
 7 января 2016 года исполнилось 75 лет действительному члену Российской академии наук, директору Института нефтехимического синтеза им. А. В. Топчиева (РАН) Саламбеку Наибовичу Хаджиеву.
7 января 2016 года исполнилось 75 лет действительному члену Российской академии наук, директору Института нефтехимического синтеза им. А. В. Топчиева (РАН) Саламбеку Наибовичу Хаджиеву.
Саламбек Наибович окончил Грозненский нефтяной институт в 1962 году. С 1967 по 1990 год работал в Грозненском нефтяном научно-исследовательском институте (ГрозНИИ), пройдя путь от старшего научного сотрудника до директора института. В 1992-1994 годах являлся генеральным директором НПО “Грознефтехим”. В 1995 году – Премьер-министр правительства Чеченской республики. В 1996 году – Председатель Государственного комитета РФ по промышленной политике. С 1997 года заведовал лабораторией Института нефтехимического синтеза им. А. В. Топчиева РАН, в настоящее время является его директором.
Член-корреспондент c 1990 года, академик c 2008 года – Отделение химии и наук о материалах.
Крупный ученый в области катализа на цеолитах, нефтепереработки и нефтехимии, в первую очередь каталитического крекинга, конверсии высокомолекулярных соединений нефти, тяжелых нефтяных остатков, превращения природного и попутного газа в моторные топлива и сырье для нефтехимии, синтеза изоалкановых и алкилароматических углеводородов, компонентов высокоплотных специальных топлив.
Исследования С.Н. Хаджиева составили научную основу новых реализованных в РФ и за рубежом промышленных процессов термокаталитических превращений высокомолекулярных углеводородов, в том числе комплексов глубокой переработки нефти Г-43-107 и КТ-1 (Москва, Уфа, Омск, Грозный, Нижнекамск, Азербайджан, Болгария, Казахстан, Литва, Украина), производства высокоплотного топлива Т-6 (Орск), синтеза алкилбензина (Болгария) и этилбензола (Салават).
Под руководством С.Н. Хаджиева выполнено и защищено более 20 докторских и кандидатских диссертаций.
Он автор более 400 научных работ, из них 163 патента и авторских свидетельств.
Главный редактор журнала «Нефтехимия».
Председатель объединенного Научного совета РАН по химии нефти, газа, угля и биомассы.
Избирался народным депутатом СССР (1989–1991), был членом Верховного Совета СССР, входил в Межрегиональную депутатскую группу, был членом Комиссии Совета Союза по бюджету, планам и финансам. В 1990 году был избран депутатом Верховного Совета Чечено-Ингушетии; в 1991 году — председатель Движения демократических реформ Чечено-Ингушетии; председатель Конгресса демократических сил народов Северного Кавказа.
Награжден орденами “Знак почета” и “Трудового Красного Знамени”.
Заслуженный нефтехимик СССР, Заслуженный работник Топливно-энергетического комплекса России.
Научный совет по катализу ОХНМ РАН и редакция Каталитического бюллетеня сердечно поздравляют
 25 февраля 2016 исполнилось 60 лет действительному члену Российской академии наук, директору Института органической и физической химии им. А.Е. Арбузова КазНЦ РАН, председателю Казанского научного центра РАН Олегу Герольдовичу Синяшину.
25 февраля 2016 исполнилось 60 лет действительному члену Российской академии наук, директору Института органической и физической химии им. А.Е. Арбузова КазНЦ РАН, председателю Казанского научного центра РАН Олегу Герольдовичу Синяшину.
По окончании Казанского государственного университета в 1978 году О.Г. Синяшин работает в Институте органической и физической химии им. А.Е. Арбузова КазНЦ РАН, с 2001 года является директором института. С 2008 года – председатель Казанского научного центра РАН.
Член-корреспондент с 1997 года, академик РАН с 2006 года – Отделение химии и наук о материалах. Выдающийся ученый в области органической и элементоорганической химии.
О.Г. Синяшиным разработаны общие методы фосфорилирования и тиилирования органических и металлоорганических соединений с участием тиопроизводных кислот трёхвалентного фосфора.
Им предложена стратегия конструирования сложных гетероциклических и каркасных функционально замещённых фосфиновых лигандов, в том числе водорастворимых оптически активных фосфиноаминокислот, и их комплексов с переходными металлами. Найден уникальный процесс самосборки неизвестных ранее макроциклических фосфинов – оригинальных объектов для супрамолекулярной химии.
На принципах биомиметики созданы катализаторы электрохимического синтеза водорода и его окисления в топливных элементах.
Развито новое научное направление – металлокомплексный катализ для селективной электрохимической активации и функционализации органических молекул.
Им изучены процессы электрохимической активации и трансформации элементного (белого) фосфора под действием органических и металлоорганических соединений, созданы научные основы высокоэффективной и экологически безопасной технологии электросинтеза таких важнейших классов соединений фосфора как третичные фосфины и гипофосфористая кислота.
