лет двадцать. Потому настроение было особенно радостным, а дискуссии -- горячими.

"Какова главная особенность проведенного мероприятия?" -- с таким вопросом я обратилась к председателю Совета научной молодежи Института катализа, председателю оргкомитета молодежной конференции по катализу Вадиму ЯКОВЛЕВУ.
-- Пожалуй, одна из главных линий форума -- подсказать молодым исследователям, как ориентироваться в современных реалиях. Ну и -- познакомиться, узнать, чем каждый занимается в области катализа.
Приехали на конференцию почти 200 человек из 28 городов России и стран ближнего зарубежья. Участвовали в ее работе и 9 настоящих иностранцев.
17 пленарных лекций прочли ведущие специалисты в области катализа. Это были глубокие, проблемные доклады. Но должен заметить, что многие молодые участники -- более сорока сообщений сделали они, также представили интересные работы.
На конференции прозвучало не только много лекций по фундаментальным направлениям катализа, но и сообщения об инновационной деятельности, трансферту технологий. Речь шла и о защите интеллектуальной собственности -- патентная деятельность, заключение лицензионных соглашений и т.д.
-- Своего рода ликбез для молодежи?
-- Сегодня эти вопросы чрезвычайно важны и во многом определяют эффективность научной работы.
-- Как я поняла -- это была не просто конференция, а школа. Какова атрибутика последней?
-- Проводилось несколько практикумов -- по химической кинетике, моделированию химических процессов на компьютерах, по решению задач для сдачи кандидатского экзамена по специальности "катализ" и т.д. Участники школы-конференции побывали в лабораториях института.
Провели также круглый стол о деятельности советов научной молодежи. Выступил я, председатели советов научной молодежи из Омска и Томска.
-- Проблемы молодежные у вас у всех одни?
-- Как и сферы деятельности -- проведение научных мероприятий и решение социальных проблем.
-- Можно ли было определить, где молодежные проблемы решаются лучше?
-- Лучше -- у нас в Институте катализа.
-- И даже жилищные вопросы?
-- Во всяком случае они решаемы. У института, помимо ведомственного жилья, есть определенная квота в общежитиях СО РАН. Но там, не побоюсь сказать, за многие годы образовалась большая неразбериха. Кто-то давно выехал из общежития, получил квартиру или отбыл за границу. А комнаты, которые за этими людьми числятся, пустуют. Вот и пытаемся решить вопрос так, чтобы и волки были сыты, и овцы целы.
-- Удается?
-- Да, многие ребята уже благодаря этой акции переехали в общежитие.
-- К молодым в институте прислушиваются?
-- Во всяком случае, с нами считаются. Мы стали более организованы, к нам обращаются чаще при решении социальных вопросов для молодежи ИК, по проведению научных и культурных мероприятий. В институте, например, мы проводим конкурс молодежных проектов. Организация конкурса и контроль за выполнением победивших проектов полностью возложены на СНМ ИК. Победившие получают определенную сумму на развитие исследований. Должен заметить, что дирекция очень нас поддерживает.
-- Спрошу еще о прошедшей недавно молодежной конференции. Сколько дней работали и в каком режиме?
-- Пять дней с девяти утра до шести вечера -- лекции, дискуссии, постерные сессии и прочая научная деятельность. Вечера -- для отдыха, программа была весьма разнообразной. Устраивали даже баньку с веничком. Собирались на шашлыки в лес выехать, да мороз помешал.
Приятно было слышать, как оценивают работу оргкомитета участники конференции: "Организация на высоком уровне!". А иностранцы добавляли: "Порядок, как у немцев".
Конечно, нам очень помогли совет научной молодежи ННЦ, дирекция Института катализа, стратегический партнер ИК АО "Катализатор", а также партнеры ИК -- ТД "Аэролайф" (г. Москва) и ООО "Фототехнологии" (г. Новосибирск) -- их представители выступили с сообщениями о деятельности коллективов.
В общем, молодежная конференция оставила у всех очень приятные впечатления.
