

15 мая исполнилось 75 лет члену-корреспонденту РАН Усеину Меметовичу Джемилеву – специалисту мирового уровня в области металлокомплексного катализа, металлоорганической химии, нефтехимии.
У.М. Джемилев с отличием закончил Казахский химико-технологический институт. В 1969 году поступил в аспирантуру Института химии Башкирского филиала АН СССР, которую закончил досрочно. В 1972 году защитил кандидатскую диссертацию, в 1977 году – диссертацию на соискание ученой степени доктора химических наук на тему “Исследование в области синтеза непредельных соединений с участием комплексов переходных металлов” на специализированном Совете по защите докторских диссертаций при ИОХ
С 1992 по 2016 г. – директор Института нефтехимии и катализа РАН (г. Уфа). Лауреат Государственной премии СССР (1990 г.), Лауреат Государственной премии РФ в области науки и техники за 2003 год (2004 г.), награжден медалью “Ордена за заслуги перед Отечеством II степени” (1999 г.). Руководитель научной школы по металлокомплексному катализу, член редколлегий научных журналов РАН "Органическая химия" и "Нефтехимия", член Научных советов по катализу РАН и нефтехимии РАН, автор и соавтор около 1500 научных статей и обзоров, более 600 авторских свидетельств и патентов, 3 монографий, 2 книг-обзоров, изданных за рубежом, научный руководитель 18 докторских и 80 кандидатских диссертаций.
Мировую известность ему принесли работы в области комплексного циркониевого катализа органических и металлоорганических реакций.
У.М. Джемилев внес крупный вклад в становление и развитие фундаментальных исследований в области каталитической активизации атомов, малых и малостабильных молекул, теломеризации сопряженных диенов, синтеза гетероциклов и гетероатомных соединений уникальной структуры, линейной и циклической олигомеризации диенов и олефинов, в том числе асимметрической.
Им разработаны каталитические методы синтеза ранее неизвестных классов металлоорганических соединений, что положило начало развитию новой области химии — химии металлоциклов непереходных металлов.
Совместно с лабораторией академика О.М. Нефедова разработана новая стратегия одностадийного каталитического синтеза высоконапряженных полициклических структур, открыты новые общие реакции конструирования соединений, построенных из трех-, четырех- и пятичленных циклов, изучена химия этого класса соединений.
Разработаны фундаментальные реакции, новые общие принципы и методы гетероциклизации сопряженных диенов и ацетиленов с получением широкого класса практически важных кислород-, азот- и серосодержащих гетероциклов.
Открытые У.М. Джемилевым реакции этилмагнирования и циклоалюминирования непредельных соединений используются в мировой практике как именные реакции («Реакции Джемилева»).
При личном участии У.М. Джемилева разработаны и внедрены в промышленность технологии получения новых мономеров, нейтрализаторов сероводорода, ингибиторов коррозии, смазочноохлаждающих жидкостей, современных препаратов для медицины и сельского хозяйства, продуктов и материалов с рекордными характеристиками для специальной техники.
Научный совет по катализу ОХНМ РАН и редакция Каталитического бюллетеня сердечно поздравляют Усеина Меметовича с юбилеем, желают ему дальнейших успехов на благо российской науки!

28 июня исполнилось 90 лет действительному члену Российской академии наук Владимиру Борисовичу Казанскому – крупному ученому в области катализа, спектроскопии, квантовой химии, химии и физики поверхности.
В.Б. Казанский окончил Химический факультет МГУ в 1954 году. С 1955 по 1966 год работал младшим и старшим научным сотрудником Института химической физики АН СССР.
С 1966 г. — зам. директора, зав. лабораторией спектральных и квантово-механических исследований каталитических реакций, зав. лабораторией радиоспектроскопических и оптических методов изучения механизма гетерогенного катализа Института органической химии им. Н.Д. Зелинского РАН.
Член-корреспондент c 1974 г., академик c 1991 г. — Отделение химии и наук о материалах.
Область научных интересов: физическая химия, изучение механизма гетерогенного катализа, спектральные исследования в области структуры активных центров катализаторов и промежуточных состояний реакционных частиц.
Разработал основы катализа (установление взаимосвязи между строением активных центров гомогенных и гетерогенных катализаторов, природой образующихся с их участием активных интермедиатов и детальным механизмом каталитических реакций). В 1960-е годы им были выполнены работы по изучению механизма гидрирования олефинов на металлах с использованием дейтерия.
Ввел в практику исследований гетерогенных катализаторов парамагнитный резонанс, ультрафиолетовую и ИК-спектроскопию диффузионного отражения, ядерный магнитный резонанс высокого разрешения.
Экспериментально изучил свойства поверхностных низкокоординированных ионов переходных металлов и установил их роль в катализе на оксидах. Исследовал механизм образования на поверхности катализаторов свободных радикалов при адсорбции и фотодиссоциации различных молекул. Определил природу избыточной каталитической активности, возникающей при ионизирующем облучении оксидных катализаторов, и выяснил, какую роль играют в радиационном катализе кислородные «дырочные» центры. Разработал новые спектральные методы изучения бренстедовской кислотности гетерогенных катализаторов. Выполнил квантово-химические расчеты структуры активных центров и состояния адсорбированных молекул.
Более поздние работы посвящены исследованию роли сольватации в кислотно-каталитических реакциях.
В.Б. Казанский создал свою научную школу — среди его учеников 32 кандидата и 12 докторов наук.
Автор более 700 научных работ, обзоров и патентов.
Главный редактор журнала «Кинетика и катализ» (по 2019 год), член редколлегий нескольких академических изданий и международных журналов.
Член бюро Научного совета РАН по катализу. Награжден орденом «Знак Почета». Лауреат премии
Научный совет по катализу ОХНМ РАН и редакция Каталитического бюллетеня сердечно поздравляют Владимира Борисовича с юбилеем и желают крепкого здоровья, успехов и благополучия!
 |
Отделение химии и наук о материалах НАУЧНЫЙ СОВЕТ ПО КАТАЛИЗУ О научно-организационной деятельности в 2020 году |
Секретариат Научного совета по катализу ОХНМ РАН (НСК) предлагает вашему вниманию сводный отчет о деятельности Совета и научных исследованиях в области катализа, выполненных научными коллективами под руководством членов Научного совета по катализу в 2020 году.
Отчет состоит из трех разделов:
Тексты отчетов, полученные от членов НСК и научно-исследовательских коллективов, практически не подвергнуты корректировке.
ОРГАНИЗАЦИОННАЯ ДЕЯТЕЛЬНОСТЬ
В 2020 году в рамках научно-организационной деятельности Научного совета по катализу ОХНМ РАН (НСК) были выполнены следующие мероприятия.
Выпущены четыре ежеквартальных сборника «Каталитический бюллетень», содержащие оперативную информацию о важнейших результатах фундаментальных и прикладных исследований в области катализа в России и за рубежом, материалы, посвященные деятельности выдающихся отечественных и зарубежных исследователей в области катализа; в сборниках дается перечень предстоящих конференций, краткие отчеты о проведенных конференциях, рабочих совещаниях и другие материалы. Членами НСК курируются журналы в области катализа: «Катализ в промышленности» (главный редактор В.Н. Пармон) и «Кинетика и катализ» (главный редактор В.И. Бухтияров). Секретариат НСК ведет переписку и текущую работу с членами Научного совета по катализу ОХНМ РАН.
Под эгидой Научного совета по катализу и при активном участии его членов организованы и проведены следующие конференции:
Изданы материалы проведенных конференций.
Продолжается сотрудничество с организациями Академий наук РФ и стран СНГ, Министерствами РФ, институтами разных ведомств и другими организациями России, дальнего и ближнего зарубежья по различным вопросам научной, научно-организационной, учебно-преподавательской и общественной деятельности в области катализа.
При активном участии членов НСК проводится работа в рамках Комплексного плана научных исследований «Ресурсо- и энергоэффективные катализаторы и процессы», цель которого – повысить на международном уровне конкурентоспособность отечественной химической науки в области межотраслевых техно-логий, при этом применяя результаты фундаментальных исследований в конкретных направлениях для последующих исследований полного цикла.
ОСНОВНЫЕ РЕЗУЛЬТАТЫ 2020 г.
Фундаментальные исследования в области создания новых каталитических систем и применения физических методов для их диагностики
Разработка новых материалов для создания катализаторов кислородной и углекислотной конверсии метана в синтез-газ.
Разработаны усовершенствованные каталитические материалы для кислородной и углекислотной конверсии метана в синтез-газ. Катализаторы на основе гидроталькитоподобных гидроксосолей [AlMg2NixCoy(OH)6.08][(NO3)nH2O] с соотношением Ni/Co = 3-7 позволяют достигать количественных выходов синтез-газа и в кислородной, и в углекислотной конверсии метана. Суммарное содержание Ni и Co в катализаторах (2% масс.) меньше, чем у большинства описанных аналогов. Полученные результаты превосходят показатели, достигнутые на аналогичном никелевом катализаторе. Катализаторы стабильно работают не менее 60 ч и не образуют углеродных нанотрубок и других углеродных отложений.
Результаты работы опубликованы:
A. G. Dedov, A. S. Loktev, V. P. Danilov, O. N. Krasnobaeva,
T. A. Nosova, I. E. Mukhin, A. E. Baranchikov, Kh. E. Yorov, Alexey G. Dedov
M. A. Bykov, I. I. Moiseev, Catalytic Materials Based on Hydrotalcite-Like Aluminum, Magnesium, Nickel, and Cobalt Hydroxides: Effect of the Nickel/Cobalt Ratio on the Results of Partial Oxidation and Dry Reforming of Methane to Synthesis Gas, Petroleum Chemistry, 2020, v. 60, No. 2, p. 194–203, Pleiades Publishing, Ltd., 2020, ISSN 0965-5441, DOI: 10.1134/S0965544120020048
[А.Г. Дедов, А.С. Локтев, В.П. Данилов, О.Н. Краснобаева,
Т.А. Носова, И.Е. Мухин, А.Е. Баранчиков, Х.Э. Ёров, М.А. Быков, И.И. Моисеев. Каталитические материалы на основе гидроталькитоподобных гидроксидов Al, Mg, Ni, Co. Влияние соотношения никель : кобальт на результаты кислородной и углекислотной конверсии метана в синтез–газ, Нефтехимия, 2020, т. 60, № 2, с. 214–224. DOI: 10.1134/S0028242120020045].
академик РАН А.Г. Дедов, академик РАН И. И. Моисеев, д.х.н., проф. А.С. Локтев, аспирант И.Е. Мухин,
к.х.н. А.Е. Баранчиков, чл.-корр. РАН В.К. Иванов,
д.х.н., проф. В.П. Данилов, О.Н. Краснобаева, Т.А. Носова
Российский государственный университет нефти и газа
(Национальный исследовательский университет) имени И.М. Губкина, г. Москва;
Институт общей и неорганической химии им. Н.С. Курнакова РАН, г. Москва;
Институт нефтехимического синтеза им. А.В. Топчиева РАН, Москва
Новые катализаторы и процессы получения полупродуктов нефтехимии из продуктов переработки возобновляемого сырья
Разработаны новые катализаторы одностадийного получения ароматических углеводородов и олефинов C2-C4 конверсией изо-бутанола, н-бутанола и этилацетата. Бутиловые спирты и этилацетат могут производиться или производятся из углеводов, полученных из биомассы. В качестве катализаторов впервые использованы синтезированные ускоренным гидротермально-микроволновым методом непромотированные и совместно промотированные цинком и хромом цеолиты HMFI, в том числе синтезированные непосредственно в протонной форме, а также синтезированный композитный материал, содержащий цеолит HMFI и карбид кремния, являющийся активным в катализе. Установлено, что синтезированные катализаторы позволяют в одну стадию получать олефины C2-C4
Показано, что, варьируя условия процесса и тип используемого катализатора, удаётся преимущественно получать либо олефины C2-C4, либо ароматические углеводороды, преимущественно бензол-толуол-ксилольной фракции.
