
Академик
Георгий Константинович Боресков.
Учёный и патриот – дела для Родины могущества

Георгий Константинович Боресков
20.04.1907 - 12.08.1984
| Академик АН СССР, выдающийся ученый-химик и
инженер, крупный организатор науки и талантливый педагог. |
| Основатель Института катализа и его директор с момента создания и до 1984 года. |
|
Работы Г.К. Борескова в области катализа, химической
кинетики и химической технологии получили мировое признание. |
Введение
20 апреля 2022 года исполнилось 115 лет со дня рождения Георгия Константиновича Борескова, весь творческий путь которого был посвящен катализу – как решению фундаментальных вопросов предвидения каталитического действия и научных основ приготовления катализаторов, так и задач химической кинетики и химической технологии, связанных с применением гетерогенного катализа на практике. Деятельность Георгия Константиновича и его научной школы достаточно ярко освещена в ряде его трудов, в том числе крупных обзорах, в воспоминаниях его коллег и учеников [1-5, 10]. Однако определённый пласт научных и инженерно-технологических разработок Георгия Константиновича, направленных на укрепление обороноспособности нашей страны, в силу объективных обстоятельств, не был в достаточной степени освещён. Пользуясь появившейся в наше время возможностью доступа к «раскрытым» источникам и опубликованным воспоминаниям современников, в представленном очерке была сделана попытка восполнить этот пробел
I. Серная кислота – «хлеб» для гидрометаллургии стратегических металлов,
химии взрывчатых веществ и ракетных топлив
Серной кислоте принадлежало и принадлежит одно из первых мест в объёме производства неорганических веществ. Так, в 2020 году в мире было произведено около 300 млн тонн кислоты, из них около 15 млн тонн – в России. Наиболее широкое применение серная кислота находит в производстве фосфорной кислоты и фосфорсодержащих минеральных удобрений, в гидрометаллургических процессах получения цветных и редких металлов, а также в технологиях синтеза компонентов углеводородных топлив, красителей и взрывчатых веществ.
Становление глубоких научных и технологических исследований получения серной кислоты из природного или техногенного сырья (сульфидные руды, сера, отходящие серосодержащие газы металлургических производств и нефтепереработки) в начале ХХ века характеризовалось определённой закрытостью ин-формации, особенно в плане публикации патентов. Для некоторых патентов проходило 10-20 лет от регистрации изобретения до его полнотекстовой публикации. Это обстоятельство в значительной степени требовало от руководства нашей страны при создании собственной сернокислотной промышленности рассчитывать «только на себя».
В те далёкие годы начала ХХ века при контактном, т.е. с использованием гетерогенного катализа, производстве серной кислоты для окисления двуокиси серы в серный ангидрид во всём мире применялся дорогостоящий платиновый катализатор. Катализатор представлял собой высокодисперсные частицы металлической платины, нанесённые, в зависимости от рецептуры, или на сульфат магния, или на асбест, или на силикагель. Хотя платиновые катализаторы проявляли высокую, достаточную для проведения технологического процесса, активность уже при температурах около 400°C, они имели очень низкую стойкость к таким контактным ядам как соединения мышьяка, сурьмы и селена, появляющиеся в диоксиде серы при её получении путём обжига сульфидных руд. Этот недостаток, из-за возникающего ограничения сырьевой базы, а также дороговизна платины существенно сдерживали рост производства серной кислоты, требующейся для новых областей применения в промышленности. Особенно это касалось областей, связанных с обороноспособностью государства (гидрометаллургия стратегических металлов: Zn, Pb, Mo, Cd, Co, U и др.; новые виды порохов для ракетных систем и мощных взрывчатых веществ, получаемых на основе продуктов нитрования смесью H2SO4/HNO3 различных органических соединений). Здесь, в качестве примера, особо следует упомянуть композит «нитроцеллюлоза + нитроглицерин + нитрогуанидин» – топливо ракетного снаряда «Катюши».
Именно с разработки нового катализатора для одного из важнейших промышленных процессов – производства серной кислоты – началась научная деятельность Георгия Константиновича Борескова. По окончании Одесского химического института он был принят в 1928 году в лабораторию катализа Одесского химико-радиологического института Наркомтехпрома на должность научного сотрудника. Самостоятельность молодого ученого проявилась с первых шагов в науке, когда он начал формировать собственную концепцию катализа, основанную на химической природе этого явления. В возрасте 25 лет он уже заведует лабораторией катализа и почти одновременно возглавляет кафедру процессов и аппаратов в Одесском химико-технологическом институте.

Г.К. Боресков – заведующий лабораторией катализа Одесского химико-радиологического института, 1932 г.
При изыскании нового гетерогенного катализатора окисления двуокиси серы в серный ангидрид Г.К. Боресков, учитывая данные о свойствах в этой реакции оксидов ряда переходных эле-ментов (железа, меди, ванадия, хрома, марганца, молибдена, свинца и др.), за основу выбрал пятиокись ванадия. В результате систематических исследований различных способов приготовления, целенаправленно варьируя носители и промоторы, Г.К. Боресков с сотрудниками смогли добиться высокой каталитической активности и устойчивости ванадий-оксидного контакта. За сравнительно короткое время в его лаборатории был создан новый высокоэффективный катализатор сложного состава, получивший название БАВ (барий-алюмо-ванадиевый). Ключевым в подборе катализаторов оказалось понимание, что активный компонент в условиях реакции находится в расплавленном состоянии. Создание катализатора явилось результатом глубокого изучения физико-химических основ каталитических процессов, детального исследования кинетики и механизма реакций. Достаточно сказать, что в 1937 году Георгий Константинович получил кинетическое уравнение окисления сернистого ангидрида на ванадиевом катализаторе, которое десятки лет использовалось во всем мире для расчета контактных аппаратов и позже уступило место более точному уравнению Борескова–Иванова [3].
Крупным успехом 25-летнего руководителя бригады учёных и специалистов был пуск промышленного контактного аппарата окисления диоксида серы на Константиновском химкомбинате в 1932 году. Превзойдя по эксплуатационным качествам все известные ранее, БАВ-катализатор совершил переворот в отечественном сернокислотном производстве. Уже в конце 30-х годов на ванадиевый катализатор перешли все заводы Советского Союза, вырабатывающие серную кислоту контактным способом. Это позволило значительно увеличить ее производство и обеспечить военную промышленность страны серной кислотой – критическим реагентом в технологии взрывчатых веществ.
В 1937 руководимая Г.К. Боресковым лаборатория катализа из Одесского химико-радиологического института была переведена в Научно-исследовательский институт удобрений и инсектофунгицидов (НИУИФ) в Москву. Лаборатория вошла в состав вновь организованного Отдела серы и серной кислоты, созданного в 1936 году по указанию наркома тяжелой промышленности Г.К. Орджоникидзе, одной из главных задач которого ставилась разработка физико-химических основ и определение технологических режимов и аппаратурного оформления получения серной кислоты различными способами. В 1937 году Г.К. Борескову была присуждена ученая степень кандидата химических наук без защиты диссертации.
Георгий Константинович первым стал широко применять химическую кинетику при решении инженерных задач по оптимизации каталитических реакторов, что явилось фундаментом нового направления науки о катализе – математического моделирования каталитических процессов. На основе научных идей Борескова были спроектированы и построены первые мощные многослойные реакторы с промежуточными охладительными поясами для получения серной кислоты. Увеличение их производительности стало возможным не только благодаря новому БАВ-катализатору, но и результатам расчетов условий проведения процесса, усовершенствованию конструкции аппаратов и оптимизации технологических режимов.
Георгий Константинович неоднократно принимал непосредственное участие в заводских испытаниях катализаторов, руководил пуском контактных аппаратов, сернокислотных цехов и катализаторных производств. Так, в 1937-1939 гг. Боресков руководил освоением аппаратов большой мощности с катализатором БАВ для производства серной кислоты в Воскресенске, в начале 1941 года управлял пуском химического цеха по производству катализатора БАВ в г. Кировограде на Урале [1].
С началом Великой Отечественной войны на Урал были эвакуированы сотни заводов и фабрик вместе с оборудованием, специалистами и рабочими. Все, что невозможно вывезти, было взорвано. Во время эвакуации Константиновского завода с юга Украины эшелон с рабочими и оборудованием попал под обстрелы и бомбежки. Производство боеприпасов оказалось под угрозой. Красноуральский химический завод стал единственной надеждой, превратившись в завод оборонного значения. Завод, по проекту самый мощный в СССР, начал строиться ещё в 1936 г., но темпы строительства были медленными, с большими задержками. Война помешала строительству, но не прекратила его. В 1941 году в сернокислотном цехе разрозненное оборудование с эвакуированных заводов удалось смонтировать. Несмотря на то, что новый метод использования отходящих газов обжиговых печей был изучен плохо, бригаде первоклассных специалистов, рабочих, инженерно-технических работников из Одессы, Константиновки, Воскресенска, Щелкова во главе с Г.К. Боресковым в тяжелейших условиях удалось подготовить к пуску один контактный узел сернокислотного производства. И уже 4 декабря 1941 года первая партия серной кислоты была получена!