Л. Юдина
"Наука в Сибири", декабрь, 2002
Suzuki coupling catalyzed by palladium shells
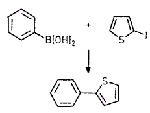
Hollow palladium spheres fabricated in the laboratory have been successfully used as a recyclable heterogeneous catalyst for Suzuki coupling reactions {J. Am. Chem. Soc., 124, 7642(2002)}. Taeghwan Hyeon, an associate professor of chemical engineering at Seoul National University, in South Korea, and coworkers prepared spherical shells that are 300 nm across and consist of 10-nm palladium particles. The large surface area of these shells makes them highly active catalysts in Suzuki cross-coupling reactions such as the one shown here. In previous studies, other groups reported that palladium nanoparticles used in these reactions agglomerated after one cycle, resulting in a loss of catalytic activity. УTo our surprise," Hyeon and coworkers write, "[our Pd spheres] maintained their catalytic activities even after seven recycles. In addition, simple filtering can retrieve the catalyst from the reaction pot." Hyeon Says this heterogeneous catalyst is nearly as active as the most popular homogeneous palladium catalyst used for Suzuki coupling. Furthermore, he believes the new catalyst could also be applied to other important carbon-carbon coupling reactions.
C & en /JuLY 1, 2002
In search of actinide catalysts
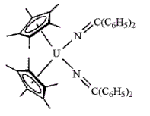
In recent years, researchers at Los Alamos National Laboratory's Chemistry Division, led by Carol J. Burns and Jaqueline L. Kiplinger, have been exploring the potential of actinide metallocene complexes as "frameworks for chemical transformations." The additional bonding possible by actinide metal f orbitals means that appropriately designed complexes could display unique catalytic activity not afforded by transition metals, Burns notes. In their latest effort, the Los Alamos chemists report the synthesis of the first f-element ketimido complex (shown) [Organometallics, 21, 3073 (2002)]. The uranium (IV) complex has unusually high thermodynamic stability, similar to uranium imido complexes the researchers have previously prepared, which they ascribe to nitrogen's π-electron donation to uranium's f orbitals. Although not reactive enough to serve as catalyst, the new complex sheds light on actinide metal orbital involvement in ligand bonding, Burns says, and will help guide future work to discover useful catalysts.
C & en /JuLY 22, 2002
Sumitomo has new process
Sumitomo has developed a process for producing fatty acid methyl esters by reacting methanol with vegetable oil heated to over 240 *C. Traditional methods for producing fatty acid methyl esters require the use of alkaline catalysts that create undesirable by-products. Fatty acid methyl esters are mostly used to make higher alcohols that, in turn, are raw materials for surfactants. Sumitomo hopes to license its technology to other companies.
Metal-catalyzed cycloadditions add up to eight-membered rings
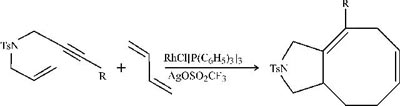
Last spring, chemistry professor Paul A. Wonder's team at Stanford University described how to build eight-membered rings with rhodium-catalyzed [5+2+1] cycloadditions [J. Am. Chem. Soc., 124, 2876 (2002)]. Now, in back-to-back papers in JACS, two other research groups show how to do the math a bit differently by using [4+2+2] cycloadditions. Like other medium-sized rings, those with eight carbons occur in many natural products and drugs but are notoriously difficult to prepare. In the approach of chemistry professor P. Andrew Evans and coworkers at Indiana University, Bloomington, intermolecular cycloaddition of heteroatom-tethered enyne derivatives with butadiene yields bicyclic heterocycles, as shown [J. Am. Chem. Soc., 124, 8782 (2002)]. Meanwhile, at Washington University, St. Louis, chemistry professor Scott R. Gilbertson's group finds that cyclization of alkynes with dieneynes gives eight-membered-ring products (page 8784). Both groups use rhodium catalysts modified with silver salts, and both note that the exact nature or amount of the silver salt profoundly affects the reactions' outcomes.