Результаты работы опубликованы:
Alexey G. Dedov, Alexander A. Karavaev, Alexey S. Loktev, Alexey S. Mitinenko, Igor E. Mukhin, Ekaterina A. Isaeva, Elena V. Rogaleva, Ilya I. Moiseev, Conversion of ethyl acetate into benzene–toluene–xylene fraction over MFI zeolite-based catalysts, Mendeleev Communications, 2020, v. 30, iss. 4, p. 459-461, IF = 1.694 Q2, https://doi.org/10.1016/j.mencom.2020.07.017; Alexey G. Dedov, Alexander A. Karavaev, Alexey S. Loktev, Alexey S. Mitinenko, Ilya I. Moiseev, Isobutanol conversion to petrochemicals using MFI-based catalysts synthesized by a hydrothermal-microwave method, Catalysis Today, 2020, v. 348, online version. https://doi.org/10.1016/
j.cattod.2020.04.064 IF=5.825, Q1; Дедов А.Г., Локтев А.С., Караваев А.А., Митиненко А.С., Исаева Е.А., Моисеев И.И., Способ получения композита на основе микропористого цеолита и карбида кремния, патент РФ № 2725586 Б.И. № 19, 2020 г. (Заявка на патент РФ №2020108036/013006 от 25.02.2020).
академик РАН А.Г. Дедов, академик РАН И. И. Моисеев,
д.х.н., проф. А.С. Локтев, к.х.н. А.А. Караваев
Российский государственный университет нефти и газа
(Национальный исследовательский университет) имени И.М. Губкина, г. Москва;
Институт нефтехимического синтеза им. А.В. Топчиева РАН, г. Москва
Исследование палладий-содержащих катализаторов в восстановительном гидродехлорировании 4-хлорфенола
С целью разработки методов очистки сточных вод проведено восстановительное гидродехлорирование 4-хлорфенола в разбавленных водных растворах (75 мг/л) в присутствии катализаторов Pd/ZrO2SiO2, FePd/ZrO2SiO2 и Fe/ZrO2SiO2 (1 масс.% палладия и 10 масс.% железа). Палладий-содержащие катализаторы эффективно и стабильно (не менее 10 циклов превращения 15 мл раствора на 0.1 г катализатора в реакторе периодического действия) превращают 4-хлорфенол в фенол, железный катализатор менее активен в этой реакции. Подробное изучение катализаторов методами ИК-спектроскопии с адсорбцией СО и восстановлением in situ, ТПВ, СЭМ-ЭДА, ПЭМ, низкотемпературной адсорбции азота позволило выявить мезопористую текстуру катализаторов, малый размер частиц палладия, уникальную способность биметаллического катализатора к восстановлению (практически полное восстановление возможно при 30 °С водородом в водной суспензии), а также хорошее восстанавливающее действие водного раствора фенола в присутствии водорода при 30 °С .
Результаты работы опубликованы:
Lokteva Ekaterina S., Shishova Vera V., Tolkachev Nikolay N., Kharlanov Andrey N., Maslakov Konstantin I., Kamaev Alexey O., Kaplin Igor Yu, Savina Irina N., Golubina Elena V., Hydrodechlorination of 4-Chlorophenol on Pd-Fe Catalysts on Mesoporous ZrO2SiO2 Support, Molecules, 2021, v. 26, № 1, 141, doi 10.3390/molecules26010141.
д.х.н., проф. Е.С. Локтева, к.х.н., доцент Е.В. Голубина, И.Ю. Каплин
Московский государственный университет имени М.В. Ломоносова,
Химический факультет, г. Москва
Разработка методов приготовления катализаторов на основе оксида церия для окисления СО
Темплатным методом приготовлены катализаторы на основе оксида церия, допированного оксидом олова и модифицированного оксидом меди, которые проявили хорошие каталитические свойства в окислении СО. В работе сравнивали результаты применения двух темплатов – СТАВ и Pluronic 123, а также способа добавления оксида меди – непосредственно в ходе синтеза двойной системы оксид церия-олова или путем последующей пропитки готового двойного оксида. Двойной оксид, приготовленный с использованием Плюроника, продемонстрировал более высокую каталитическую эффективность по сравнению с полученным с использованием СТАВ, благодаря повышенной дефектности решетки и мобильности кислорода. Модификацию медью предпочтительно проводить в ходе синтеза двойного оксида, причем для модифицированных медью оксидов использование СТАВ более предпочтительно. Это вызвано положительным действием образования смешанного оксида CuCeSnOx, способного обеспечивать поверхностные центры Сu+, и низкой стабильностью карбонатных группировок на поверхности этой системы, что обеспечивает низкотемпературные каталитические свойства.
Результаты работы опубликованы:
Kaplin Igor Yu, Lokteva Ekaterina S., Tikhonov Artem V., Zhilyaev Kirill A., Golubina Elena V., Maslakov Konstantin I., Kamaev Alexey O., Isaikina Oksana Ya, Templated Synthesis of Copper Modified Tin-Doped Ceria for Catalytic CO Oxidation, Topics in Catalysis, 2020, v. 63, № 1-2, p. 86-98, doi 10.1007/s11244-020-01251-w.
д.х.н., проф. Е.С. Локтева, к.х.н., доцент Е.В. Голубина, И.Ю. Каплин
Московский государственный университет имени М.В. Ломоносова,
Химический факультет, г. Москва
Высокоселективная кросс-конденсация на основе продуктов, получаемых из возобновляемого сырья
Обнаружено, что в сверхкритическом состоянии этанол превращается в 1-бутанол в присутствии наноразмерных биметаллических Pd- и Au- и Cu-содержащих катализаторов, модифицированных металлами I, II, VIII групп Периодической таблицы и ряда редкоземельных элементов, нанесенных на поверхность γ-оксида алюминия. При практической исчерпывающей селективности выход 1-бутанола достигает 60%. В развитии реакции самоконденсации этанола были разработаны каталитические реакции кросс-конденсации различных оксигенатов, такие как взаимодействие этанола с изопропанолом в пентанол-2 и практически важная реакция ванилина с изопропанолом или ацетоном в зингерон или дегидрозингерон, являющиеся важными компонентами ряда медицинских препаратов. Экспериментально и теоретически были исследованы особенности механизма реакций кросс-конденсации и определены факторы, влияющие на стабильность в работе каталитических систем.
Результаты работы опубликованы:
S.A. Nikolaev, M.V. Tsodikov, A.V. Chistyakov, P.A. Chistyakova, D.I. Ezzhelenko, M.I. Shilina, PdCu nanoalloy supported on alumina: A stable and selective catalyst for the conversion of bioethanol to linear a-alcohols, Catalysis Today, 2020, DOI: 10.1016/
j.cattod.2020.06.061; П.А. Чистякова, А.В. Чистяков, М.В. Цодиков, Гетерогенно-каталитическое получение зингерона и дегидрозингерона, Нефтехимия, 2020, т. 60, № 5, с. 1-8, DOI: 10.31857/S0028242120050068; С.А. Николаев, М.В. Цодиков, А.В. Чистяков, П.А. Чистякова, Д.И. Эзжеленко, И.Н. Кротова, Влияние промотора M (M = Au, Ag, Cu, Ce, Fe, Ni, Co, Zn) на активность Pd-M/Al2O3 катализаторов конверсии этанола в a-спирты, Кинетика и Катализ, 2020, т. 61, № 6, с. 864-872, DOI: 10.31857/S045388112006012X.
к.х.н. А.В. Чистяков, д.х.н., проф. М.В. Цодиков
Институт нефтехимического синтеза им. А.В. Топчиева РАН, г. Москва
Скоростные превращения высокоустойчивого возобновляемого сырья в светлые продукты нефтехимии и энергоносители
В лаборатории каталитических нанотехнологий ИНХС РАН развиваются научные основы плазменно-каталитического превращения высокоустойчивых компонентов растительной биомассы и остаточных нефтяных фракций, стимулированных микроволновым излучением, в светлые продукты. Для этих исследований была разработана оригинальная установка.
Найдено, что формирование на поверхности лигнина древесного происхождения железосодержащих каталитических компонентов до 0,5% приводит к существенному увеличению поглощения микроволнового излучения с быстрым возникновением пробойных эффектов и генерированием плазмы (чистый лигнин не поглощает МВИ в достаточной степени для генерирования плазмы).
Показано, что в плазменно-каталитическом режиме, стимулированном микроволновым излучением, протекают с высокой интенсивностью процессы углекислотного риформинга лигнина древесного происхождения в синтез-газ и гидрогенизационного превращения в жидкие ароматические углеводороды (т.н. монолигнолы). Время протекания полного превращения высокоустойчивого субстрата составляет 20-25 минут, конверсия лигнина по обоим направлениям достигает 65%. Селективность по синтез-газу состава Н2/СО ~ 1 составляет 94%; при протекании гидрогенизационного процесса выход светлых ароматических монолигнолов составляет 50%. На основе углеродного остатка превращения лигнина разработан способ получения однороднопористого адсорбента, проявляющего высокую хемосорбционную способность по отношению к ароматическим углеводородам. Разработанный подход перспективен для практически полной утилизации лигнина, количество которого в отходах достигает 150 млн т/год.
Результаты работы опубликованы:
P. A. Zharova , O. V. Arapova, G. I. Konstantinov, A. V. Chistyakov, and M. V. Tsodikov, Kraft Lignin Conversion into Energy Carriers under the Action of Electromagnetic Radiation, Journal of Chemistry, 2019, 1-9, doi.org/10.1155/2019/6480354; О.В. Арапова, А.В. Чистяков, М.В. Цодиков, И.И. Моисеев, Лигнин – возобновляемый ресурс углеводородных продуктов и энергоносителей (обзор), Нефтехимия, 2020, т. 60, № 3, с. 251–269, DOI: 10.1134/
S0028242119010118; Alexander I. Netrusov, Vladimir V. Teplyakov, Mark V. Tsodikov, Andrey V. Chistjakov, Polina A. Zharova, Maxim G. Shalygin. Laboratory scale production of hydrocarbon motor fuel components from lignocellulose: Combination of new developments of membrane science and catalysis, Biomass and Bioenergy, 2020, 135:105506 (Doi: 10.1016/j.biombioe.2020.105506; О.В. Арапова, А.В. Чистяков, Т.А. Паланкоев, Г.Н. Бондаренко, М.В. Цодиков, Переработка лигнина в жидкие продукты в присутствии Fe и Ni под воздействием микроволнового излучения, Нефтехимия, 2020, т. 60, № 5, с. 1–7, DOI: 10.31857/S0028242120050020; M. V. Tsodikov, S. A. Nikolaev, A. V. Chistyakov, O. V. Bukhtenko, A. A. Fomkin, Formation of adsorbents from Fe-containing processing residues of lignin, Microporous and Mesoporous Materials, 2020, 160, 10089. DOI: doi.org/10.1016/j.micromeso.2020.110089, Патент РФ №2724253 (13).
к.х.н. Г.И. Константинов, к.х.н. А.В. Чистяков, д.х.н., проф. М.В. Цодиков
Институт нефтехимического синтеза им. А.В. Топчиева РАН, г. Москва
Математическая модель для изучения влияния процессов массо- и теплопереноса на свойства колебательных режимов в реакции окисления СО на металлах платиновой группы
Методом математического моделирования изучено влияние процессов массо- и теплопереноса на свойства колебательных режимов в реакции окисления СО на металлах платиновой группы. На базе точечной модели, построенной в рамках механизма Лэнгмюра-Хиншельвуда с учетом процессов окисления-восстановления катализатора, построена распределенная модель типа реакция–диффузия–конвекция для трехмерной области (3D-RDC). Созданная математическая модель хорошо описывает экспериментальные данные, полученные с помощью метода PLIF.
Показано, что высокая реакционная способность гидратированного оксида титана в образовании титанатов ЩЗЭ в условиях обработки в среде водного флюида при температурах ≤ 400 °С позволяет проводить их синтез внутри сферических гранул пористого α-Al2O3. Определены условия синтеза, в которых возможно регулирование распределения нанесенной фазы по глубине гранулы носителя.