Сотрудники лаборатории катализа Научно-исследовательского
института удобрений и инсектофунгицидов, 1942 г.
Сидят, слева направо: В.В. Илларионов, Т.И. Соколова,
Г.К. Боресков (зав. лабораторией), В.А. Дзисько.
«Было адски трудно, – вспоминает о пусковом периоде бывший технолог цеха М.С. Шевчук. Но мы все хорошо понимали, как нужны были фронту олеум, аккумуляторная кислота и другая продукция нашего сернокислотного цеха. Работали круглосуточно, не считались со временем. Слесари и механики сутками не отходили от холодильников, которые безудержно текли. Не выдерживали стальные трубы, истончались в бумагу. Подчас под резервуарами с кислотой, страшной своими свойствами, заменяли и монтировали трубопроводы. Цех нельзя было остановить, он наращивал мощность. Монтажники, котельщики в условиях большой загазованности собирали контактные узлы. Аппараты то затухали, то загорались, их жизнь поддерживалась теплом огромного количества дров. В цехе не хватало мужчин, они были па фронте. Вместо них женщины и подростки взялись за освоение сложных технологических станций. Не один десяток кубометров дров пришлось перетаскать им для того, чтобы поддерживать нужную температуру в аппаратах. В ночь на 20 декабря 1941 года налилась олеумом первая 15-тонная цистерна!» [Из архивных материалов Историко-производственного музея Красноуральского медеплавильного комбината].
Из воспоминаний В.А. Дзисько, сотрудника лаборатории Г.К. Борескова, о запуске первого контактного сернокислотного аппарата «Бригада разбилась на группы, в обязанности которых входило обследование и наведение порядка во всем комплексе цехов, входящих в производство серной кислоты Красноуральского завода. Мы с Георгием Константиновичем были «приставлены» к контактному аппарату. Работали в сменах по два человека 12 часов. Неуютно выглядел огромный, почти темный и пустой цех (тогда приходилось экономить электроэнергию). Очень холодно – цех рассчитан на работу шести аппаратов, а включен только один, и тот еле «дышит». Отопление было (как сказали бы теперь) «местное», т.е. с помощью «мангала» – железного барабана, в котором горел кокс. Подойдешь, погреешь руки – и снова к приборам. Еще хуже была сильная загазованность. Цех ведь был новый, необкатанный. Из многих фланцев, а то и просто дырок, били струи газа. Но самое страшное было ходить ночью по территории завода, когда по ходу работы надо было обойти все корпуса. Строительство вели в спешке – война; многочисленные траншеи, нарытые строителями, остались незасыпанными, и для перехода перебросили узкие дощечки. Падение с такого мостика в траншею в мороз под 50°С могло окончиться весьма печально. К трудностям на работе прибавлялось еще весьма скудное питание, а жить Георгию Константиновичу, как и всем нам, пришлось в необустроенном бараке. Тем не менее, я никогда не слышала от него жалоб на бытовые неудобства. Георгий Константинович всегда был человеком долга, гражданином в высоком смысле этого слова. На том этапе истории нашей страны лозунг «Все для фронта, все для победы!» был не просто словами, а отражал самое главное в жизни в самом высоком смысле этого слова!» [2].
За достижения и заслуги по разработке и реализации
новой схемы производства контактной серной кислоты
Г.К. Боресков в 1942 году был удостоен звания Лауреата Сталинской премии,
а за цикл работ по сернокислотному катализу
в 1944 году был награжден Орденом «Знак Почёта».
Во время войны Георгий Константинович подготовил и в 1946 году защитил докторскую диссертацию «Теория сернокислотного катализа».
Позже, в 1954 году, результаты исследований каталитического окисления двуокиси серы были обобщены и проанализированы Г.К. Боресковым в его монографии «Катализ в производстве серной кислоты» [6], отразившей на примере этой реакции все аспекты катализа: кинетику и механизм процесса, свойства и структуру катализатора, конструирование и расчет промышленного аппарата. Такое уникальное сочетание сделало эту книгу незаменимой для специалистов в области гетерогенного катализа.
«Основная тенденция в развитии нашей науки – это переход от феноменолоrических исследований к исследованиям на молекулярном уровне. Одна из систем, которой занимался Георгий Константинович, это окисление SО2 в SО3 на гетерогенных катализаторах. Он это сделал настолько глубоко, что, по существу, это один из первых в мире примеров системы, где механизм изучен на атомно-молекулярном уровне» – из доклада К.И. Замараева «Идеи Г.К. Борескова и современная наука и практика катализа» [2].

Приглашение на празднование 25-летия сернокислотного цеха
Красноуральского химического завода, направленное в 1966 году
Г.К. Борескову, который руководил пуском цеха 4 декабря 1941 года.
II. Атомный проект СССР
Г.К. Боресков в послевоенные годы внес большой вклад в решение ряда задач, имеющих важное значение для восстановления народного хозяйства, укрепления научного и оборонного потенциала страны.
По окончании Великой Отечественной войны в мае 1945 года Г.К. Боресков был командирован в составе делегации крупных российских специалистов-химиков в Германию для ознакомления с новейшим оборудованием на химических заводах гигантского концерна «I.G. Farben» в рамках работы комиссии по промышленным репарациям. В целом СССР потерял около 30% своего национального богатства, ущерб необходимо было возмещать. Репарации должны были не только возместить ущерб, который нанесла Германия СССР, но и подорвать ее военный потенциал. Особое внимание уделялось демонтажу предприятий, занимавшихся производством ФАУ, научно-исследовательских учреждений, работавших над атомной проблематикой, вооружением и боевой техникой, особенно ракетной.

Подполковник Г.К. Боресков, май 1945 года.
Поскольку на немецкие военные заводы не допускались гражданские лица, на время поездки всем членам делегации были присвоены воинские звания (Георгию Константиновичу – подполковника) и выдано новейшее отечественное табельное оружие. В первые мирные дни передвигаться по территории Германии было небезопасно, оружие все обязаны были вернуть по возвращении в Москву. В военном билете Г.К. Боресков числился «рядовым запаса».

Военный билет Г.К. Борескова.
В 1946 году Г.К. Боресков был приглашён в Научно-исследовательский физико-химический институт им. Л.Я. Карпова (НИФХИ), который в первые послевоенные годы был включён в работы над Атомным проектом СССР. Перед институтом была поставлена задача разработки научно-технических основ промышленного производства тяжёлой воды, необходимой для отечественных атомных реакторов.
Справка: Для создания ядерного оружия необходим плутоний – химический элемент, практически отсутствующий в природе и синтезирующийся искусственно, в специальных реакторах из природного урана, следуя ядерной реакции 238U+(n) → 239U – (е–) → 239Np – (е–) → 239Pu. Для эффективного протекания этой реакции необходимо, чтобы энергия нейтрона, n, возникающего при спонтанном делении ядер урана (главным образом, имеющегося в природном уране в малых (0,7%) количествах изотопа 235U), имела величину, достаточную для проникновения нейтрона в ядро 238U, но меньшую, чем это необходимо для расщепления этого ядра на осколки. В качестве таких «замедлителей» наиболее подходящими являются или графит, или тяжёлая вода, D2O, имеющая преимущество в том, что при её использовании нет необходимости обогащать природный уран, увеличивая в нём содержание изотопа 235U. Теоретически тяжёлую воду можно получить из природной воды, в которой содержание дейтерия в среднем составляет величину 0,0145–0,0146 ат.%, путём многократной дистилляции. Однако из-за небольшой разницы температур кипения (100°C для Н2О; 100,7°C для HDO и 101,4°C для D2O) коэффициент изотопного разделения составляет всего 1,07, что делает такой технологический подход для получения воды с высоким, более 90%, содержанием дейтерия нереализуемым на практике (для использования в атомных реакторах требуется D2O с содержанием дейтерия не менее 99,8%). Гораздо большую величину коэффициент изотопного разделения (5-7) проявляет при электролизе воды – из-за более отрицательного значения потенциала восстановления 2D+ + 2e– → D2 (относительно потенциала процесса 2Н+ + 2е– → H2) при катодном потенциале, оптимальном для образования протия, дейтерий накапливается в водной фазе, тогда как газовая фаза (водород) обогащается протием. Однократный эффект разделения повторяется в последовательно соединенных электролизерах, в каждый следующий из которых подается электролит с увеличенной концентрацией дейтерия из предыдущего, в то время как образующийся обедненный по дейтерию водород после превращения его путём сжигания в воду подается в один из предыдущих электролизеров. Существенное концентрирование дейтерия может быть осуществлено путём ректификации жидкого электролитического водорода, состоящего из Н2, HD и D2, которые значительно различаются точками кипения (19,2 К для Н2; и 23,6 К для D2). Проводя при этом реакцию изотопного обмена 2HD ↔ H2 + D2, окончательно выделяют D2, сжигание которого приводит к образованию D2O [7].
Работая в НИФХИ, Г.К. Боресков организовал и возглавил лабораторию технического катализа. В лаборатории решались задачи разработки каталитических методов разделения изотопов вoдорода, была показана эффективность введения в процесс первичного обогащения воды электролизом стадии газофазного «водяной пар/водород» изотопного обмена «протий/дейтерий». Для осуществления этой стадии был разработан специальный никель-хромовый катализатор, эффективность работы которого была проверена в большом масштабе, а в феврале 1948 года был пущен цех для получения этого катализатора на Московском государственном опытном заводе НИУИФ [8].