Впервые экспериментально получен и охарактеризован предсказанный ранее только теоретически фосфиноксид H3PO, являющийся важным интермедиатом процесса трансформации молекулы P4.
Под руководством О.Г. Синяшина успешно развивается химия элементного фосфора в части изучения полифосфидов щелочных металлов, что позволяет глубже понять механизмы раскрытия молекулы белого фосфора и последующего образования практически значимых веществ.
О.Г. Синяшин внёс существенный вклад в органическую химию фуллеренов. Под его руководством синтезировано большое число новых органических производных фуллерена C60, в том числе новых классов соединений – фуллероимидазопиримидинов.
В ряду предложенных производных фуллеренов обнаружены соединения, перспективные для создания высокоэффективных органических солнечных батарей.
Руководит созданной им научной школой.
Подготовил четырех докторов наук и 15 кандидатов наук.
Автор и соавтор более 800 научных публикаций, в том числе монографии и четырёх глав в монографиях, а также 46 патентов.
Член редакционной коллегии научных журналов Mendeleev Communications, Heteroatom Chemistry, Известия АН. Серия химическая.
Член комиссии РАН по работе с молодежью.
Член секции Межведомственного совета по присуждению премий Правительства РФ в области науки и техники.
Заместитель председателя Научного совета по органической и элементоорганической химии РАН.
Член Бюро Совета по координации деятельности региональных отделений и региональных научных центров РАН.
Член Международного союза по теоретической и прикладной химии (IUPAC).
Член Американского химического общества.
Лауреат премии имени А.Н. Несмеянова.
Научный совет по катализу ОХНМ РАН и редакция Каталитического бюллетеня поздравляют Олега Герольдовича с юбилеем, желают ему дальнейших успехов на благо российской науки!

Отчет о научно-организационной деятельности в 2015 году
Секретариат Научного совета по катализу ОХНМ РАН (НСК) предлагает Вашему вниманию сводный отчет о деятельности Совета и научных исследованиях в области катализа, выполненных научными коллективами под руководством членов Научного совета по катализу.
Отчет состоит из трех разделов:
Тексты отчетов, полученные от членов НСК и научно-исследовательских коллективов, практически не подвергнуты корректировке.
В 2015 году в рамках научно-организационной деятельности Научного совета по катализу ОХНМ РАН (НСК) были выполнены следующие мероприятия.
1. Выпущены четыре ежеквартальных сборника «Каталитический бюллетень», содержащие оперативную информацию о важнейших результатах фундаментальных и прикладных исследований в области катализа в России и за рубежом, материалы, посвященные деятельности выдающихся отечественных и зарубежных исследователей в области катализа, дается перечень предстоящих конференций, краткие отчеты о проведенных конференциях, рабочих совещаниях и другие материалы.
2. Под эгидой Научного совета по катализу и при активном участии его членов организованы и проведены следующие конференции:
Изданы материалы проведенных конференций.
Секретариат НСК ведет переписку и текущую работу с членами Научного совета по катализу ОХНМ РАН.
Продолжается сотрудничество с организациями Академий наук РФ и стран СНГ, Министерствами РФ, институтами разных ведомств и другими организациями России, дальнего и ближнего зарубежья по различным вопросам научной, научно-организационной, учебно-преподавательской и общественной деятельности в области катализа.
Фундаментальные исследования в области создания новых каталитических систем и применения физических методов для их диагностики
Каталитическое исследование реакции Сузуки
При исследовании реакции Сузуки было установлено, что малоактивные арилгалогениды, не вступающие в реакцию в присутствии наночастиц палладия, начинают реагировать, если одновременно участвует более активный арилйодид. Обнаруженное явление вовлечения в реакцию малоактивных субстратов более активными можно интерпретировать как прямое доказательство образования более активных мелких наночастиц палладия путем вымывания (leaching) атомов палладия из исходного нанопалладиевого катализатора. Данное явление наблюдали с различными типами наночастиц палладия, что подтверждает общий характер эволюции различных палладиевых наноматериалов в процессе каталитической реакции. Явление вовлечения можно использовать в качестве надежного теста, указывающего на гомогенный характер механизма каталитической реакции и, таким образом, оно может рассматриваться как первый невозмущающий способ доказательства перераспределения палладия путем его вымывания из исходного гетерогенного катализатора и последующего формирования новых, более реакционноспособных малых наночастиц. Кроме того, обнаруженное явление имеет существенное практическое значение, поскольку указывает на пути контролирования и повышения каталитической активности наночастиц палладия.