Изучено превращение этанола на Cu-Ce-Zr системах и показано, что значительные количества Н2 наблюдаются в присутствии катализаторов, на поверхности которых эффективно образуется высокотемпературный формиатный комплекс. Образование водорода сопровождается при этом образованием СО. Наличие формиатного комплекса на носителе не приводит к образованию водорода. Это означает, что существование формиатов на кластерах CuO является необходимым условием получения водорода.
д.х.н., проф. В.Н. Корчак, д.х.н. М.М. Слинько, д.х.н. М.Ю. Синев,
к.х.н. Е.А. Лагунова, Ю.А. Гордиенко, д.х.н. В.А. Матышак, к.х.н. О.Н. Сильченкова
Институт химической физики им. Н.Н. Семенова РАН, г. Москва
Очистка газовых и жидких смесей от соединений серы
Разработан новый тип адсорбентов-катализаторов для удаления соединений серы из газовых и жидких углеводородных смесей на основе поликатионных оксидных наночастиц (Mn, Zn, Cu), инкапсулированных в матрицу цеолитов. В качестве адсорбентов они также исключительно эффективны для очистки углеводородов от непредельных соединений (ацетиленов и диенов). Их адсорбционная емкость по соединениям серы в 5-20 раз превосходит лучшие из цеолитных адсорбентов. При сочетании адсорбционной и каталитической активности они способны практически полностью удалять соединения серы из газовых или жидких смесей. Так, при использовании в десульфуризации природного газа содержание соединений серы снижается от 50 ppm до 10-20 ppb, т.е. в 5000 раз! Традиционные системы достигают только 10-20 кратного снижения. Аналогично при очистке жидких углеводородов концентрация тиолов снижается от 40 ppm до 60 ppb (в 800 раз), тогда как традиционные адсорбенты способны снижать содержание тиолов лишь в 30 раз. Катализатор-адсорбент полностью регенерируем.
проф., д.х.н. Л.М. Кустов, д.х.н. А.А. Грейш, к.х.н. П.В. Соколовский
Институт органической химии им. Н.Д. Зелинского РАН, г. Москва
Гидродеоксигенирование глицерина в 1,2-пропандиол
Разработаны новые методы получения нанесенных золотосодержащих биметаллических катализаторов путем редокс-нанесения золота на монометаллический катализатор с предварительно сформированными наночастицами Cu, Pd, Pt, Ru, позволяющие получать высокодисперсные биметаллические наночастицы Au/Me или Au/MeOx. Синтезированы низкопроцентные биметаллические золотосодержашие катализаторы с ультранизким содержанием золота – 0.025-0.2% масс., биметаллические золотосодержащие системы. Система Au/RuO2/θ-Al2O3 показала высокую эффективность в реакции гидродегидроксилирования глицерина в 1,2-пропандиол.
проф., д.х.н. Л.М. Кустов, к.х.н. Е.А. Редина, к.х.н. О.А. Кириченко, асп. К.В. Виканова
Институт органической химии им. Н.Д. Зелинского РАН, г. Москва
Разработка и исследование нового катализатора синтеза Фишера-Тропша
Впервые исследовано влияние графитового скелета как нового типа теплопроводящего компонента гранулированных катализаторов высокоэкзотермических процессов. На этой основе разработан и внедрен в промышленном масштабе новый катализатор синтеза Фишера-Тропша ИНФРА S2. По результатам исследования опубликована статья в журнале «Applied Catalysis A».
Также были проведены исследования синергетических явлений между кобальтовыми и кислотными (по Бренстеду) активными центрами во вторичных превращениях углеводородов в ходе синтеза Фишера-Тропша. Впервые установлено две отчетливые температурные области (170-210 °С и 220-260 °С), в которых наблюдаются существенно различные механизмы превращений. Опубликована статья в журнале Catalysis Today.
д.х.н. В.З. Мордкович
Технологический институт сверхтвердых и новых углеродных материалов, г. Троицк, г. Москва
Утилизация монооксида углерода в непрерывном производстве стали из железной руды
Утилизация отходящих газов, образующихся в процессе непрерывной выплавки низкоуглеродистой стали из железной руды, является актуальной проблемой как с экономической, так и с экологической точек зрения. Рассмотрен способ утилизации выбросных газов, содержащих до 60,0÷90,0 об. % монооксида углерода. Способ предусматривает получение водорода путем каталитической конверсии монооксида углерода с водяным паром.
В связи с тем, что процесс каталитической конверсии монооксида углерода с водяным паром протекает с выделением большого количества тепла, утилизацию больших количеств СО предложено осуществлять в каскаде нескольких последовательно соединенных реакторов с промежуточным охлаждением проконвертированного газа. Катализаторы, которые могут эксплуатироваться в таких жестких условиях проведения этого процесса, должны иметь высокую каталитическую активность, термостабильность и механическую прочность.
Для осуществления процесса каталитической конверсии с водяным паром был выбран разработанный нами промотированный диспрозием (до 2,0 масс. % DyO) медь-цинк-алюмо-кальциевый катализатор серии НТК-10-2ЛФ (НИАП-06-04) с содержанием меди 27,0 ± 4,0 масс. % CuO и цинка 40,0 ± 4,0 масс. % ZnO. Уменьшенное содержание меди и увеличенное содержание цинка в катализаторе, а также введение в его состав диспрозия в качестве промотора способствуют меньшей рекристаллизации активного компонента при высоких температурах процесса, которые получаются в случае переработки больших количеств монооксида углерода. Сравнение каталитической активности по степени превращения СО показывает, что она практически одинакова во всем температурном интервале (280÷550 °С). Показано, что катализатор НТК-10-2ЛФ, содержащий DyO, проявляет высокую термостабильность до 550 °С.
Катализатор может эксплуатироваться при объемных скоростях до 10000÷15000 ч–1 в температурном диапазоне до 550 °С. Отработана технология приготовления катализаторов НТК-10 (НИАП-06-04) в различной геометрической форме (таблетки, кольца, экструдаты). Катализатор достаточно устойчив при длительном воздействии воды и водяного пара.
Исследование выполнено при финансовой поддержке РФФИ и Тульской области в рамках научного проекта № 19-48-710014.
к.т.н., доцент В.Н. Ефремов, д.х.н., проф. Е.З. Голосман
ООО “НИАП-КАТАЛИЗАТОР”, г. Новомосковск
к.т.н., доцент П.И. Маленко, ассистент Е.А. Протопопов
Тульский государственный университет, г. Тула
Синтез платинаорганических катализаторов для гидросилилирования полидиметилсилоксановых олигомеров
Получены первые представители платинаорганических комплексов с ортозамещёнными диалкиларилфосфиновыми лигандами, являющиеся активными катализаторами процессов гидросилилирования полидиметилсилоксановых олигомеров с настраиваемой каталитической активностью, эффективность которых в десятки раз превосходит показатели современных мировых аналогов.
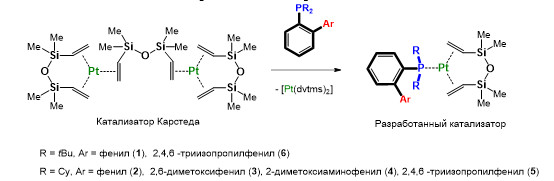
Схема образования платинаорганических катализаторов процесса гидросилилирования
Впервые показано, что ортозамещённые диалкиларилфосфины (лиганды Бухвальда) при взаимодействии с комплексами платины(0) на основе 1,3-дивинил-1,1,3,3-тетраметилдисилоксана (dvtms) способны образовывать активные платинаорганические катализаторы процессов гидросилилирования (аддитивной сшивки) жидких силиконовых смесей, содержащих винил-терминированные и гидридные полидиметилсилоксановые олигомеры, с образованием силиконовых полимеров (жидких силиконовых резин) с заранее заданными свойствами, высоко востребованных в современной промышленности и медицине. Установлено, что варьирование природы заместителей в фосфорорганическом лиганде приводит к изменению каталитической активности полученных комплексов и может быть использовано для настройки каталитических свойств катализатора и характеристик получаемых силиконовых полимеров за счёт экранирования аксиального положения платинового центра каталитически активной формы лабильным ортозаместителем ароматического фрагмента при атоме фосфора.
Результаты работы опубликованы: Lukin R.Y., Kuchkaev A.M., Sukhov A.V., Bekmukhamedov G.E., Yakhvarov D.G., Platinum-catalyzed hydrosilylation in polymer chemistry, Polymers, 2020, vol. 12, Art. 2174; Lukin R.Y., Emelyanov D.A., Kachmarzhik A.D., Sukhov A.V., Sinyashin O.G., Yakhvarov D.G., Effect of Buchwald-type ligands on platinum catalyzed hydrosilylation of vinyl terminated polydimethylsiloxane, Mendeleev Commun., 2019, vol. 29, p. 458-460.
д.х.н., проф. РАН Д.Г. Яхваров, Г.Э. Бекмухамедов, А.М. Кучкаев,
Р.Ю. Лукин, А.В. Сухов, д.х.н., проф. А.А. Карасик, академик РАН О.Г. Синяшин
Институт органической и физической химии им. А.Е. Арбузова ФИЦ КазНЦ РАН, г. Казань
Новый синтез гранулированных цеолитов ZSM-5 высокой степени кристалличности с иерархической пористой структурой – путь к созданию высокоэффективных катализаторов для нефтехимии и промышленного органического синтеза
С помощью методов химического и рентгеноструктурного анализа, адсорбционных измерений, низкотемпературной адсорбции азота, сканирующей электронной микроскопии, ВМУ ЯМР 27Al изучены закономерности кристаллизации в растворах силиката натрия гранул, состоящих из кристаллов ZSM-5 и частиц синтетического аморфного алюмосиликата. На основе полученных результатов разработан способ синтеза гранулированного цеолита NaZSM-5 (Si/Al=15) высокой фазовой чистоты и степени кристалличности 100% с иерархической пористой структурой. Способ включает стадии смешения 60% масс. порошкообразного цеолита ZSM-5 и 40%мас. аморфного алюмосиликата, увлажнения смеси водой и механической грануляции в гранулы диаметром 1.6±0.2 мм и длинной 3-6 мм, сушки при 150ºС в течение 4 часов в атмосфере воздуха и кристаллизацию полученных гранул в реакционной смеси состава: 2,2Na2O ∙2,3R ∙Al2O3 ∙70SiO2 ∙444H2O (где R - темплат) при 150-160ºС с предварительной выдержкой при 30 ºС в течение 24 ч. Предложена экспериментально обоснованная стадийная схема формирования (диаметр: 1.6-2.0 мм, длина: 3.0-6.0 мм) указанного выше цеолитного материала
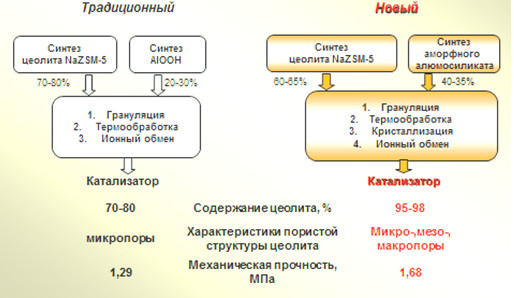
к.х.н. О.С. Травкина, к.х.н. Р.З. Куватова, к.х.н. И.Н. Павлова,
д.х.н., проф. Б.И. Кутепов, чл.-корр. РАН У.М. Джемилев
Институт нефтехимии и катализа УФИЦ РАН, г. Уфа
Гетерогенно-каталитический синтез промышленно важного 2-метил-5-этилпиридина на цеолитах FAU (H-Y и H-Yh) с различной пористой структурой
С целью разработки перспективного для промышленности метода получения 2-метил-5-этилпиридина (МЭП) осуществлен его синтез с использованием цеолитных катализаторов структурного типа FAU с различной пористой структурой: высокодисперсного микропористого HNа-Y и иерархического HNа-Yh. Осуществлен синтез 2-метил-5-этилпиридина реакцией ацетальдегида с аммиаком в автоклаве при 130-160 °C, мольном соотношении CH3СOH:NH3 = 1:3, содержании катализатора 1-20 масс. % на исходный ацетальдегид. Установлено, что в зависимости от условий реакции и кислотности цеолитов в присутствии изученных катализаторов селективность образования МЭП достигает 91-100%. Изучение стабильности действия катализаторов показало, что глубокодекатионированный цеолит HNа-Ymmm обеспечивает 100% селективность образования МЭП в течение 4-5 циклов при небольшом снижении активности, в то время как цеолит HNа-Y активен только в первом цикле. Разработанные катализаторы перспективны для промышленного использования.