Лаборатория технического катализа Научно-исследовательского
физико-химического института им. Л. Я. Карпова (НИФХИ), 1952 г., Москва.
Сидят (справа налево): М.Г. Щеголева, В.А. Дзисько,
Г.М. Маркова, зав. лаб., д.х.н. Г.К. Боресков, А.Г. Филиппова,
М.Г. Слинько. Стоит, первый справа – К.И. Матвеев.
После американских атомных бомбардировок Хиросимы и Нагасаки к программе создания советского атомного оружия было привлечено более двухсот тысяч человек в десятках научных, производственных и строительных организациях по всей стране. Начался форсированный этап Атомного проекта СССР, который курировал Л. Берия. Несмотря на то, что в СССР научно-технологические исследования по получению тяжелой воды для уран-тяжеловодного реактора начались с мая 1945 года, первый промышленный реактор этого типа уже в 1951 году был введен в строй.
За работы по созданию катализаторов изотопного обмена водорода
с водой для промышленных установок получения тяжёлой воды
в 1953 году Г.К. Боресков был удостоен
Сталинской премии (переименована в 1963 году в Государственную премию СССР).
В 1949 году в Московском химико-технологическом институте им. Д.И. Менделеева (МХТИ) для подготовки специалистов химиков-технологов для молодой атомной промышленности, электронной и других отраслей новой техники был создан Инженерный физико-химический факультет. В качестве руководителя кафедры разделения и применения изотопов на факультет был приглашен д.х.н., профессор Г.К. Боресков. Боресков опрeделил профиль новой специальности, создал и читал специальный курс «Физико-химические основы и технология разделения изотопов», который включал как разработку и применение изотопных методов в исследовании гетерогенного катализа и катализаторов, так и создание катализаторов для технологии стабильных изотопов. Семинарские занятия и курсовые работы на кафедре вел молодой кандидат химических наук М.Г. Слинько. Среди первых выпускников Инженерного физико-химического факультета был Р.А. Буянов, который после распределения на Чирчикский электрохимический комбинат (Узбекская ССР) участвовал в строительстве промышленного объекта по выделению дейтерия (с последующим получением из него тяжёлой воды) методом ректификации жидкого водорода. Этот метод был успешно реализован в 1954 году, и такое крупное научно-техническое достижение было отмечено присуждением в 1960 году коллективу учёных и инженеров Ленинской премии, среди награждённых – М.Г. Слинько1 и Р.А. Буянов2 .
Из воспоминаний академика А.П. Александрова о разработке и освоении низкотемпературных процессов выделения дейтерия на Чирчикском электрохимическом комбинате мeтодом ректификации (воспоминания А.П. Александрова необычны по форме, т.к. они – точная запись его устной речи): «Так я, значит, собрал всех и произнес следующую речь. Вот, товарищи, я познакомился с тем предложением, которое сделал Петр Леонидович <академик Капица> относительно производства дейтерия путем ректификации жидкого водорода. <…> Я понимаю так, нам сейчас нужно разобраться в физике этого дела, нужно сделать у себя сначала небольшую лабораторную установочку. Потом большую полупроизводственную установку. <…> И в это же время будем разрабатывать проект крупного завода. Пусть у нас потом половина этого проекта окажется негодной, мы ее переделаем, но, во всяком случае, сэкономим очень много времени. Значит, все были несколько изумлены таким подходом. “Слушайте, а как с точки зрения термодинамики?” Они говорят, что при этих температурах жидкого водорода устойчив такой-то (я уже сейчас не помню, кто– орто или пара, кажется, орто). Тогда я говорю: “Так давайте попробуем сделать таким образом, чтобы у нас был специальный какой-то участок, где мы превращаем, делаем конверсию орто в пара или пара в орто. Чтоб нам уже получить равновесный состав этого водорода, и тогда с ним обращаться, чтобы не было никаких тепловых помех при этом процессе <ректификации>”. Ну, они все согласились <…>. И тогда, значит, я договорился с Карповским институтом по этому поводу, что он берется такие катализаторы разработать. <Они были созданы в лаборатории технического катализа под руководством будущего академика Г.К. Борескова>
Заседание происходило так. Там было много народу. Председательствовал Берия, как всегда на этих заседаниях. Это называлось Спецкомитет. Берия сидел за столом, таким перпендикулярным, а от него шел длинный стол за которым все сидят. Слева от него сидел Махнёв <генерал-майор госбезопасности, начальник секретариата Спецкомитета>, ближе всех к нему, и он, собственно, и представлял все материалы. Махнёв докладывает. Вот, значит, товарищ Александров представил проект завода для получения тяжелой воды <в Чирчике>. Берия, значит, берет в руки бумагу: “А товарищ Александров знает, что взорвалась опытная установка в Дзержинске?” Махнёв говорит: “Знает”. А я сижу прямо против Махнёва, тоже рядом прямо с Берией. Он не ко мне обращается, к Махнёву: “Он свою подпись не снимает?” Он <Махнёв> говорит: “Нет, не снимает”. Потом он <Берия > так посидел: “А он знает, что если завод взорвется, он поедет, где Макар тэлята гоняет?” Он немного по-русски не очень-то говорил. Я говорю, что да, это я себе представляю. “Вы подпись не снимаете, товарищ Александров?” Я говорю: “Нет, не снимаю.” – “Строить завод” – Берия написал резолюцию: “ЗА, ЛБ”. Всё.
Завод стоимостью что-то около сотни миллионов рублей. И, как-никак, впервые в мире был водородный холод в промышленном масштабе здесь реализован. Американцы это делали изотопным обменом. Высокотемпературным изотопным обменом. Термодинамически был гораздо более выгодный тот процесс, который мы разработали. Но он технически труднее. Но сейчас и то, и другое делают у нас. Этот завод еще работает в Чирчике до сих пор и ни разу не взорвался.»
------------------------------------------------------------------------------
1 М.Г. Слинько – член-корреспондент АН СССР, зам. директора Института катализа (1959-1976). Во время работы в лаборатории Г.К. Борескова в НИФХИ (1946-1955) занимался проблемами получения тяжелой воды и защитой атомных установок от взрыва горючей смеси, образующейся в результате радиолиза воды. Разработал многоступенчатый электролитический метод получения тяжелой воды в сочетании с каталитическим изотопным обменом дейтерием между водой и водородом. Принимал участие в пусковых работах первой АЭС в Обнинске, определяя стационарную концентрацию гремучей смеси в первом контуре ядерного реактора (1954) [9].
2 Р.А. Буянов – член-корреспондент РАН, зам. директора Института катализа (1961-1995). В составе группы ученых Института физических проблем АН СССР участвовал в разработке и проектировании крупного промышленного объекта по выделению дейтерия методом ректификации жидкого водорода. Затем был направлен на Чирчикский электро-химический комбинат, где руководил строительством и пуском этого объекта (1950-1954). Разработал и внедрил в промышленность катализаторы низкотемпературной конверсии ортоводорода в параводород для производства и хранения жидкого параводорода – ракетного топлива, на котором в 1987 г. совершил полет космический корабль «Буран».
3 А.П. Александров – Академик АН СССР, выдающийся российский физик, один из основателей советской ядерной энергетики, Президент АН СССР (1975-1986), директор ИФП АН СССР, директор ИАЭ им. И.В. Курчатова.

Первая дирекция Института катализа, г. Новосибирск, 1963 г.
Слева направо: Ф.Т. Калинченко, к.х.н. Р.А. Буянов,
чл.-корр. АН СССР Г.К. Боресков, д.х.н. М.Г. Слинько.
Награждены за крупный вклад в Атомный проект СССР:
Г.К. Боресков – Сталинская премия (1953 г.),
Р.А. Буянов и М.Г. Слинько – Ленинская премия (1960 г.).
III. Горючие кристаллы для быстрых и мощных ракет
Герой Социалистического Труда, кавалер трех Орденов Ленина и четырех Государственных премий академик Г.К. Боресков основным делом своей жизни считал создание Института катализа, который он возглавлял со дня основания в 1958 году и до последних дней жизни. С 1991 года Институт катализа СО РАН носит имя Георгия Константиновича Борескова.