C & en /JuLY 29, 2002
Large-scale carbon nanofibre synthesis
Carbon nanotubes and their counterparts with no central channel, carbon nanofibres, are being investigated for their special electronic and structural properties in device applications, and as nanotips in atomic force microscopy. In many cases, it is not enough to make a bunch of carbon nanotubes/nanofibres. More importantly, they have to be grown at predetermined locations on a substrate, with control over length, diameter, shape, chemical composition and orientation. It was recently noted that the angle of alignment is in fact influenced by an applied electric field. V. Merkulov et al have devised a process where an electric field can help in the fabrication of oriented carbon nanofibres in a large-scale synthesis (Appl Phys Lett 2002, 80, 4816).

Electric fields lines control the direction a nanofibre grows during FECVD
The authors set out by making catalyst nanodots (composed of a Ni-Fe alloy) on a silicon wafer using electron-beam lithography and metal evaporation. The carbon nanofibres were then grown from these catalyst sites by plasma-enhanced chemical vapour deposition (PECVD) of acetylene gas, in the presence of ammonia. As the plasma is generated between two electrodes, the carbon nanofibres tend to grow perpendicular to the cathode, which is normally the substrate. When an additional sample holder (with the substrate on top) is positioned in between the electrodes, the field lines located far enough from the edges will still be aligned perpendicular to the substrate's surface. However, around the corners the electric field lines bend significantly, and carbon nanofibres that are allowed to grow there will do so at an angle to the surface (see Figure). So, by choosing where to place the substrate, the alignment angle can be varied by up to 40*. Even kinked nanostructures are possible if the substrate is repositioned half-way through the PECVD process.
Another strategy how to control the fabrication of aligned carbon nanofibres has been devised by S. Huang, L. Dal and A. Mau (Adv Mater 2002, 14, 1140). By patterning a specially developed metal-containing photoresist followed by a calcination/reduction step, they were able to generate catalyst nanodots anywhere they wanted on a substrate. Aligned carbon nanofibres grew from these dots during acetylene pyrolysis. Having demonstrated the principle, the authors became a bit more imaginative with their choice of photomask, in their case a conventional black-and-white film. Rather than using a regular array of dots, they drew chemical structures, formulae and orbital shapes, photographed a high-quality printout on paper, then replicated a reduced image with carbon nanofibres on a substrate.
Chemistry&Industry - 1 October 2002
HTTP://PUBS.ACS.ORG/CEN
"Synetix Award for Innovative Catalysis" and "Francois Gault Lectureship 2004" will be granted during the EUROPACAT-IV meeting to researchers who have significantly contributed over the past five years to innovation in catalysis.
The former award, sponsored by Synetix, consists of an award of $2000 to a researcher who has contributed especially to the opening of new areas for applied and industrial catalysis. The Synetix Award for Innovative Catalysis for the year 2002 was granted to Professor Pierre Jacobs.
The Francois Gault Lectureship 2004 consists of a series of lectures given throughout Europe and will be assigned also to a researcher who has significantly contributed to the progress of research on catalysis, from fundamental to applied aspects. The Francois Gault Lecturer 2002 has been Professor Avelino Corma Canós .
The nominations for candidates should be sent by the end of February 2003 to the secretary of EFCATS (prof. Outi Krause) with a letter of support. The nominations should be made preferably by the national catalysis associations. The Synetix Award will be decided by a board nominated by EFCATS and Synetix. The Francois Gault Lecturer 2004 will be decided in the EFCATS council meeting to be held in Innsbruck.
EFCATS Ph. D. Award will instead consists of one or more awards of ?1000 each (depending on the quality of the submissions) for innovative PhD theses in catalysis published in the period June 2002-June 2003. The nominations should be sent by the supervisor of the thesis and should include (i) a copy of the thesis (+ a short English summary), (ii) an accompanying letter from the PhD supervisor and (iii) a comment letter from an external referee. The deadline for nominations is July 15, 2003. Also these awards will be assigned during EUROPACAT-IV.