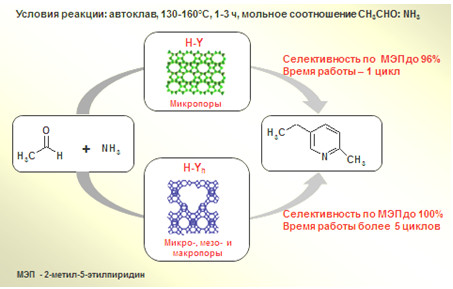
д.х.н. Н.Г. Григорьева, к.х.н. С.В. Бубеннов,
м.н.с. Н.А. Филиппова, чл.-корр. РАН У.М. Джемилев
Институт нефтехимии и катализа УФИЦ РАН, г. Уфа
Новая реакция в синтезе замещенных пирролов и пиридинов, катализируемая Cp2ТiC2
С целью разработки нового универсального метода синтеза практически важных пирролов и пиридинов впервые осуществлена реакция гетероциклизации терминальных ацетиленов с арил- и алкилнитрилами и EtAlCl2 в присутствии катализатора Cp2ТiC2. (10 мол. %) и восстановителя – мелкодисперсного порошка металлического Mg в условиях (растворитель ТГФ, 0 0 °С, 8 ч) при соотношении исходных мономеров – ацетилена и нитрила – 2:1. Установлено, что арилзамещенные нитрилы вступают в реакцию с терминальными ацетиленами и EtAlCl2 в описанных выше условиях с образованием замещенных пирролов с выходами 40-77%. В случае алкилзамещенных нитрилов в разработанных условиях удается направить реакцию гетероциклизации в сторону образования соответствующих замещенных пиридинов с выходами 50-65%. Разработанные универсальные способы синтеза замещенных пирролов и пиридинов перспективны для применения как в лабораторной практике, так и в промышленности.
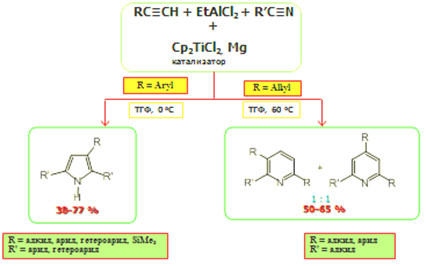
чл.-корр. РАН У.М. Джемилев, к.х.н. Л.О. Хафизова,
к.х.н. М.Г. Шайбакова, асп. Н.А. Рихтер
Институт нефтехимии и катализа УФИЦ РАН, г. Уфа
Новая стратегия в синтезе пятичленных фосфорорганических соединений – фосфоланов с применением реакции Джемилева
В развитие проводимых в ИНК УФИЦ РАН исследований в области химии фосфорорганических соединений разработан оригинальный однореакторный метод синтеза фосфоланов и фосфоленов, основанный на применении на ключевой стадии синтеза реакции каталитического циклоалюминирования линейных и циклических олефинов, дизамещенных ацетиленов с помощью AlEt3 в присутствии катализатора Cp2ZrCl2 с получением интермедиатных замещенных алюминациклопентанов или алюминациклопентенов, которые in situ реагируют с РХ3 или РХ5 с образованием целевых замещенных монофосфоланов и бис-фосфоланов, а также фосфоленов с достаточно высокими выходами 65-85%. Разработанная реакция имеет общий характер и перспективна не только в лабораторной практике, но и для разработки промышленных технологий получения широкого ассортимента полезных веществ – лекарственных препаратов, средств защиты растений, экстрагентов, лигандов для каталитических систем.
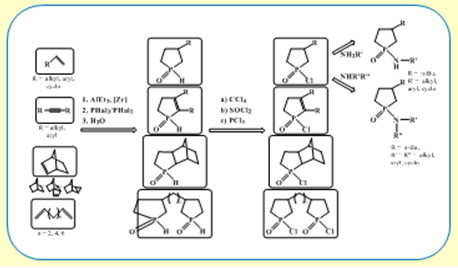
проф. РАН, д.х.н. В.А. Дьяконов, к.х.н. А.А. Махаматханова, чл.-корр. РАН У.М. Джемилев
Институт нефтехимии и катализа УФИЦ РАН, г. Уфа
Исследования механизмов каталитических реакций, базирующиеся на новом методе анализа дифференциальной селективности, использующем фазовые траектории реакций
Продолжены исследования механизмов каталитических реакций, базирующиеся на новом методе анализа дифференциальной селективности, использующем фазовые траектории реакций. Доказана обратимость стадии активации нереакционноспособных арилхлоридов палладиевыми катализаторами в реакции Сузуки-Мияуры, что принципиально отличается от ранее установленного необратимого характера активации более реакционноспособных арилиодидов и арилбромидов. Более того, обратимость активации арилхлоридов сильнее всего влияет на протекание реакции с наименее реактивными дезактивированными арилхлоридами. Полученные данные полностью опровергают возможность лимитирования скорости реакции на стадии активации субстрата, что предполагалось в течение последних трех десятилетий и исключает медленную активацию арилхлоридов как главный фактор их низкой реакционной способности в реакции Сузуки-Мияуры.
Доказано, что закономерности дифференциальной селективности реакций арилирования алкенов (реакция Мицороки-Хека) и реакции прямого C-H арилирования индолов однозначно указывают на неадекватность «классического» линейного механизма. Обнаруженная независимость дифференциальной селективности по продуктам превращения двух конкурирующих алкенов в реакции Хека или двух конкурирующих индолов в реакции прямого арилирования от арильного заместителя и галогена в арилирующем реагенте, а также от природы противоионов добавляемых галогенидных солей указывает на активацию субстратов интермедиатами, не содержащими фрагментов арилгалогенида. Показано, что совокупность экспериментальных данных согласуется с нелинейным кооперативным механизмом катализа с участием двух различных форм катализатора в активации двух реагирующих субстратов.
Все результаты были получены в реальных каталитических условиях без использования каких-либо модельных экспериментов.
д.х.н., проф. А.Ф. Шмидт, к.х.н., доцент А.А. Курохтина,
к.х.н. Е.В. Ларина, к.х.н. Е.В. Ярош, аспирант Н.А. Лагода
Иркутский государственный университет, г. Иркутск
Причины антибатной зависимости частоты оборотов гидрирования ненасыщенных соединений от концентрации палладиевого катализатора
Обнаружен антибатный характер зависимости частоты оборотов гидрирования ненасыщенных соединений (алкинов, ацетиленовых спиртов и алкенов) под действием Pd-P от концентрации катализатора. Совокупностью кинетических методов и электронной микроскопии дискриминированы гипотезы о причинах антибатной зависимости частоты оборотов: диссоциация поликристаллических Pd-P частиц, смещение равновесия катализатор – стабилизатор и агрегирование – дезагрегирование Pd-P частиц. Установлено, что основной причиной влияния концентрации катализатора на кажущуюся частоту оборотов в диапазоне 0.125 – 1 ммоль⋅л–1 является агрегирование Pd-P частиц в растворе. В зависимости от природы субстрата действие этого фактора на активность различно. На примере гидрирования стирола показа-но соответствие предложенной кинетической модели экспериментальным данным и определены константы скоростей отдельных стадий.
Результаты работы опубликованы:
Скрипов Н.И., Белых Л.Б., Стеренчук Т.П., Левченко А.С., Шмидт Ф.К., Причины антибатной зависимости частоты оборотов гидрирования ненасыщенных соединений от концентрации палладиевого катализатора, Кинетика и катализ, 2021, т. 62, № 2, с. 245-253.
д.х.н., проф. Ф.К. Шмидт, д.х.н., проф. Л.Б. Белых,
к.х.н., доцент Н.И. Скрипов, к.х.н. Т.П. Стеренчук, магистрант А.С. Левченко
Иркутский государственный университет, г. Иркутск
Эволюция каталитической системы HAuCl4/ДМСО в процессе катализа гидроаминирования пропаргиловых эфиров арабиногалактана имидазолами и бензимидазолом
Для лабильных природных полисахаридов катализаторы на основе золота являются альтернативой обычно используемым в таких реакциях щелочным катализаторам, поскольку известно, что щелочи вызывают деструкцию (пилинг) макромолекулы сахара. С целью синтеза новых фармакологически перспективных функционализированных и золотосодержащих полисахаридов впервые осуществлено гидроаминирование пропаргиловых эфиров арабиногалактана имидазолами и бензимидазолом в присутствии каталитической системы HAuCl4/ДМСО.
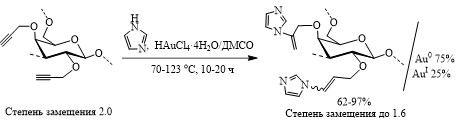
Методом рентгеновской фотоэлектронной микроскопии показано восстановление Au(III) в условиях гидроаминирования с образованием Au(I) (∼25%) и преимущественно Au(0). Золото в указанных валентных состояниях содержится в виде частиц размером 190-640 нм. Формирование Au(0) and Au(I) в ходе гидроаминирования может объясняться известной восстанавливающей способностью матрицы арабиногалактана. Гидрохлориды имидазолпроизводных арабиногалактана демонстрируют высокую бактериостатическую активность в отношении тестовых грамположительных микроорганизмов, подтверждая тем самым перспективность новых биоактивных производных на основе данного природного полисахарида, выделяемого в пилотных масштабах из древесины сибирской лиственницы.
Результаты работы опубликованы:
Carbohydrate Polymers. 2020. V. 246. Article number 116638. DOI: 10.1016/j.carbpol. 2020.116638. IF 7.182, Q1
академик РАН Б.А. Трофимов, к.х.н. Л.А. Грищенко, д.х.н. Л.Н. Паршина, д.х.н. Л.И. Ларина,
к.х.н. Л.А. Беловежец, д.х.н., доцент И.В. Клименков, д.х.н., проф. А.Ю. Устинов
Иркутский институт химии им. А.Е. Фаворского СО РАН, г. Иркутск
Изучение гидрирования диметилацетилендикарбоксилата в нулевом магнитном поле
Применение ЯМР в сильном магнитном поле для изучения химических превращений в условиях, близких к используемым в промышленности, затруднительно. В частности, металлический реактор предотвращает проникновение радиочастотных волн, необходимых для регистрации сигнала ЯМР. Кроме того, спектр ЯМР неоднородных образцов недостаточно информативен. Для решения этой проблемы в данной работе предложено использовать ЯМР в нулевом (сверхнизком) магнитном поле, поскольку металл не оказывает экранирующего эффекта на низких частотах ЯМР. Для создания достаточного сигнала ЯМР использован метод индуцированной параводородом поляризации ядер. В работе исследована двухстадийная реакция гидрирования диметилацетилендикарбоксилата в диметилмалеат и диметилсукцинат в контейнере из титана. Результаты показали, что с помощью ЯМР в нулевом поле можно изучать химические превращения в металлическом контейнере и наблюдать кинетику процесса с высоким спектральным разрешением при непрерывном барботировании параводорода через исследуемый раствор, что невозможно в сильных полях. Спектроскопия ЯМР в нулевом поле может найти широкое применение в области катализа для наблюдения кинетики химических превращений и изучения механизмов химических реакций в реальных условиях. Исследования проведены совместно с коллегами из института Гельмгольца в Майнце, Германия, и поддержаны грантом РФФИ (№ 19-29-10003).