Лаборатория окисления, Институт катализа СО АН СССР, 1967 г.
1-й ряд (слева направо): Г.М. Полякова, Т.В. Андрушкевич, Б.И. Попов,
Г.К. Боресков (зав. лабораторией), В.В. Поповский, Г.А. Иванова,
Т.М. Юрьева, Н.И. Попова; 2-й ряд: М.М. Данилова, Ю.В. Лихачева,
О.Н. Кимхай, Н.Н. Сазонова, Л.Н. Шкуратова, Е.Е. Вермель,
К.Д. Осипова, Л.Н. Качан, Н.Г. Скоморохова; 3-й ряд: В.П. Щукин,
А.А. Иванов, Г.Л. Семин, Ю.А. Зверев, С.А. Веньяминов,
А.Е. Черкашин, В.А. Сазонов, Г.Д. Коловертнов, В.И. Чигрина,
П.Г. Цырюльников, А.Н. Питаева, Э.Э. Рачковский, С.Г. Шубников.
Георгий Константинович с самого начала активно участвовал в организации Сибирского отделения, координировал в рамках программы «Сибирь» работы Института катализа, среди которых крупнейшие – «Нефть и газ Западной Сибири», «Угли Кузбасса и Канско-Ачинского месторождения». С момента создания под руководством Г.К. Борескова в 1965 году кафедры катализа и адсорбции в Новосибирском государственном университете ведется целенаправленная подготовка специалистов для Института катализа и других организаций.
Г.К. Боресков являлся одним из активнейших авторов проекта решения ЦК КПСС об использовании разработок ученых СО РАН для оборонных работ. Пожалуй, одна из самых «закрытых» работ, в которой Георгий Константинович принимал участие и как учёный, и как организатор, была связана с созданием в 1970-1980-х годах технологии получения продукта специального назначения, имевшего в то время шифрованное название «катализатор ЦН». По сути этот продукт являлся микрокристаллами α-AlH3 – одним из самых «энергомощных» горючих для твёрдых ракетных топлив. Отметим, что шифрованное название «катализатор Циглера-Натта» создавало надёжную легенду для прикрытия участия Института катализа в этой работе. Исследования по «настоящим» катализаторам Циглера-Натта для полимеризации олефинов Институтом действительно проводились, и результаты этих исследований публиковались. Привлечение к этим работам Института катализа во многом определилось инициативой руководства института, в настоящее время известного как ФНЦП «Алтай», и, прежде всего, Геннадия Викторовича Саковича, ныне академика РАН, познакомившегося с Г.К. Боресковым ещё в далёком 1957 году. Для понимания химии образования этого соединения потребовалось применить всю имевшуюся в то время в Институте катализа мощь физических методов исследования. По поручению Г.К. Борескова исследования легли на плечи будущих академиков РАН и директоров Института катализа Кирилла Ильича Замараева и Валентина Николаевича Пармона.
Из воспоминаний академика В.Н. Пармона: «Как заместителю Кирилла Ильича по только что созданной в Институте катализа лаборатории, мне пришлось быстро втягиваться во многие проблемы Института и узнать многое из того, за что, как мы полагаем, совсем недавно Институт катализа, наравне с огромным Ростехом, был включён в санкционные списки США. Правда, это было очень давно, а в санкционные списки включили недавно. Так вот, в один прекрасный день в Институт в стандартных армейских сундуках, под многочисленной вооружённой охраной, принесли нечто такое, о чём и говорить-то строго запрещалось. А в кабинете Кирилла Ильича, тогда ещё совсем молодой и очень энергичный создатель этого «нечто», Геннадий Викторович Сакович, давал чёткие пояснения, что именно хотелось бы выяснить с помощью новейших методов исследования, доступных в то время практически только в Институте катализа. Задача была понятной, но очень сложной. Тем не менее, за её решение взялась большая бригада сотрудников Института. Поставленные Геннадием Викторовичем задачи были решены, а сам уникальнейший продукт был поставлен на производство и обеспечил мощный рывок в нашей ракетной технике».
Справка: Твёрдое ракетное топливо (ТРТ) является композитом, состоящим из частиц твёрдого горючего, частиц твёрдого окислителя и связующего – резиноподобного материала, в массе которого эти частицы должны быть равномерно распределены. К каждому из твёрдых компонентов, помимо их энергетических характеристик, выдвигаются жёсткие требования, касающиеся плотности, формы и размера их кристаллов, а также устойчивости к условиям проведения с ними необходимых технологических операций и хранения. С точки зрения энергетики таких ТРТ, огромный интерес в качестве горючего представлял гидрид алюминия, энтальпия образования которого (в виде кристаллов в наиболее плотной альфа-модификации) была близка к нулю (–11,4 кДж/моль), т.е. при горении α-AlH3 в кислороде выделяется практически такая же тепловая энергия, что и при горении соответствующей стехиометрической смеси алюминия и водорода! Хотя гидрид алюминия достаточно просто может быть получен по протекающей в среде этилового эфира реакции AlCl3 + 3LiAlH4 → 4AlH3 + 3LiCl, из раствора при удалении эфира он выделяется в форме достаточно устойчивого эфирата. Разрушение этого комплекса, в котором атом алюминия находится в тетраэдрическом окружении атомов водорода, при повышении температуры, которая ограничена температурой разложения гидрида алюминия на алюминий и водород (~ 140°C), не может без перестройки координационной сферы атомов алюминия привести к образованию кристаллов α-AlH3, в которых атом алюминия находится в октаэдрическом окружении атомов водорода. Таким образом, для получения гидрида алюминия в виде кристаллов в альфа-модификации необходимо было разработать принципиально новый метод проведения реакции AlCl3 с LiAlH4 – проведения в условиях, способствующих формированию полиядерных алюмогидридных комплексов-кластеров, содержащих октаэдрический алюминий, как зародышей для роста кристаллов α-AlH3. Такой технологический подход, получивший название «кинетический метод эпитаксиальной кристаллизации продукта», был разработан и осуществлён в 1982 году в промышленном масштабе в Советском Союзе, что обеспечило мощный рывок нашей страны в новых видах ракетного вооружения.
Государственная премия СССР (1986 г.) была присуждена
Гергию Константиновичу Борескову посмертно,
в составе авторского коллектива с участием В.Ф. Комарова,
Б.Н. Переходы, А.И. Горбунова и др.
за цикл работ по технологии специальных продуктов
(за разработку и внедрение качественного "катализатора ЦН"
на опытном производстве
на ИГМЗ, г. Исфара, и на комбинате "Навоиазот").

Из воспоминаний Почетного директора ФНПЦ «Алтай», Героя Социалистического труда, академика Г.В. Саковича о сотрудничестве с Г.К. Боресковым:
«Нам, отраслевым ученым, импонировала полная самоотдача делу, стремление Георгия Константиновича общими усилиями "докопаться" до сути и найти пути решения. Мне не приходилось слышать от него оценку "фундаментальности" исследований. Как настоящий ученый, он понимал, что порой в прикладных исследованиях могут быть такие "фундаментальные" изюминки, раскрытие которых обеспечит настоящий прогресс страны.
Один пример. В середине семидесятых годов нашей стране потребовался новый продукт, так называемый "катализатор ЦН". Синтез его был известен из мировой литературы и воспроизведен в СССР. Было решено организовать производство этого продукта на химкомбинате "Навоиазот" Минхимпрома. В ходе отработки технологии выяснилось, что качество продукта было неудовлетворительным. К усовершенствованию процесса были подключены многие научные институты европейской части СССР. Наше объединение решило для ускорения параллельно своими силами создать опытно-промышленное производство в другой точке страны. Вскоре производство было запущено, но качество продукта также не отвечало всем нашим требованиям.
Совместным решением Минмаша и СО АН СССР был создан координационный Совет, которому были даны полномочия привлекать все творческие силы ФНПЦ "Алтай" и Сибирского отделения АН для решения этой проблемы. Сопредседателями комиссии были назначены академик Г.К. Боресков и я. По настоянию Георгия Константиновича наши заседания проходили с чередованием председательства и, регулярно, с протокольным поручением заданий и проверкой исполнения. Его умение ставить вопросы, вникать в суть ответов, не оставляя отвечающему шанса уходить "в сторону" или прятаться за общие фразы, вскоре сузило круг участников – мы привлекли новых, более творческих сотрудников. Такая форма руководства для меня была нормой, и Георгий Константинович воспринял её как должное. Я видел, что Г.К. Борескову, как истинному ученому, такой стиль и темп работы по душе. Он легко соглашался посмотреть "на месте" результаты наших совместных усилий. Спецрейсами мы посещали опытное производство в г. Исфаре, г. Навои. Несмотря на свои годы, он с удовольствием ходил по цехам, с большим удовлетворением рассматривал паутину массопроводов, каскад реакторов, подчеркивая: "Это – великое научное и инженерное творчество".
За сравнительно короткий срок, благодаря работе нашего координационного Совета, была решена задача получения качественного "катализатора ЦН" с внедрением сначала на опытном производстве на ИГМЗ (г. Исфара), а затем и на комбинате "Навоиазот". В ее основе лежало научное и технологическое обоснование эпитаксиальной кристаллизации продукта на принципах топохимии. Общему успеху содействовала также высокая организация работы: от поиска до внедрения. Буквально в считанные недели научные идеи проверялись и воплощались в технологии конкретных производств.

Правление химкомбината "Навоиазот", г. Навои, 1978 год.
Слева направо: В.Ф. Комаров, Г.К. Боресков, А.И. Горбунов, Г.В. Сакович.
Георгий Константинович Боресков имел большой авторитет в академических кругах. К его мнению очень внимательно относился М.А. Лаврентьев. С ещё большим почтением встречал его и беседовал с ним В.А. Коптюг. Очевидным было то, что они глубоко осознавали, что у Георгия Константиновича всегда есть свои аргументированные убеждения по затронутым вопросам, причем не легковесные, а основополагающие, и что от этих убеждений он, в силу своего патриотического воспитания, не отступит. А его патриотизм имел глубинные российские корни.»
Литература
1. Музыкантов В.С., Яблонский Г.С. Георгий Константинович Боресков. Библиография ученых СССР, Изд-во Наука, Москва, 1982, 119 с.
2. Андрушкевич Т.В., Юрьева Т.М., Михайлова И.Л., Музыкантов В.С. Георгий Константинович Боресков (Наука Сибири в лицах). Книга воспоминаний, Изд-во СО РАН, Новосибирск, 2007, 355 с.
3. Андрушкевич Т.В., Бухтияров В.И. Научное наследие Георгия Константиновича Борескова. Кинетика и катализ, 2019, том 60, № 2, с. 152–168.
4. Куперштох Н.А. Академик Г.К. Боресков: катализ как судьба. Управление наукой: теория и практика. 2021, том 3, № 4, с. 254–276.
5. Пармон В.Н. Жизнь, отданная катализу. Вестник Российской академии наук, ТОМ 77, № 5, 2007, с. 434-441.
6. Боресков Г.К. Катализ в производстве серной кислоты. М.-Л.: Госхимиздат, 1954, 348 с.
7. Круглов А.К. Как создавалась атомная промышленность в СССР. М.: ЦНИИАтоминформ, 1995. 380 c.
8. Банникова Н.Ф. Научные достижения Научно-исследовательского физико-химического института им. Л.Я. Карпова в послевоенные годы. Самар. нац. исслед. ун-т им. С. П. Королева – Самара, АНО "Издательство СНЦ", 2019.
9. Михаил Гаврилович Слинько – служение науке и Отечеству (Наука Сибири в лицах): сб. статей: посвящается 100-летию со дня рождения М.Г. Слинько / отв. ред. В.Н. Пармон, Новосибирск: Изд-во СО РАН, 2014, 539 с.
10. Ilina L.Y., Zibareva I.V., Vedyagin A.A. Scientific Legacy of Georgy K. Boreskov: Bibliometric and Thematic Analysisю Reaction Kinet-ics, Mechanisms and Catalysis. 2017, V. 122, No. 2, p. 685-697.
Материал подготовили:
В.А. Лихолобов, Л.Я. Старцева
Институт катализа им. Г.К. Борескова СО РАН
 |
“Нефтепереработка: катализаторы и гидропроцессы” 26-30 апреля 2021 г., в режиме онлайн http://conf.nsc.ru/STS_4/en |
Научно-технологический симпозиум “Нефтепереработка: катализаторы и гидропроцессы” проводится раз в два года и признается мировым научным и производственным сообществом как мероприятие высокого международного уровня. Симпозиум является практически единственным европейским форумом по гидропроцессам, каталитическим гидрогенизационным технологиям нефтепереработки. В 2021 году, с учетом неблагоприятной эпидемиологической ситуации, IV Научно-технологический симпозиум “Нефтепереработка: катализаторы и гидропроцессы” (Catalytic Hydroprocessing in Oil Refining, HydroCat IV) прошел с 26 по 30 апреля в режиме онлайн на платформе Zoom.
Соорганизаторами Cимпозиума стали Институт катализа СО РАН (Новосибирск, Россия), Институт химических процессов и энергетических ресурсов CPERI (Салоники, Греция) и ПАО «Газпром нефть» (Санкт-Петербург, Россия), выступившее также генеральным спонсором симпозиума. Председателем симпозиума традиционно стал академик Валентин Пармон (ИК СО РАН, Новосибирск, Россия). Экспертный Совет возглавили д.т.н., профессор Александр Носков (ИК СО РАН, Новосибирск, Россия) и д-р Ангелос Лаппас (Институт химических процессов и энергетических ресурсов CPERI, Салоники, Греция).
Научная программа симпозиума охватывала следующие направления:
В работе IV Научно-технологического симпозиума “Нефтепeреработка: катализаторы и гидропроцессы” приняли участие более 100 специалистов в области нефтехимии из 13 стран мира, среди которых были представители России, Греции, Франции, Канады, Германии, Китая, Мексики, Кувейта, Испании, Бельгии, Сербии, Азербайджана и Узбекистана. В рамках научной про-граммы было представлено 3 пленарных и 3 ключевых лекции известных и признанных в мире специалистов, 43 устных и 41 стендовый доклад. Научная программа Симпозиума была сoставлена таким образом, что принять участие в онлайн-сессии и выступить с устным сообщением смогли все участники, проживающие в различных временных поясах, от Мексики и Канады до Китая и Западной Сибири. Авторы стендовых докладов могли разместить свои доклады на официальном сайте симпозиума, а также выступить устно с краткими флэш-презентациями своих работ.
В ходе симпозиума были представлены следующие пленарные и ключевые лекции:
PL-1. Проф. Вильгельм Швигер
Иерархические цеолиты в переработке углеводородов
(Hierarchical zeolites in processing of hydrocarbons)
Фридрих-Александровский университет Эрлангена-Нюрнберга, Германия
PL-2. Доктор Мохан С. Рана
Последние достижения в гидропереработке нефтяных остатков
(Recent advances in residue hydroprocessing)
Кувейтский институт научных исследований, Сафат, Кувейт
PL-3. Проф. Ангелике Лемониду
Интенсификация парового риформинга для производства водорода
(Intensification of steam reforming for hydrogen production)
Университет Аристотеля в Салониках, Греция
KL-1. Доктор Стелла Безергиани
Каталитические гидропроцессы: эффективный способ для направленной декарбонизации топлива
(Catalytic hydroprocessing: an effective mode for direct fuels decarbonization)
Центр исследований и технологий Эллады / CERTH
Институт химических процессов и энергетических ресурсов / CPERI, Греция
KL-2. Проф. Джорис Тибо
Моделирование конверсии сложных смесей на основе фундаментальных принципов
(Simulating complex mixtures conversion from first principles)
Университет Гента, Гент, Бельгия
KL-3. Доктор Владимир Данилевич
Данилевич В., Надеина К., Авдеенко Е., Климов О., Носков A.
Оксиды алюминия как носители катализаторов гидроочистки
(Aluminum oxides as supports for hydrotreating catalysts)
Институт катализа СО РАН, Новосибирск, Россия
По итогам Симпозиума традиционно был выпущен спецвыпуск журнала Catalysis Today, в который вошло 26 научных статей, прошедших предварительный отбор, а также полноценное рецензирование с участием более 100 экспертов в области нефтепереработки из многих стран мира. Журнал Catalysis Today имеет высокий импакт-фактор (6,766) и входит в первый публикационный квартиль, что, безусловно, является признанием Симпозиума форумом высокого уровня.
Подробно ознакомиться с тезисами пленарных, ключевых, устных и стендовых докладов можно в сборнике тезисов докладов Симпозиума, который размещен на официальном сайте Симпозиума и доступен по ссылке: http://conf.nsc.ru/files/conferences/STS_4/644741/ABSTRACTS-STS-IV-2021.pdf
Организаторы Симпозиума выражают глубочайшую признательность ПАО «Газпром нефть» (Санкт-Петербург, Россия), выступившему в качестве генерального спонсора симпозиума и оказавшему значительную поддержку в его организации.
На заключительном заседании Симпозиума было отмечена крайняя важность и актуальность проведения мероприятий в области разработок новых, более совершенных каталитических систем для процессов гидроочистки и гидрокрекинга, которые являются ключевыми процессами современной нефтепереработки. Актуальность исследований обусловлена необходимостью удовлетворять растущие экологические требования к качеству выпускаемых заводами нефтепродуктов. Организаторы и участники выразили общую надежду на то, что следующее мероприятие состоится в очном режиме и позволит устанавливать деловые контакты с еще большей эффективностью.
А.Н. Загоруйко, О.В. Климов, А.С. Носков, С.С. Логунова
XI Научно-практическая конференция
“Сверхкритические флюиды: фундаментальные основы, технологии, инновации”
(XI Scientific and Practical Conference “Supercritical
Fluids: Fundamentals, Technologies, Innovations”)
21-25 июня 2021 г.,
Новосибирск, Россия
http://scftec.isc-ras.ru
С 21 по 25 июня 2021 г. в Новосибирском Академгородке прошла XI Научно-практическая конференция “Сверхкритические флюиды: фундаментальные основы, технологии, инновации”. Она продолжила серию встреч специалистов в этой актив-но развивающейся области исследований, начало которым было положено в 2004-2006 годах тремя конференциями, проведёнными в Ростове-на-Дону под эгидой Ростовского Государственного Университета (ныне – Южный Федеральный Университет) и компании ГОРО. От конференции к конференции несколько менялось её название, но неизменными оставались сосредоточенность на обсуждении многообразия флюидных систем и их свойств и сочетание фундаментальной и практической составляющих в программе.
Наиболее важные традиции получили продолжение и на этот раз. Несмотря на известные сложности, конференции удалось сохранить статус мероприятия с международным участием; о том, в какой форме это было реализовано – чуть ниже. Сохранена структура программы, включающей приглашённые и ключевые лекции, устные доклады и стендовые сообщения. Было обеспечено широкое представительство различных регионов и научных центров России и участие большого числа молодых специалистов. Это позволило совместить работу Конференции со Школой молодых учёных (также традиционной – 12-й по счёту), в рамках которой были организованы две полноценных сессии и проведен конкурс представленных на них работ. В конференции приняли участие 130 человек из 16 городов мира: Архангельск – 8, Барнаул – 7, Бордо – 1, Иваново – 13, Казань – 4, Лилль – 1, Лондон – 1, Махачкала – 2, Москва и Троицк – 50, Новосибирск – 18, Омск – 1, Саратов – 4, Тверь – 4, Турку – 1, Эрланген – 1. Из них молодых ученых – 82 человека.
Конечно же, были организованы экскурсионная и культурная программы, обеспечившие возможность общения участников Конференции на тесном неформальном уровне.
Были, однако, и серьёзные изменения по сравнению с предыдущими конференциями. Это было первое мероприятие такого масштаба, прошедшее без участия безусловного лидера сверхкритического сообщества России – Валерия Васильевича Лунина. И именно его памяти Конференция и была посвящена. Хотя и на предыдущей Конференции (в Ростове-на-Дону) Валерий Васильевич уже не мог непосредственно присутствовать по состоянию здоровья, он был безусловным её вдохновителем, участвовал в формировании научной программы и был незримо с нами на всех сессиях и других мероприятиях. Теперь же нам оставалось только постоянно вспоминать его и благодарить за всё, что он сделал для многих из присутствовавших лично и для всего сообщества в целом...