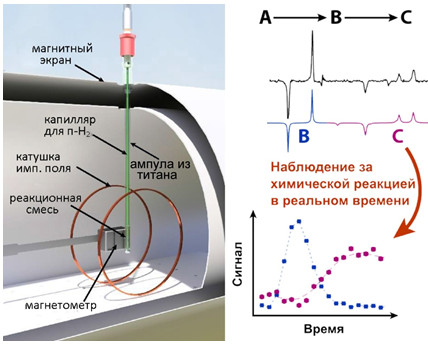
Результаты работы опубликованы:
D. B. Burueva, J. Eills,
J. W. Blanchard, A. Garcon, R. Picazo-Frutos, K. V. Kovtunov,
I. V. Koptyug, D. Budker. Chemical reaction monitoring using zero-field nuclear magnetic resonance enables study of heterogeneous samples in metal containers, Angewandte Chemie Internation Edition, 59, 17026-17032 (2020); https://onlinelibrary.wiley.com/doi/10.1002/anie.202006266.
к.х.н. Д.Б. Буруева, др. Дж. Ийлс, др. Дж.У. Бланшард, др. А. Гарсон,
Р. Пиказо-Фрутос, д.х.н. К.В. Ковтунов, д.х.н. проф. И.В. Коптюг, проф. др. Д. Будкер
Международный томографический центр СО РАН, г. Новосибирск
Институт Гельмгольца в Майнце, г. Майнц, Германия
Синтез и охарактеризование нового металл-органического каркаса на основе титана для фотокаталитических приложений
В работе, проведенной в соавторстве с KAUST (Saudi Arabia), предложен синтез и проведена характеризация нового устойчивого металл-органического каркаса на основе титана, АСМ-1. Его структура включает бесконечные цепи Ti-O и органические лиганды-фотосенсибилизаторы, где комбинация высокоподвижных фотогенерируемых электронов и высокой локализации дырок на органическом линкере приводят к большим временам жизни состояний с разделенными зарядами. С помощью метода ЭПР (МТЦ СО РАН) проведены исследования и подтвержден фото-индуцированный перенос электрона с органического линкера на титан. Энергетическая структура данного каркаса благоприятна для широкого спектра фотокаталитических приложений, от генерации водорода до селективного окисления органических субстратов.
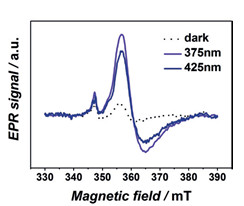
Спектры ЭПР Ti3+ (gx,y = 1.944; gz = 1.902) в ACM-1 до фотооблучения и
после облучения длиной волны 375/425 нм.
Результаты работы опубликованы:
A. Cadiau, N. Kolobov, S. Srinivasan, M. Goesten, H. Haspel, A. Bavykina, M. Tchalala,
P. Maity, A. Goryachev, A. Poryvaev, M. Eddaoudi, M. Fedin, O. Mohammed, J. Gascon, A new Titanium Metal Organic Framework with visible-light responsive photocatalytic activity, Angew. Chem. Int. Ed., 59 (2020) 13468-13472.
асп. А.С. Порываев, д.ф.-м.н., проф. РАН М.В. Федин
Международный томографический центр СО РАН, г. Новосибирск
Разработка палладиевых катализаторов для селективного окисления арилалканов
Предложено семейство катализаторов на основе комплексов палладия(II) с N4-донорными лигандами, позволяющих селективно окислять арилалканы по бензильному положению надуксусной кислотой при низких загрузках катализатора (< 1% мол.). В зависимости от условий реакция может протекать с преимущественным образованием 1-арилалканолов либо соответствующих кетонов с высокой селективностью (до 98 %). Изучен механизм каталитического действия предложенных систем. Полученные результаты позволяют сделать вывод, что реакция бензильного окисления протекает в соответствии с рекомбинационным механизмом, аналогичным механизму действия металлоферментов семейства P450, а частицей, ответственной за скорость-лимитирующий отрыв бензильного атома водорода, выступает терминальный палладий-кислородный комплекс. Противоречие теоретическому запрету на существование терминальных металл-оксо комплексов поздних переходных металлов («oxo-wall») следует считать кажущимся: данные квантовохимического моделирования предсказывают триплетное состояние данной частицы с локализацией двух неспаренных электронов на Pd-O фрагменте, что позволяет интерпретировать её как оксильный комплекс палладия(III).
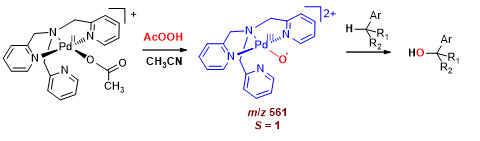
Результаты работы опубликованы:
Д.П. Лубов, А.А. Брылякова, Д.Г. Самсоненко, Д.Г. Шевень, Е.П. Талзи, К.П. Брыляков, Dalton Trans, 2020, 49, р. 11150-11156.
Д.П. Лубов, к.х.н. А.А. Брылякова, к.х.н. Д.Г. Самсоненко,
к.х.н. Д.Г. Шевень, д.х.н., проф. Е.П. Талзи, д.х.н., проф. РАН К.П. Брыляков
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Разработка способа прямого энантиоселективного С-Н гидроксилирования арилалканов пероксидом водорода
Разработан способ прямого энантиоселективного С-Н гидроксилирования арилалканов по бензильному положению «зелёным» окислителем – пероксидом водорода с высокой энантиоселективностью (до 97 %), использующий каталитические системы на основе хиральных негемовых комплексов марганца (0.2-0.4% мол.) в варьируемых условиях. Достигаемые показатели выход/энантиоселективность являются рекордными для известных процессов асимметрического окисления с использованием синтетических катализаторов на основе комплексов переходных металлов. С помощью данного способа выполнено энантиоселективное гидроксилирование ряда производных 3,4-дигидрохинолинона и аналогов, являющихся распространёнными структурными единицами биологически активных соединений.
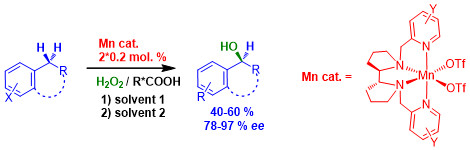
Результаты работы опубликованы:
Р. В. Оттенбахер, Е. П. Талзи, К. П. Брыляков, J. Catal., 2020, 390, р. 170-177.
к.х.н. Р.В. Оттенбахер, д.х.н., проф. Е.П. Талзи, д.х.н., проф. РАН К.П. Брыляков
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Биметаллические электрокатализаторы на основе Ni для реакции окисления водорода
Комплексное исследование серии электрокатализаторов 60% Ni/KB, 57% Ni20Cu1/KB и 59% Ni10Mo1/KB с применением различных физико-химических (в том числе in situ) методов позволило впервые показать, что степень окисленности поверхности катализатора (наличие центров Ni/NiOx) определяет его активность в реакции окисления водорода. Электронное влияние второго металла на свойства никеля, считавшееся ранее ключевым параметром в биметаллических NiM/C катализаторах, практичеcки не сказывается на удельной активности металлических (полностью восстановленных) образцов. На основе полученных данных сформулированы новые подходы к разработке эффективных электрокатализаторов на основе никеля.
Результаты работы опубликованы:
Oshchepkov A.G., Braesch G., Bonnefont A., Savinova E.R., Chatenet M., Recent Advances in the Understanding of Nickel-Based Catalysts for the Oxidation of Hydrogen-Containing Fuels in Alkaline Media, ACS Catalysis, 2020, V. 10, № 13, р. 7043-7068, DOI: 10.1021/acscatal.0c00101; Oshchepkov A.G., Bonnefont A., Savinova E.R., On the Influence of the Extent of Oxidation on the Kinetics of the Hydrogen Electrode Reactions on Polycrystalline Nickel, Electrocatalysis, 2020, V. 11, № 2, р.133-142, DOI: 10.1007/s12678-019-00560-3; Kuznetsov A.N., Oshchepkov A.G., Cherstiouk O.V., Simonov P.A., Nazmutdinov R.R., Savinova E.R., Bonnefont A., Influence of the NaOH Concentration on the Hydro-gen Electrode Reaction Kinetics of Ni and NiCu Electrodes, ChemElectroChem, 2020, V. 7, № 6, р. 1438-1447, DOI: 10.1002/
celc.202000319; Braesch G., Oshchepkov A.G., Bonnefont A., Asonkeng F., Maurer T., Maranzana G., Savinova E.R., Chatenet M., Nickel 3D Structures Enhanced by Electrodeposition of Nickel Nanoparticles as High Performance Anodes for Direct Borohydride Fuel Cells, ChemElectroChem, 2020, V. 7, № 7. р. 1789-1799, DOI: 10.1002/celc.202000254; Braesch G., Wang Z., Sankarasubramanian S., Oshchepkov A.G., Bonnefont A., Savinova E.R., Ramani V., Chatenet M., A High Performance Direct Borohydride Fuel Cell Us-ing Bipolar Interfaces and Noble Metal-Free Ni-Based Anodes, Journal of Materials Chemistry A, Materials for Energy and Sustainability, 2020, V. 8, № 39, р. 20543-20552, DOI: 10.1039/d0ta06405j.
к.х.н. А.Г. Ощепков, к.х.н. П.А. Симонов, А.Н. Кузнецов,
к.х.н. О.В. Шерстюк, д.х.н., проф. РАН Д.В. Козлов
Институт катализа им. Г.К. Борескова СО РАН,
г. Новосибирск
Фотоактивные самоочищающиеся тканевые материалы
Разработан метод создания самоочищающихся тканевых материалов путём закрепления на поверхности тканевых волокон фотоактивного компонента на основе нанокристаллического диоксида титана, допированного азотом и модифицированного переходными металлами. Предложенный подход позволяет получать текстильные материалы, отличительной особенностью которых является способность под действием солнечного света или света искусственных источников освещения осуществлять окислительную деструкцию химических веществ, макромолекул, вирусов и бактерий, обеспечивая тем самым очистку и обеззараживание собственной поверхности и окружающего пространства, что позволяет использовать их для изготовления защитной одежды и специальных изделий для работы с опасными биологическими и химическими загрязнителями с целью снижения риска распространения опасных инфекций и создания безопасных условий работы сотрудников.

Фотоактивные тканевые материалы предназначены для изготовления: средств индивидуальной защиты (костюмов, халатов, спецодежды) для работников медицины и здравоохранения, биохимических лабораторий; защитной одежды при работе с боевыми отравляющими и токсичными органическими веществами; защитной одежды при работе на опасных производствах и ликвидациях последствий чрезвычайных ситуаций; антибактериальных тканей; самоочищающихся тканей в интерьере помещений; тканевых фильтров в системах очистки и обеззараживания воздуха.
Результаты работы опубликованы:
M. Solovyeva, D. Selishchev, S. Cherepanova, G. Stepanov, E. Zhuravlev, V. Richter, D. Kozlov, Self-cleaning photoactive cotton fabric modified with nanocrystalline TiO2 for efficient degradation of volatile organic compounds and DNA contaminants, Chem. Eng. J., 388 (2020) 124167, doi:10.1016/j.cej.2020.124167.
к.х.н. Селищев Д.С., д.х.н., проф. РАН Д.В. Козлов
Институт катализа им. Г.К. Борескова СО РАН,г. Новосибирск
Оптимизация химического состава цеолитов Y и ZSM-5 для реакций крекинга углеводородов бензинового ряда
Изучены цеолиты Y с содержанием оксидов РЗЭ от 0,5 до 10 масс. %, а также ZSM-5, модифицированные фосфором. На их основе получены катализаторы, обеспечивающие повышенную активность и селективность образования легких алкенов при крекинге углеводородов бензинового ряда. Максимальный выход легких алкенов достигается при превращении н-алканов с относительно высокой молекулярной массой (н-додекан, н-гексадекан). Крекинг гексена-1 и циклогексена в сравнении с алканами протекает с более высокими выходами легких алкенов. Присутствие серосодержащих соединений способствует увеличению выхода легких алкенов при крекинге алканов за счет протекания реакций переноса водорода.