Как известно, 2021 год объявлен в Российской Федерации Годом науки и технологий. И именно в этом году впервые ни одно ведомство, ни один фонд не оказали проведению Конференции финансовой поддержки. Все затраты были покрыты из оргвзносов участников. Именно в этом году был ликвидирован Российский Фонд Фундаментальных Исследований, который на протяжении многих лет поддерживал работы в области “сверхкритики”. До настоящего момента продолжается выполнение ряда грантов, за счёт средств которых многие участники Конференции смогли компенсировать свои затраты на поездку в Новосибирск. Организаторами конференции в этом году выступили Институт катализа СО РАН (Новосибирск), Институт общей и неорганической химии РАН (Москва), Институт химии растворов РАН (Иваново), Московский государственный университет имени М.В. Ломоносова.
Неоценимой была помощь в организации Конференции со стороны традиционного спонсора – компании ЗАО “ШАГ”, а также принимающей организации – ФИЦ Институт катализа СО РАН им. Г.К. Борескова, с помощью которого удалось существенно сократить расходы на аренду помещений для проведения сессий Конференции. Хорошо известно, что Институт катализа на сегодня является одной из немногих (а может быть, и единственной) академической организацией в России, способной полностью обеспечить организацию крупномасштабных научных форумов международного уровня. Причём в “послужном списке” ИК – мероприятия (в том числе “серийные”), организуемые не только в России, но и за её пределами. На этот раз в последний день все заседания прошли в здании Института катализа, что позволило совместить полезное с ещё одним полезным (и одновременно приятным) – экскурсией по лабораториям Института.
Конференция проводилась одновременно с научным семинаром “Передовые методы химической визуализации и колебательной спектроскопии для решения актуальных задач в области катализа и химической технологии”, что позволило участникам познакомиться и с этим направлением работы Института катализа СО РАН.

Серьёзной "новацией" стало также то, что значительная часть пленарных лекций, в первую очередь – иностранных лекторов, была организована в дистанционном формате с использованием технологий “удалённого присутствия”. К счастью, эта часть программы прошла без технических сбоев, и участники таких сессий по обе стороны линии связи имели возможность полноценного диалога – не только доложить и выслушать подготовленные материалы, но и задать вопросы, ответить на них, кратко прокомментировать услышанное.
Традиционно информационная поддержка Конференции была обеспечена редакцией журнала “Сверхкритические Флюиды: Теория и Практика” и порталом “Сверхкритические Флюиды” (www.scftec.ru).
Что касается научной программы Конференции, то она была весьма насыщенной и заняла пять рабочих дней – c 21 июня (день заезда, регистрации и открытия Конференции) по 25 июня (день закрытия Конференции и экскурсии по Институту катализа).
Конференцию открыл директор Института Катализа СО РАН, академик Валерий Иванович Бухтияров, с приветственным словом выступил научный руководитель ИК СО РАН академик Валентин Николаевич Пармон.
В.И. Бухтияров начал пленарную сессию конференции рассказом о жизни и работе Валерия Васильевича Лунина. Его непременно вспоминали и пленарные лекторы, представившие свои выступления как очно (А.Г. Насибулин, Сколковский Институт науки и технологии, Москва, Россия; Aalto University School of Science, Espoo, Finland, “Single-walled carbon nanotubes: from synthesis to applications”; С.Г. Казарян, Department of Chemical Engineering, Imperial College London, United Kingdom, “In situ vibrational spectroscopy and spectroscopic imag-ing for supercritical fluids”), так и дистанционно:
– C. Aymonier, Institute of Condensed Matter Chemistry of Bordeaux, France, “Supercritical fluids for advanced functional materials”;
– A.S. Braeuer, Technische Universität Bergakademie Freiberg, Germany, “Vibrational spectroscopy for studying mass transfer in SCF”;
– Д.Ю. Мурзин, Åbo Akademi University, Turku, Finland, “Hydrogen generation by catalytic aqueous and supercritical water reforming”;
– A. Idrissi, University of Lille, France, “Spectroscopy and modelling of supercritical ammonia”.
Ключевые лекции, как правило, открывавшие утренние сессии Конференции, представили:
– Д.А. Зимняков, Саратовский государственный университет имени Ю.А. Гагарина, Саратов, Россия, “СКФ синтез высокопористых полимерных матриц: фундаментальные особенности и технологические аспекты формирования, развития и стабилизации полимерных пен”;
– И.М. Абдулагатов, Институт проблем геотермии ДагНЦ РАН, Объединённый Институт Высоких Температур РАН, Махачкала, Дагестан, Россия, “Критические и сверхкритические явления в бинарных смесях, содержащих CO2”;
– О.Б. Бельская, Центр новых химических технологий ИК СО РАН, Институт катализа СО РАН, Омск, Россия, “Переработка органоминерального сырья с использованием СКФ”;
– А.Ю. Манаков, Институт неорганической химии им. А.В. Николаева СО РАН, Новосибирск, Россия, “Газовые гидраты и критические явления в газах – точки соприкосновения”;
– А.Ю. Барняков, Институт ядерной физики СО РАН, Новосибирск, Россия, “Аэрогели в физике высоких энергий”;
– Н.В. Меньшутина, Российский химико-технологический университет имени Д.И. Менделеева, Москва, Россия, “Аэрогели: из лаборатории в промышленность”;
– А.А. Ведягин, Институт катализа СО РАН, Новосибирск, Россия, “Применение сверхкритических условий при синтезе наноструктурированных оксидных материалов”.