к.т.н. В.П. Доронин, к.х.н. О.В. Потапенко,
Т.П. Сорокина, д.х.н. А.В. Лавренов,
Центр новых химических технологий Института катализа им. Г.К. Борескова СО РАН, г. Омск
Активный и сверхактивный кислород на металлах в сопоставлении с оксидными системами
Выполнен сравнительный анализ результатов исследований кислорода на поверхности металлов и кислорода на поверхности оксидов. Принято считать, что, подобно оксидам, реакционная способность кислорода на металлах определяется его энергией связи с поверхностью. Попытка подтвердить это утверждение путем сопоставления скорости окисления Н2 с теплотой адсорбции кислорода показала несоразмерно высокую каталитическую активность металлов, которая на много порядков превышает ожидаемые результаты. Обычно эта несоразмерность объясняется присутствием на металлах так называемого «горячего» или, в общем случае, сверхактивного кислорода, который способен вести окисление в области криогенных температур, но не участвует в измерении теплоты адсорбции. Сравнение свойств сверхактивного кислорода на металлах со свойствами анион-радикала О•– на оксидах показывает ясно выраженное сходство этих частиц и дает основание заключить, что кислород на металлах также имеет радикальную природу. Это объясняет его сверхвысокую реакционную способность без привлечения идеи об энергетически возбужденном состоянии.
Результаты низкотемпературных реакций сверхактивного кислорода позволяют сформулировать новую трактовку правила селективности. Для катализатора селективного окисления важна не сама по себе высокая или низкая активность поверхностного кислорода, а согласованность между скоростями стадий образования и десорбции продуктов. Это расширяет возможности для поиска новых подходов в разработке селективных катализаторов, включая потенциальную возможность создания таких катализаторов на основе металлов.
Результаты работы опубликованы:
Gennady I. Panov, Eugeny V. Starokon, Dmitry P. Ivanov, Larisa V. Pirutko, Alexandr S. Kharitonov, Active and super active oxygen on metals in comparison with metal oxides, Catalysis Reviews, Published online: 23 June 2020. DOI: 10.1080/01614940.2020.1778389.
д.х.н., проф. Г.И. Панов, к.х.н. Е.В. Староконь,
к.х.н. Д.П. Иванов, к.х.н. Л.В. Пирютко, д.х.н. А.С. Харитонов
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Композитные катализаторы для конверсии дизельного топлива в синтез-газ
Разработана общая стратегия дизайна каталитических систем для процессов получения водорода из дизельного топлива, которая заключается в использовании композитных катализаторов типа «наночастицы металлов/наночастицы активного оксида/структурный оксидный компонент/структурированная металлическая подложка» и описан подход для их направленного синтеза. Структурированная металлическая подложка обеспечивает эффективный отвод/подвод тепла для экзо-/эндотермических реакций, обладает хорошими гидродинамическими характеристиками и облегчает масштабирование технологии. Структурный оксидный компонент (оксид алюминия) обеспечивает термическую и коррозионную устойчивость и высокую удельную поверхность каталитического покрытия, выполняя защитную функцию для металлической подложки. Активный оксидный компонент (преимущественно оксиды церия-циркония) повышает устойчивость к зауглероживанию за счет кислородной подвижности и поддерживает высокую дисперсность активного компонента за счет сильного взаимодействия металл–носитель. Наночастицы металлов участвуют в активации молекул-субстратов.
В качестве теплопроводящей подложки использованы фехралевые (FeCrAl) сетки, сформованные в цилиндрические блоки заданных размеров, на которые отжигом формировали слой α-Al2O3 и затем по методу Байера наносили покрытие η-Al2O3 с «дышащей» игольчатой морфологией. Далее методами пропитки и/или осаждения наносили смешанный оксид церия-циркония. Для нанесения наночастиц Pt, Rh, Ru размером 1–2 нм был разработан метод сорбционно-гидролитического осаждения.
к.х.н. В.Д. Беляев, д.т.н. А.Н. Загоруйко, к.т.н. С.В. Зажигалов, к.х.н. Н.А. Кузин,
к.т.н. А.В. Куликов, к.х.н. Д.И. Потемкин, к.х.н. В.Н. Рогожников, асп. Н.В. Рубан,
к.х.н. П.А. Симонов,
к.х.н. П.В. Снытников, д.х.н., проф. В.А. Собянин
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Моделирование процесса каталитического сжигания иловых осадков в среде ASPEN Plus
Получены результаты моделирования установки сжигания осадка сточных вод производительностью 6 тонн/час с использованием ASPEN Plus. Программа ASPEN Plus позволяет рассчитывать материальные и тепловые потоки технологических установок с использованием встроенных термодинамических пакетов.
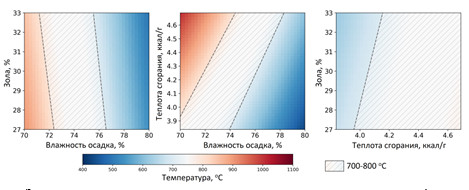
Зависимость температуры сжигания от характеристик осадка
Показано, что технология каталитического сжигания может быть использована для эффективной утилизации механически обезвоженного осадка влажностью 75 % в автотермическом режиме (без использования дополнительного топлива). При этом установка позволяет получать 3.07 МВт тепловой энергии. Показано, что влажность осадка и его теплота сгорания существенно влияют на процесс горения. При влажности менее 72 % необходима дополнительная подача воды, чтобы избежать перегрева слоя катализатора. В случае увеличения влажности более 76 % требуется дополнительная подача топлива (например, бурого угля).
Результаты работы опубликованы:
Fedorov A.V., Dubinin Y.V., Yeletsky P.M., Fedorov I.A., Shelest S.N., Yakovlev V.A., Combustion of Sewage Sludge in a Fluidized Bed of Catalyst: ASPEN PLUS Model, Journal of Hazardous Materials, V. 405, 5 March 2021, р. 124196, DOI:10.1016/j.jhazmat.2020.124196.
А.В. Федоров, к.х.н. Ю.В. Дубинин, к.х.н. П.М. Елецкий, д.х.н. В.А. Яковлев
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Кинетическое исследование и оптимизация процессов пероксидного каталитического окисления древесины
Разработка новых экологически безопасных методов получения целлюлозы из древесного сырья является весьма актуальной задачей. Впервые изучено влияние породы древесины и природы органического растворителя на протекание «зеленых» процессов пероксидной делигнификации древесной биомассы в присутствии суспендированного катализатора TiO2 в интервале температур 70-100 °С. Процессы каталитического пероксидного окисления хвойной (ель, пихта, сосна, лиственница) и лиственной (осина, береза) древесины, осуществляемые в среде «уксусная кислота – вода», характеризуются сходными кинетическими закономерностями. Эти процессы хорошо описываются уравнениями первого порядка. Достаточно высокая энергия активации процессов каталитической пероксидной делигнификации (76-87 кДж/моль) указывает на отсутствие значительных внешне-диффузионных ограничений.
Однако для глубокой делигнификации хвойной древесины, лигнин которой имеет гваяцильную природу, требуются более высокие концентрации пероксида водорода и уксусной кислоты, чем в случае лиственной древесины, в лигнине которой преобладают сирингильные структуры.
Методами ИКС, РФА, твердофазной 13С ЯМР и СЭМ установлено, что целлюлоза, полученная при оптимальных условиях процессов пероксидной делигнификации, имеет структуру микрокристаллической целлюлозы.
Обнаружено, что природа органического растворителя оказывает существенное влияние на протекание процесса каталитической пероксидной делигнификации древесины. При пероксидном окислении в среде «муравьиная кислота – вода» в качестве основных продуктов образуются целлюлоза и растворимый низкомолекулярный лигнин, в то время как в среде «уксусная кислота – вода» образуются целлюлоза и низкомолекулярные органические кислоты.
На основе оптимизированных процессов каталитического пероксидного окисления древесины предложен одностадийный метод получения микрокристаллической целлюлозы, который исключает использование экологически опасных серо- и хлор-содержащих делигнифицирующих агентов.
Результаты работы опубликованы:
Boris N. Kuznetsov, Irina G. Sudakova, Natalya V. Garyntseva, Valery E. Tarabanko, Nikolay V. Chesnokov, Laurent Djakovitch, Franck Rataboul, Kinetic Studies and Optimization of Heterogeneous Catalytic Oxidation Processes for the Green Biorefinery of Wood, Topics in Catalysis, 2020, 63 (1-2), р. 229-242, https://doi.org/10.1007/s11244-020-01244-9.
д.х.н., проф. Б.Н. Кузнецов, д.х.н. В.Е. Тарабанько,
д.х.н. Н.В. Чесноков, к.т.н. И.Г. Судакова, к.х.н. Н.В. Гарынцева
Институт химии и химической технологии СО РАН,
г. Красноярск
Каталитический гидрогенолиз нативного и органосольвентного лигнинов древесины осины в среде сверхкритического этанола
Каталитический гидрогенолиз в среде сверхкритических органических растворителей является перспективным методом деполимеризации древесных лигнинов. Нами впервые сопоставлены свойства бифункциональных катализаторов Ru/углерод, Pt//ZrO2, NiCuMo/SiO2 в процессах гидрогенолиза древесины и этаноллигнина осины в среде сверхкритического этанола при температуре 250 °С. Наиболее активными в процессах деполимеризации лигнина являются катализаторы Ru/углерод и Pt//ZrO2, которые обеспечивают высокую конверсию древесины (до 78 % масс.), значительный выход жидких продуктов (до 50,6 % масс.) и низкий выход твердого остатка (до 22 % масс.). Эти катализаторы увеличивают содержание мономерных соединений в жидких продуктах с 10,5 % (некаталитическое гидрирование) до 50,4 % от массы лигнина. При каталитическом гидрировании этаноллигнина, выделенного из древесины осины, выход жидких продуктов ниже по сравнению с древесиной. Это, вероятно, обусловлено меньшим содержанием слабых эфирных β-О-4 связей в структуре этаноллигнина относительно нативного лигнина древесины.
По данным метода ГПХ, катализаторы смещают молекулярно-массовое распределение жидких продуктов гидрогенолиза древесины и этаноллигнина осины в область пониженных ММ. Согласно данным ГХ-МС, в составе жидких продуктов преобладают алкилпроизводные метоксифенолов (в основном пропилсирингол и пропилгваякол). Твердые продукты каталитического гидрогенолиза древесины осины содержат, в основном, целлюлозу (до 82,2 % масс.).
Таким образом, каталитический гидрогенолиз в сверхкритическом этаноле в присутствии бифункциональных катализаторов Ru/углерод и Pt//ZrO2 позволяют осуществить фракционирование древесной биомассы на целлюлозу и жидкие углеводороды, обогащенные пропилсиринголом и пропилгваяколом. Мономерные продукты каталитического гидрогенолиза нативного лигнина древесины осины имеют перспективы использования в качестве компонентов моторных топлив и топливных добавок.
Результаты работы опубликованы:
B. N. Kuznetsov, V. I. Sharypov, S. V. Baryshnikov, A. V. Miroshnikova, O. P. Taran, V. A. Yakovlev, A. V. Lavrenov, L. D. Djakovitch, Catalytic hydrogenolysis of native and organosolv lignins of aspen wood to liquid products in supercritical ethanol medium, Catalysis Today, 2020, DOI:10.1016/
j.cattod.2020.05.048.