Место проведения конференции и научные приоритеты основного организатора обусловили некоторую специфичность программы устных выступлений, которая включала большое количество докладов о каталитических реакциях в среде и с участием СКФ, по приготовлению катализаторов и носителей в СКФ условиях, в частности, доклады:
–– В.Г. Матвеевой (Тверь) “Получение биотоплива второго поколения с применением сверхкритических растворителей”;
–– О.П. Паренаго (Москва) “Пути синтеза катализаторов с использованием сверхкритических сред”;
–– М.Г. Сульмана (Тверь) “Структура бифункциональных катализаторов, синтезированных с использованием субкритической воды”;
–– Н.С. Нестерова (Новосибирск) “Сверхкритический диоксид углерода – среда для получения гетерогенных катализаторов с уникальными характеристиками”;
–– М.Н. Cимонова (Новосибирск) “Катализаторы углекислотной конверсии метана: синтез в сверхкритических спиртах и исследование каталитической активности”;
–– А.А. Филиппова (Новосибирск) “Восстановительные пре-вращения анизола в суб- и сверхкритическом 2-PrOH в присутствии высоконаполненных никелевых катализаторов”;
–– А.М. Воробья (Москва) “Катализаторы на основе кобальтата самария, полученные с использованием метода сверхкритического антисольвентного осаждения”;
–– А.В. Смирнова (Москва) “Самоконденсация ацетона на станнатах стронция и бария в сверхкритических условиях”;
–– А.А. Степачёвой (Тверь) “Использование сверхкритических растворителей в процессах гидроконверсии модельных соединений тяжелых нефтяных фракций”;
–– А.В. Припахайло (Москва) “Влияние концентрации наноразмерных частиц оксида железа на эффективность процесса субкритической деасфальтизации тяжелой нефти”;
–– О.В. Манаенкова (Тверь) “Каталитическая конверсия полисахаридов в субкритической воде”.
Традиционно в рамках Конференции была проведена Всероссийская школа-конференция молодых ученых “Сверхкритические флюидные технологии в решении экологических проблем”. Было заслушано 27 докладов молодых ученых, и также традиционно были награждены лучшие выступавшие:
–– Премией им. Ю.Е. Горбатого – Илья Всеволодович Новиков, (Сколковский институт науки и технологий, Москва) за работу “Метод RESS для создания эластичных композитов на основе однослойных углеродных нанотрубок и термопластичного полиуретана";
–– Премией имени В.Н. Баграташвили – Вадим Викторович Зефиров (МГУ имени М.В. Ломоносова, Москва) за работу “Модификация полимерных матриц в среде сверхкритического диоксида углерода для электрохимических приложений”;
–– Премией имени В.В. Лунина – Татьяна Александровна Иванова (МГУ имени М.В. Ломоносова, Москва) за доклад “Механизм высвобождения нитроксильных радикалов из матриц поли-D,L-лактида в процессе их деградации”.
Специальной премией Оргкомитета Конференции была награждена Ирина Сергеевна Шаврина (Северный (Арктический) федеральный университет, Архангельск) за работу и доклад “Деполимеризация технических лигнинов в среде сверхкритических растворителей”.
Кроме того, премией за лучший доклад молодого учёного, представленный от Института катализа СО РАН, награждена Екатерина Андреевна Смаль, выступившая с докладом “Влияние метода приготовления и добавок Ti и Nb на структурные особенности катализаторов на основе смешанных оксидов Ce-Zr и их активность в реакции УКМ”.
Организаторы конференции также отметили специальным дипломом Эрнеста Ефимовича Саид-Галиева, как самого преданного и активного участника СКФ сообщества России, внесшего неоценимый вклад в проведение ВСЕХ конференций цикла.

Проведение следующей конференции запланировано на 2023 год в городе Тверь, на базе Тверского государственного университета.
Статьи, вошедшие в два номера журнала “Сверхкритические флюиды: Теория и практика” (том 16, № 2 и том 16, № 3, 2021 г.) представляют собой расширенные тезисы докладов, представленных в ходе работы конференции.
Материал подготовили:
О.О. Паренаго
Институт общей и неорганической
химии им. Н.С. Курнакова РАН, Москва;
М.Ю. Синев
Институт химической физики
им. Н.Н. Семенова РАН, Москва
Cheap catalysts recycle polystyrene into valuable products
Two groups report upcycling the plastic into benzoic acid using light and oxygen
Polystyrene, the polymer commonly used to make foam packaging, insulation, and food containers, is notoriously difficult to recycle. Instead of turning the material into new polystyrene products, breaking it down chemically into more valuable substances could be a more cost-effective alternative. So far, though, chemical recycling has been energy-intensive and expensive. Two groups of researchers have now independently come up with simple, low-cost processes that use light to drive the catalytic breakdown of polystyrene into commodity chemicals.
One group uses an acid as a catalyst and violet-blue light to cleave the strong carbon-carbon and carbon-hydrogen bonds in polystyrene (J. Am. Chem. Soc. 2022, DOI: 10.1021/jacs.2c01410). The other uses white light to trigger an iron chloride catalyst to break the bonds (J. Am. Chem. Soc. 2022, DOI: 10.1021/jacs.2c01411).
Older methods of chemically cracking polystyrene’s bonds tend to be complex, use harsh chemicals and high temperatures, and “almost inevitably produce a soup of many compounds that are difficult to separate, so the value is decreased,” says Jianliang Xiao, a chemist at the University of Liverpool who led the acid catalyst study.
The two teams set out to create simple processes that generate just a few products that can be easily harvested from the reaction mixture. Both reactions use oxygen, light-emitting diodes (LEDs), and readily available catalysts to generate benzoic acid—which can be worth about twice as much as polystyrene by weight, according to market research firm ChemAnalyst.
Xiao says that his graduate student Zhiliang Huang made the “surprising discovery” that acids work better than previously studied metal catalysts to cleave bonds in polystyrene. When irradiated with a high-power blue-violet LED, the acid reacts with the polystyrene to form reactive oxygen species, which then cause a chain reaction that breaks up polystyrene’s strong bonds. The resulting formic acid and benzoic acid were easy to separate from the reaction mixture.
Meanwhile, Cornell University chemist Erin E. Stache and her graduate student Sewon Oh chose iron chloride as a catalyst because chlorine radicals are known to break strong C–H bonds. Under white-light irradiation and oxygen-rich air, those broken C–H bonds created peroxy radicals, which in turn cleave C–C bonds, degrade the polymer, and make benzoic acid.
Although the processes are similar, each has its advantages. The acid-catalyzed process gives a benzoic acid yield of about 50% and even higher yields of formic acid as a side product, while the iron catalyst achieves 23% benzoic acid yield. However, the acid catalyst is double the price of the iron chloride, Stache says. And Xiao adds that Stache’s team’s use of white light might reduce cost and energy use.
In tests, the methods worked for a range of commercial polystyrene products, including disposable cup lids, food containers, and polystyrene foam. Both teams demonstrated that their recycling processes could work in a flow reactor, which would be needed for commercialization.
The downside of the techniques is their use of light, says Frank Leibfarth, a chemist at the University of North Carolina at Chapel Hill. “Scaling up photochemistry is challenging, especially on the scale that commodity polymers are produced,” he says. “The development of a method that works thermally would have a higher potential for making it to market.”
Still, the upcycling of polystyrene into defined products with a clear market using inexpensive, earth-abundant catalysts is an important advance, Leibfarth says. Tackling the challenge of plastic waste will require many technologies, and these approaches to turn polystyrene into benzoic acid “could be one piece of that puzzle”.
Electrons catalyze molecular assembly
Simple catalysts can push molecular recognition and assembly into high gear
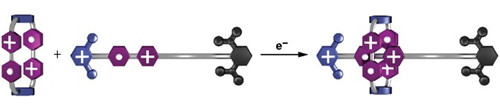
A ring-shaped host and dumbbell-shaped guest, each containing radical (white dot)
and cationic (+) sites, quickly form a complex when catalyzed by electrons.
A few electrons are all it takes to significantly boost the rate of supramolecular assembly of one molecule threading through another. The finding extends electron catalysis, which is used in synthetic covalent chemistry, to noncovalent chemistry, and may lead to new complex forms of materials.
Non-covalent bonding processes, such as molecular recognition and supramolecular assembly, occur widely in chemistry and biology. The rates of these phenomena, which can be sluggish, can be jumpstarted, but doing so generally requires complex catalytic systems.
On Wednesday at the American Chemical Society Spring 2022 meeting, Yang Jiao of Northwestern University reported that the assembly rate of a host-guest complex can be increased by a factor of 640 in the presence of simple catalysts—electrons.
Speaking in a session organized by the Division of Organic Chemistry, Jiao described a study involving the assembly of a complex consisting of a ring-shaped molecular host and a dumbbell-shaped guest. The host contains two bipyridinium (BIPY) radical cations. The guest consists of three units; a BIPY radical cation binding site in the center, which drives assembly with the host via radical-pairing interactions; a bulky diisopropylphenyl group on one end that cannot be threaded through the ring; and a dimethylpyridinium (PY) cation on the other end. Under normal conditions, repulsion between the PY cation and the BIPY radical cations prevents the host and guest from assembling.
The researchers found that catalytic quantities of various types of chemical electron sources, including metals, metal complexes, and common reducing agents, rapidly increase the reaction rate with little dependence on the type of source. Applying electric current to a solution of the molecules also catalyzed the assembly process. Adding electrons to the system lowers Coulombic repulsion, enabling the PY end of the dumbbell to thread the ring, as shown by quantum calculations.
Jiao and others, including Northwestern’s Sir J. Fraser Stoddart and William A. Goddard III of the California Institute of Technology, recently published this work in Nature (2022, DOI: 10.1038/s41586-021-04377-3).
Redox-driven formation of mechanically interlocked molecules has been known for a long time, but “the involvement of electron catalysis during the process is remarkable and eye-opening,” said Rafal Klajn of the Weizmann Institute of Science. He hypothesizes that the catalytic activity could be further increased if either the ring or the thread component were surface-immobilized.
Chemical & Engineering News
|
June
6-8, 2022 19th Nordic Symposium on Catalysis Espoo, Finland |
https://19nsc.fi/ |
|
June
6-10, 2022 11th European Conference on Solar Chemistry and Photocatalysis: Environmental Applications (SPEA11) Turin, Italy |
https://www.spea11.unito.it/ |
|
27
июня 2022 г. III Международная научно-техническая конференция «Новые материалы и технологии глубокой переработки сырья – основа инновационного развития экономики России» ВИАМ, Москва, Россия |
https://conf.viam.ru/conf/364 |
|
June
27-29, 2022 Annual Meeting of German Catalysts Weimar, Germany |
https://dechema.de/en/katalytiker2022.html |
|
June
27-30, 2022 19th International Symposium on Relations between Homogeneous and Heterogeneous Catalysis (ISHHC 19) University of Oslo, Norway (Digital conference) |
https://www.mn.uio.no/kjemi/english/ research/groups/catalysis/ishhc19/ |
|
27
июня – 3 июля 2022 г. 16-е Совещание с международным участием Фундаментальные проблемы ионики твердого тела» (ФПИТТ-2022) Черноголовка, Московская область, Россия |
https://fpssi16.altes.su |
|
1-3 июля 2022 г. VI Всероссийский научный симпозиум «Физикохимия поверхностных явлений и адсорбции» г. Плёс, Ивановская обл., Россия (без оргвзноса) |
ads-cat@yandex.ru
https://yadi.sk/i/miR6VnX4EqXtqw 8-905-059-40-24 |
|
July
3-8, 2022 12th Triennial Congress of the World Association of Theoretical and Computational Chemists (WATOC 2020) Vancouver, Canada |
http://watoc2020.ca |
|
July
3-6, 2022 International Conference of the International Association for Spectral Imaging (IASIM 2022) Esbjerg, Denmark |
https://2020.iasim.net/ |
|
July
11-13, 2022 1st Forum of Young Researchers on Heterogeneous Catalysis ( YOURHETCAT 2022) Szeged, Hungary |
https://www.catalysis.hu/yourhetcat-2022/ |
|
11-13
июля 2022 г. Кузнецовские чтения - 2022. VI семинар по проблемам химического осаждения из газовой фазы ИНХ СО РАН, Новосибирск, Россия |
http://www.niic.nsc.ru/science/conferences-inx/ 909-conferences-2022/3565-kuznetsovskie-chteniya-2022 |
|
July
17-22, 2022 28th IUPAC Symposium on Photochemistry Amsterdam, Netherlands |
https://photoiupac2022.amsterdam |
|
July
18-22, 2022 2nd International Conference on Noncovalent Interactions (ICNI 2021-2022) Strasbourg, France |
http://icni2021.unistra.fr/ |
|
July
18-22, 2022 26th IUPAC International Conference on Chemistry Education (ICCE 2020) Cape Town, South Africa |
https://iupac.org/event/26th-iupac-international- conference-on-chemistry-education/ |
|
July
24-28, 2022 84th Prague Meeting on Macromolecules – Frontiers of Polymer Colloids Prague, Czech Republic |
https://www.imc.cas.cz/sympo/84pmm/ |
|
July
24-29, 2022 9th Tokyo Conference on Advanced Catalytic Science and Technology (TOCAT9) Fukuoka, Japan |
https://tocat.catsj.jp/9/ |
|
July
24-29, 2022 XXII International Symposium on Homogeneous Catalysis ( XXII ISHC) Lisbon, Portugal |
https://xxii-ishc.events.chemistry.pt/ |
|
July
25-27, 2022 International Conference “Materials and Nanomaterials” (MNs-22) Rome, Italy |
https://nanomaterials-europe.eu/ |
|
July
31 – August 4, 2022 15th International Conference on Catalysis in Membrane Reactor (ICCMR-15) Tokyo, Japan |
http://iccmr15.org/index.html |
|
August
28 – September 2, 2022 44th International Conference on Coordination Chemistry Rimini, Italy |
https://www.iccc2022.com/ |
|
September
5-9, 2022 9th IUPAC International Conference on Green Chemistry (ICGC-9) Athens, Greece |
https://greeniupac2022.org/ |
|
5-9
сентября 2022 г. X международная конференция им. В.В. Воеводского (VVV-2022) Новосибирск, Россия |
www.vvv2022.com |
|
16-25
сентября 2022 г. XIV Симпозиум «Современная химическая физика» Туапсе, Россия |
www.chemicalphysics.ru |
|
September
21-23, 2022 2nd International Conference on Unconventional Catalysis, Reactors & Applications (UCRA 2022) Warwick, United Kingdom |
www.ucra2022.org |
|
25
сентября - 1 октября 2022 г. IV Съезд аналитиков России Москва, Россия |
http://analystscongress.ru/iv/default.aspx |
|
26-30
сентября 2022 г. Международная конференция "Химия нефти и газа" Томск, Россия |
http://petroleum-chemistry.ru/ |
|
27-30
сентября 2022 г. Школа-конференция молодых ученых «Неорганические соединения и функциональные материалы» (ICFM-2022) Новосибирск, Россия |
http://niic.nsc.ru/science/conferences-inx/ 909-conferences-2022/3774-vi-shkola- konferentsiya-molodykh-uchenykh- neorganicheskie-soedineniya-i- funktsionalnye-materialy-icfm-2022 |
|
3-7
октября 2022 г. XXIII Международная Черняевская конференция по химии, аналитике и технологии платиновых металлов Новосибирск, Россия |
https://chernyaev2022.affinaz.ru/ |
|
31
октября – 2 ноября 2022 г. Всероссийская научно-техническая конференция «Проблемы науки. Химия, химическая технология и экология» Новомосковск, Россия (без оргвзноса) |
gez75@yandex.ru 301665 Тульская область, г. Новомосковск, ул. Дружбы, д. 8 Новомосковский институт (филиал) ФГБОУ ВО «Российский химико-технологический университет имени Д.И. Менделеева» |
|
October
31 – November 3, 2022 International Conference “Synchrotron Radiation Techniques for Catalysts and Functional Materials Novosibirsk, Russia (no registration fee) |
http://conf.nsc.ru/SRTCFM-2022/en |
|
November
9-10, 2022 6th International Conference on Catalysis and Chemical Engineering (CatChem 2022) Dubai, UAE |
https://catchemconference.com |
|
November
18-19, 2022 5th International Symposium on Hydrogen Energy and Energy Technologies (HEET 2022) Osaka, Japan |
http://www.heet-18.org/ |
|
January
9-11, 2023 10th Annual Global Congress of Catalysis 202 (GCC-2023) Sapporo, Japan |
https://www.bitcongress.com/gcc2023-japan/ |
|
June
19-23, 2023 IV International Symposium «Applied Nanotechnology & Nanotoxicology» (ANT-2022) Kaliningrad, Russia |
http://conf.nsc.ru/ant-2022/en |
|
August
27 – September 1, 2023 15th European Congress on Catalysis (EuropaCat 2023) Prague, Czech Republic |
https://www.europacat2023.cz/ |
| 2023 г VI German-Russian Seminar “Bridging the Gap between Model and Real Catalysis Berlin, Germany |
http://conf.nsc.ru/rgs-2022/en/ |
|
2023 XII International Conference “Mechanisms of Catalytic Reactions” (MCR-XII) |
http://conf.nsc.ru/mcr2022/en |
|
2023 VII International Conference on Structured Catalysts and Reactors (ICOSCAR7) |
http://conf.nsc.ru/ICOSCAR7/en |
|
2023 6th International Conference “Catalysis for Renewable Sources: Fuel, Energy, Chemicals” (CRS-6) Bar, Montenegro |
http://conf.nsc.ru/CRS6/en |