д.х.н., проф. Б.Н. Кузнецов, д.х.н., проф. РАН О.П. Таран,
к.х.н. С.В. Барышников, А.В. Мирошникова
Институт химии и химической технологии СО РАН, г. Красноярск
Катализатор для переработки широкой фракции легких углеводородов в ароматические соединения
Для превращения модельной смеси алканов С1-С6, имитирующей состав широкой фракции легких углеводородов (ШФЛУ, состав (% мас.): CH4 – 8,4; C2H6 – 12,8; C3H7 – 38,9; C4H10 – 36,0; С5Н12-С6Н14 – 3,9), в ароматические соединения получен цеолит-содержащий катализатор на основе синтезированного галлоалюмосиликата структурного типа ZSM-5 с добавкой 20 % масс. связующего вещества (псевдобемита). Катализатор обеспечивает степень превращения исходного сырья не менее 80 %, выход и селективность образования ароматических углеводородов более 40 и 50 %, соответственно. Селективность образования целевого продукта при многоцикловом использовании катализатора изменяется незначительно, составляя более 50 % при его стабильной активности. Результаты дифференциально-термического анализа, электронной микроскопии и термодесорбции аммиака указывают на то, что при окислительной регенерации катализатора происходит полное удаление кокса с его поверхности и практически полное восстановление кислотности. Полученные данные свидетельствуют о возможности длительной эксплуатации Ga-содержащего цеолитного катализатора в процессе переработки углеводородных газов в жидкие продукты.
дд.х.н., проф. А.В. Восмериков, к.х.н. Л.Н. Восмерикова
Институт химии нефти СО РАН, г. Томск
Исследование структурных и каталитических свойств модифицированного лантаном вольфрамированного диоксида циркония
Синтезированы и охарактеризованы методами РФА, ДСК и РФЭС твердые растворы катионов лантана в диоксиде циркония на основе неравновесной тетрагональной модификации. Установлено, что в системах La/ZrO2 и La/W/ZrO2 при температурах формирования 600-800 °С реализуются два фактора стабилизации неравновесной модификации ZrO2: наноразмерный эффект (размер кристаллитов 18-25 м) и образование твердых растворов. Методом РФЭС показана преимущественная локализация La в приповерхностных слоях.
При повышении температуры (900 °С и выше) метастабильная модификация ZrO2 стабилизирована за счет термической устойчивости объемных и/или приповерхностных твердых растворов катионов лантана в диоксиде циркония.
Показано промотирующее влияние добавок лантана на каталитические свойства вольфрамированного диоксида циркония. В реакции гидроизомеризации смеси гептана с бензолом достигнуто увеличение (на 10-15 %) выхода продуктов с повышенными октановыми числами.
Результаты работы опубликованы:
S. A. Naumova, A. V. Obukhova, L. I. Kuznetsova, J. of Siberian Federal University, Chemistry, vol. 13, iss. 1, p. 133-141.
д.х.н., проф. П.Н. Кузнецов
Институт химии и химической технологии СО РАН, г. Красноярск
к.х.н., доцент Ф.А. Бурюкин
Институт нефти и газа, Сибирский федеральный университет, г. Красноярск
Разработка и усовершенствование промышленных катализаторов и технологий
Создание новой кормовой добавки «Энергосорб»
В течение нескольких лет в «СФК АГРО», «СФК Удобрение» (Смоленск, Челябинск) и «НИАП-КАТАЛИЗАТОР» (Новомосковск) осуществлялись работы по созданию препарата (фунгицида) для защиты растений. За основу препарата был взят полупродукт при производстве медьсодержащих катализаторов. После отработки технологии, выбора оптимальных сырьевых компонентов и внесения добавок был получен препарат, получивший название «МедьАгро». Экологичный препарат «МедьАгро» прошел испытания в различных НИИ сельского хозяйства и уже эффективно используется в десятках областей РФ. Эта работа была отмечена Национальной экологической премией имени В.И. Вернадского.
Также проводилась разработка на основе полупродуктов при производстве медных катализаторов для лечения животных от болезней бактериального происхождения. Десятки тонн препарата, получившего название «X-Hooves» были поставлены для масштабных испытаний в НИИ и эксплуатации в 300 сельхозпредприятий. Было подтверждено высокое качество препарата.
Опыт приготовления препаратов «МедьАгро» и «X-Hooves», а также многолетний опыт при разработке рецептуры и технологии производства промышленных катализаторов позволили в относительно короткие сроки создать новую высокоэффективную кормовую добавку, получившую название «Энергосорб».
Авторами разработана технология непрерывного безотходного процесса производства энергетической кормовой добавки «Энергосорб», при которой, в частности, вместо высокотоксичной серной кислоты используется уксусная кислота, что обеспечило безопасность производства и ликвидацию сточных вод.
В качестве исходных компонентов при производстве кормовой добавки «Энергосорб» использовали: жидкое натриевое стекло, уксусную кислоту, пропиленгликоль, глицерин, модифицированный крахмал холодного загустевания. Достоинство способа производства состоит в том, что все необходимые для синтеза кормовой добавки «Энергосорб» компоненты непрерывно подаются в реактор синтеза, в котором происходит, в частности, химическое взаимодействие кремнийсодержащего соединения в виде стекла натриевого жидкого с уксусной кислотой.
В результате химического синтеза в реакторе непрерывного действия получают полуфабрикат кормовой добавки в виде коллоидной системы, состоящей из продуктов химического взаимодействия (тригидрат ацетата натрия и аморфный диоксид кремния), глицерина и пропиленгликоля. Существенно упрощается технология, исключается трудоемкая и дорогостоящая стадия промывки и фильтрации осадка, и получается однородная гомогенная структура кормовой добавки, не требующая дополнительной стадии пропитки аморфного диоксида кремния пропиленгликолем и глицерином.
Экспериментальная проверка эффективности энергетической кормовой добавки «Энергосорб» проводилась в фермерских хозяйствах Смоленской области, Республики Мордовии и в других регионах РФ, где ее применяли для кормления коров. Была подтверждена ее высокая эффективность, проявляющаяся в увеличении жирности молока, улучшении его органолептических качеств, повышении удоя коров. Подтверждение высокой эффективности «Энергосорб» стало основанием для создания первой очереди промышленной установки по производству энергетической кормовой добавки мощностью 1000 т/г в п. Верхнеднепровский (Смоленская обл.).
Результаты работы опубликованы:
Голосман Е.З., Ефремов В.Н., Промышленные инновационные катализаторы и лечебные препараты на основе полупродуктов при производстве катализаторов, с. 169-179, в кн.: Теория и практика гетерогенных катали-заторов и адсорбентов, под ред. О.И. Койфмана, М.: ЛЕНАНД, 2020. 640 с.
О.П. Фирсов, д.т.н., проф. Ю.В. Загашвили, Е.Н. Бородако,
М.Н. Скарлыгин, к.т.н., доцент В.Н. Ефремов,
д.х.н., проф. Е.З. Голосман
ООО “СФК АГРО”, г. Смоленск
ООО «ВТР», Санкт-Петербург
ООО «СФК Удобрение», Челябинск
ООО “НИАП-КАТАЛИЗАТОР”, г. Новомосковск
Технология утилизации отработанных катализаторов дегидрирования изобутана
Проводились научно-исследовательские работы по поиску возможных технологий и способов утилизации отработанных алюмохромовых катализаторов дегидрирования изобутана. Предложена технология уменьшения содержания Cr(VI) в отработанных промышленных алюмохромовых катализаторах с 0,7 до ~ 0,03 %.
к.т.н. Д.В. Качалов, А.С. Шуткин, к.э.н. А.В. Кужин,
А.И. Рубец, к.х.н. В.И. Титов
ОАО НИИ «Ярсинтез», г. Ярославль
Tandem catalyst converts propane to propylene
Nanoparticles unite platinum and indium oxide catalysts for better yields at lower temperature
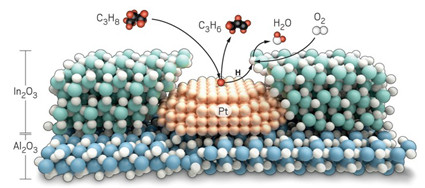
The tandem catalyst uses platinum to turn propane (C3H8) into propylene (C3H6)
before handing hydrogen atoms to indium oxide for conversion into water.
By combining the catalytic powers of platinum and indium oxide in finely tuned nanoparticles, researchers have improved a reaction that converts propane into propylene, an important process in the petrochemical industry (Science 2021, DOI: 10.1126/science.abd4441).
The proof-of-concept work shows that nanostructured tandem catalysts—which host different reactions side-by-side on a single nanoparticle—have the potential to play a bigger role in industrial processes, says Justin M. Notestein of Northwestern University, who led the work with his colleague Peter C. Stair.
The team targeted propylene production because of its significance in the chemical industry—global output reached 110 million metric tons last year, much of it destined for polypropylene plastics. Changes to the raw materials supplying steam crackers have shrunk the supply of propylene, while the method developed to replace this supply, propane dehydrogenation (PDH), is energy intensive and expensive. PDH plants convert propane to propylene at temperatures of 600 °C or more, and these conditions generate sooty carbon deposits that quickly deactivate the catalysts.
To solve these problems, researchers have spent decades developing the oxidative dehydrogenation of propane (ODHP), in which the hydrogen freed from propane is immediately combined with oxygen to create water. This pulls the reaction equilibrium in the right direction, requires lower temperatures, and makes less catalyst-corrupting carbon. “Given the scale at which this technology is needed, that could mean tremendous energy and cost savings,” says Ive Hermans of the University of Wisconsin-Madison, who has developed boron-based catalysts for ODHP. However, ODHP catalysts also have a nasty habit of turning propylene into CO and CO2, which means they still cannot beat the propylene output from PDH.
That’s where the new tandem catalyst comes in. It contains two catalysts, each targeting a different stage of the reaction, to increase propylene production and reduce the formation of unwanted byproducts.
To make the catalyst, the Northwestern team dotted 2 nm wide clumps of platinum onto 100 nm particles of alumina. Then they coated each particle with a 2 nm thick shell of indium oxide, using a process called atomic layer deposition. Heating the particles opened up 1.4 nm pores in the shell, exposing about half of the platinum atoms on the surface beneath.
During the 450 °C ODHP reaction, platinum plucks hydrogen from propane to release propylene before indium oxide takes over to combine the hydrogen atoms with oxygen. This process converts about 40% of the available propane, giving a mix of products that is about 75% propylene and 25% CO2 with virtually no carbon. Overall, Notestein says, this system offers the best balance between conversion and selectivity for any ODHP catalyst. “To be honest, I did not expect this system to work nearly as well as it did,” he says.
The indium oxide shell stabilizes the platinum nanoparticles, which improves the catalyst’s longevity. Since the reaction operates at a constant temperature in a single vessel, Notestein says it could enable much simpler reactor designs than typical PDH systems. “From what I’ve seen, it looks very exciting,” Hermans says. Notestein adds that tandem catalysts might also offer a less energy-intensive route to produce ethylene.
The tiny laboratory system used in this study is a long way from a gigantic propylene plant, though, and atomic layer deposition is a laborious way to build a catalyst. “A major challenge with the scale-up process will be the production of the catalyst,” says Jinlong Gong of Tianjin University, who has developed PDH catalysts. Notestein hopes that more straightforward synthesis methods could be developed to create similar nanostructures.
Phosphonium salt boosts electrochemical Haber-Bosch reaction
An electrochemical Haber-Bosch process achieves record efficiency and longevity with the aid of a fast proton shuttle
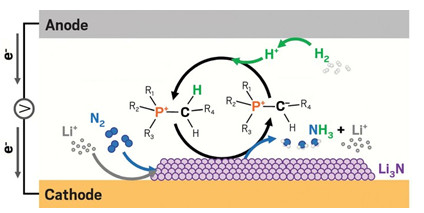
A phosphonium cation helps transfer protons to the cathode of an electrochemical cell where
nitrogen is reduced to ammonia through a Li3N intermediate. The cation is regenerated at the anode.
R1, R2, and R3 are hexyl groups and R4 is a tridecyl group.
Aphosphonium salt speeds the delivery of protons and boosts the performance of an electrochemical Haber-Bosch process to record levels (Science, 2021 DOI: 10.1126/science.abg2371).
The Haber-Bosch process is one of the most important and energy-consuming chemical reactions in the world. If it could be done electrochemically, the process could be powered by renewable electricity instead of fossil fuels, burned to provide the high temperatures and pressures in most Haber-Bosch reactors. The electrochemical process directly reduces nitrogen to ammonia. It first activates dissolved nitrogen gas with lithium ions at the cathode, forming lithium nitride, an unstable, transient species. Then, protons produced at the anode replace lithium and convert lithium nitride to ammonia.
For decades, this process has been studied only in labs, suffering from low efficiency and slowness. Bryan H. R. Suryanto, Alexandr N. Simonov, Douglas R. MacFarlane, and their colleagues at Monash University identified one target for improvement: if protons could move faster from the anode to the cathode, ammonia production could speed up.
Typically, ethanol serves as a proton shuttle. But ethanol molecules diffuse slowly across the cell and get consumed in the process. Effectively, that means ammonia is synthesized from ethanol, which is not sustainable, Simonov said.
Instead, the team turned to phosphonium salts. Lithium nitride is a strong, proton-seeking base, and phosphonium cations are known to give up a proton from the phosphorus atom’s neighboring carbon atoms to form ylides, molecules with opposite charges on adjacent atoms, MacFarlane explained. If the ylide could in turn pick up protons from the anode, it would regenerate the phosphonium cation. The phosphonium cations thus had the potential to be a recyclable proton shuttle, driven by their charge to move quickly towards the negatively charged cathode.
The researchers tested their idea using trihexyltetradecylphosphonium salt in a simple electrochemical cell. Over a 20-hour experiment, the cell produced ammonia at a rate of 53 nmol per second per square centimeter of the electrode’s surface area with 69% faradaic efficiency, a measure of how efficiently electrons are converted to products in an electro-chemical reaction. In comparison, the previous high record was 30 nmol s–1 cm–2 with 35% faradaic efficiency, which was sustained for under an hour (Nat. Catal., 2020 DOI: 10.1038/s41929-020-0455-8).
These “much-improved” performances represent important steps forward, said Karthish Manthiram at the Massachusetts Institute of Technology, who led the 2020 study. “To get this kind of performance at this duration is really very special.” More importantly, the novel demonstration that phosphonium ions could be effective proton shuttles “opens up a lot of new space,” Manthiram said.
When the researchers extended the reaction time to 93 hours, the overall performance dropped closer to that from Manthiram’s study. Still, the fact the reaction lasted 93 hours was “remarkable,” Manthiram says.
One likely reason for the performance loss over time is buildup of ammonia gas in the system, explained MacFarlane. The researchers opted for a fixed-volume cell configuration for this proof-of-concept study. The researchers are now working to test the process on a larger scale using a flow setup, which would continuously remove ammonia and avoid this problem. Another “elephant-in-the-room” problem that plagues the field more broadly is the use of tetrahydrofuran as a solvent, said Suryanto. Tetrahydrofuran is electrochemically unstable and polymerizes over time, slowing diffusion during long-term experiments.
Reduction carves path to chiral compounds
Asymmetrical Zn is key to compounds with 4 different carbon groups
Many pharmaceuticals need to be a certain shape to fit into the binding pocket of the enzyme they target. Synthesizing molecules with the desired chirality requires controlled addition of functional groups, which is challenging. Now Pengwei Xu and Zhongxing Huang from the University of Hong Kong have made this process a little easier. They found a way to transform malonic esters into chiral compounds with four different carbon substituents (Nat. Chem. 2021, DOI: 10.1038/s41557-021-00715-0). The chemists created six classes of molecules containing a variety of functional groups, which wasn’t possible with prior methods.
Previously, researchers used pig liver esterases to transform malonic esters into chiral compounds. But these enzymes don’t work well when the esters have bulky side groups or multiple side groups similar in size, which limits the enzymes’ utility. The team used cheap starting materials to make a library of malonic esters of different sizes. The researchers used tetradentate prolinol ligands and a dinuclear diethylzinc catalyst to reduce one ester group to make over 70 compounds. These include amino and hydroxyl esters, alcohols, and diols, all in moderate to good yields and as high as 98% enantioselectivity. This catalyst works in the presence of multiple functional groups, including amines, olefins, and thioethers, Huang says.
The diethylzinc catalyst spontaneously ignites in air, but Huang says the team is researching alternatives. This tool will allow researchers to make a wider variety of complex natural products more easily and is a good companion to the existing enzyme method, Huang says.
Spontaneously arising electric fields affect reactions on catalyst particles in solution
Finding could provide handle for controlling reactions and sorting out mechanisms
As gaseous reagents react on Pt particles in liquids, electric fields arise spontaneously and affect the reaction rate.
Electric fields arise spontaneously at the surface of solid catalysts im-mersed in liquids, and this common yet largely overlooked phenomenon directly affects the rates of a large class of reactions. Using a meticulous set of techniques, chemists have been able to measure this elusive effect for the first time (ACS Cent. Sci. 2021, DOI: 10.1021/acscentsci.1c00293).
Researchers have known for years that charged moieties in enzymes can cause electric fields to arise at their active sites and that the fields drive biochemical reactions. A similar process should occur at the surface of catalytic particles in solution because of the charges carried by electrons and ions that come and go during reactions. But because there’s no simple way to wirelessly monitor electrical events occurring at particles submerged in solvents, the phenomenon has remained out of sight and out of mind.
To measure these tough-to-see electric fields and study their influence on heterogeneous catalytic reactions, Thejas S. Wesley, Yuriy Román-Leshkov, and Yogesh Surendranath of the Massachusetts Institute of Technology applied techniques from spectroscopy, electrochemistry, and other areas to a test reaction—solution phase ethylene hydrogenation using a platinum catalyst.
First, the team applied infrared spectroscopy to a reporter molecule to show that the extent of spontaneous polarization at the interface between Pt and water can readily be tuned by varying the pH, a measure of ion concentration. Polarization is caused by accumulation of charge (ions) at the interface and is directly related to electric fields that arise there.
Then the group ran the reaction in a cell containing Pt catalyst particles and a Pt electrode that tracked a voltage corresponding to pH-dependent changes in polarization. They found that the reaction rate changed systematically with changes in pH.
The results show that in aqueous solution, where proton transfer causes Pt particles to become polarized and spontaneously sets up electric fields, the fields directly influence reaction rates. The group found similar results when it repeated the experiment in aprotic organic solvent. In that case, the polarization and electric fields result from electron transfer.
Controlling electric fields could provide a handle for controlling catalytic reactions, and the work provides insights into the underlying mecha-nisms of heterogeneous catalysis, Surendranath says.
“This is very creative work” that will inspire follow-up research, says Jahan Dawlaty, a catalysis specialist at the University of Southern California. It makes sense that electric fields should arise at catalyst particles in solution, he says, but the effect “has never been studied before in a beautiful and systematic way.”
Chemical & Engineering News
|
July
19-23, 2021 30th International Conference on Photochemistry (ICP2021) (virtual event) Geneva, Switzerland |
https://www.icp2021.ch |
|
August
13-20, 2021 48th World Chemistry Congress (virtual event) Montreal, Canada |
https://www.cheminst.ca/conference/ccce2021/ |
|
16-20
августа 2021 г. ХI Всероссийская научная конференция и школа «Аналитика Сибири и Дальнего Востока», посвященная 100-летию со дня рождения И.Г. Юделевича Новосибирск, Россия |
http://conf.nsc.ru/asfe-11
|
|
8-10
сентября 2021 г. VIII Всероссийская научно-техническая конференция молодых ученых «Перспективы создания и применения конденсированных высокоэнергетических материалов» Бийск, Россия |
http://www.ipcet.ru/images/news/2021-04-09/inform2021.pdf |
|
September
12-17, 2021 XXIV International Conference on Chemical Reactors CHEMREACTOR-24 (virtual event) Milan, Italy |
http://conf.nsc.ru/CR-24/en/ |
|
September
13-15, 2021 4th European Conference on Metal Organic Frameworks and Porous Polymers (EuroMOF 2021) (virtual event) Krakow, Poland |
https://dechema.de/en/EuroMOF2021.html |
|
September
20-23, 2021 13th European Congress of Chemical Engineering and 6th European Congress of Applied Biotechnology (ECCE 13 & ECAB 6) (virtual event) Berlin, Germany |
http://ecce-ecab2021.eu/ |
|
20-25
сентября 2021 г. IV Российский конгресс по катализу «РОСКАТАЛИЗ» Казань, Россия |
http://conf.nsc.ru/RusCat-2021/ru
|
|
24
сентября – 4 октября 2021 г. XXXIII Симпозиум «Современная химическая физика» Туапсе, Россия |
http://www.chemicalphysics.ru
chemphysics-2021@yandex.ru |
|
13-16
октября 2021 г. Международная научно-техническая конференция «КАТАЛИЗ: переработка углеводородного сырья и экология» Ташкент, Узбекистан |
http://conf.nsc.ru/tashkent-2020/ru |
|
17-20
October, 2021 4th International Symposium on Multiscale Multiphase Process Engineering (MMPE) Berlin, Germany |
https://dechema.de/en/mmpe2020.html |
|
18-21
октября 2021 г. I Международная научнотехническая конференция «Химия и химическая технология: теоретические и прикладные исследования» Чирчик, Узбекистан |
kkt2021@yandex.ru
+998909929066 |
|
December
16-21, 2021 International Chemical Congress of Pacific Basin Societies (Pacifichem 2021) Honolulu, Hawaii, USA |
http://www.chemistry.or.jp/en/events/pacifichem.html |
|
|
|
|
2022
6th International Conference “Catalysis for Renewable Sources: Fuel, Energy, Chemicals” (CRS-6) Lisbon, Portugal |
http://conf.nsc.ru/CRS6/en |
|
2022
г. XIV Конференция «Металлургия цветных, редких и благородных металлов» в рамках XII Международного конгресса и выставки «Цветные металлы и минералы» Красноярск, Россия |
https://nfmsib.ru/ |
|
May
25-27, 2022 New Trends in Polymer Science: Health of the Planet,Health of the People Turin, Italy |
https://polymers2022.sciforum.net/ |
|
May-June,
2022 10th International Symposium “Molecular Order and Mobility in Polymer Systems” (MOMPS-X) Saint Petersburg, Russia |
http://momps2020.macro.ru/ |
|
June,
2022 International BioEPR School-conference 2021 Novosibirsk, Russia |
http://www.bioepr2020.ru |
|
6-10
June, 2022 11th European Conference on Solar Chemistry and Photocatalysis: Environmental Applications (SPEA11) Turin, Italy |
http://www.spea11.unito.it/home |
|
July
3-8, 2022 12th Triennial Congress of the World Association of Theoretical and Computational Chemists (WATOC 2020) Vancouver, Canada |
http://watoc2020.ca |
|
July
18-22, 2022 2nd International Conference on NonCovalent Interactions (ICNI 2021-2022) Strasbourg, France |
http://icni2021.unistra.fr/ |
|
July
18-22, 2022 26th IUPAC International Conference on Chemistry Education (ICCE 2020) Cape Town, South Africa |
https://iupac.org/event/26th-iupac-international-conference-on-chemistry-education/ |
|
July
24-28, 2022 84th Prague Meeting on Macromolecules – Frontiers of Polymer Colloids Prague, Czech Republic |
https://www.imc.cas.cz/sympo/84pmm/ |
|
August
28 – September 2, 2022 44th International Conference on Coordination Chemistry Rimini, Italy |
https://www.iccc2020.com/ |
|
September,
2022 7th International Conference on Metal-Organic Frameworks and Open Framework Compounds Dresden, Germany |
https://dechema.de/en/MOF2020.html |
|
September
– October, 2022 9th IUPAC International Conference on Green Chemistry (ICGC-9) Athens, Greece |
http://www.greeniupac2020.org |
|
August
27 – September 1, 2023 15th European Congress on Catalysis (EuropaCat 2021) Prague, Czech Republic |
https://www.europacat2023.cz/ |