Результаты работы опубликованы: A.N. Kashin, O.G. Ganina, A.V. Cheprakov, IP. Beletskaya. The Direct Non-Perturbing Leaching Test in the Phosphine-Free Suzuki-Miyaura Reaction Catalyzed by Palladium Nanoparticles. ChemCatChem, 2015, 7, 2113-2121; A.S. Sigeev, A.S. Peregudov, A.V. Cheprakov, I.P. Beletskaya. The Palladium Slow-Release Pre-Catalysts and Nanoparticles in the “Phosphine-Free” Mizoroki–Heck and Suzuki–Miyaura Reactions. Advanced Synthesis & Catalysis, 2015, Vol. 357, 417-429.
академик И.П. Белецкая
Институт физической химии и электрохимии им. А.Н. Фрумкина РАН, г. Москва
Получение in situ никель-вольфрамовых катализаторов гидрирования
В ИНХС РАН впервые для гидрирования ароматических углеводородов было предложено использовать ненанесенные никель-вольфрамовые катализаторы, полученные in situ в углеводородном сырье из маслорастворимых прекурсоров – гексакарбонила вольфрама и никель(II) 2-этилгексаноата. Показано, что в условиях реакции гидрирования в присутствии серы из маслорастворимых прекурсоров возможно формирование частиц катализатора размером около 100 нм с высокой долей промотированной сульфидной фазы (Ni-W-S). Мольное соотношение W:Ni, обеспечивающее максимальную каталитическую активность, составляет для маслорастворимых прекурсоров 1:2. Синтезированный катализатор проявляет высокую активность в реакции гидрирования ароматических углеводородов. На примере гидрирования гомологов нафталина с одним, двумя и тремя метильными заместителями показано, что при высоких конверсиях замещенных нафталинов выход декалинов превышает 50%. Впервые показана возможность проведения гидроочистки легких газойлей каталитического крекинга в присутствии разработанных ненанесенных сульфидных систем: при температуре 350°C и давлении водорода 5,0 Мпа в течение 10 часов общее содержание серы снижается с 3500 до 58 мг/кг (ГДС = 98,3%), а общая доля ароматических углеводородов – с 55,5 до 21% мас. (степень деароматизации составляет 62%) при практически полном отсутствии в продуктах поли- и бициклических ароматических углеводородов.
академик С.Н. Хаджиев, д.х.н. А.Л. Максимов, И.А.
Сизова
Институт нефтехимического синтеза им. А.В. Топчиева РАН, г. Москва
Высокоселективные катализаторы конверсии этанола в альфа-спирты
Бутанол-1 является важным продуктом современной химической индустрии, использующимся как высококачественная добавка к моторным топливам, а также как исходное сырье для органического синтеза. Однако микробиологические и каталитические методы его получения на сегодняшний день не позволяют достигнуть высокой селективности и заметного выхода.
Разработаны бикомпонентные Au-Ni/Al2O3 и Au-Cu/Al2O3 оригинальные каталитические системы. Впервые в присутствии этих катализаторов в сверхкритическом состоянии этанола достигнуты рекордные показатели в прямом его превращении в бутанол-1. Найдено, что в присутствии Au-Ni/-содержащей каталитической системы выход спиртов С4-С8 составляет 55% при суммарной селективности 85% и селективности по бутанолу-1 65%; в присутствии Au-Cu системы при выходе спиртов С4-С8 35% селективность по спиртам достигает исчерпывающего значения при селективности по бутанолу-1 80%. Эти результаты заметно превышают показатели, представленные в мировой литературе. Методами РФЭС и ПЭМ ВР показано, что в биметаллических системах размеры частиц золота составляют 2,5 нм и не подвергаются агломерации при длительном испытании (свыше 50 ч).
проф. М.В. Цодиков
Институт нефтехимического синтеза им. А.В. Топчиева РАН, г. Москва
Разработка высокоэффективных катализаторов для кислородной и углекислотной конверсии метана в синтез-газ
В РГУ нефти и газа имени И.М. Губкина совместно с Химическим факультетом МГУ разработаны новые катализаторы для кислородной и углекислотной конверсии метана в синтез-газ, позволяющие достигать 100% селективности по синтез-газу при 100% конверсии метана, стабильные на протяжении более 140 часов. Сущность разработки – использование в качестве прекурсора катализаторов сложнооксидных материалов, которые при контакте с исходными реагентами и продуктами реакции образуют стабильные наноразмерные частицы никеля и кобальта, диспергированные в матрице оксидов редкоземельных элементов. Оптимальное сочетание компонентов позволяет предотвратить закоксовывание катализаторов. Новизна подтверждена четырьмя положительными решениями по заявкам на выдачу патентов РФ. Катализаторы предполагается использовать для реализации инновационных процессов получения синтез-газа кислородной и углекислотной конверсией метана.
чл.-корр.
РАН А.Г. Дедов, академик И.И. Моисеев,
д.х.н., проф.
А.С. Локтев, к.х.н. Г.Н. Мазо, д.х.н. О.А. Шляхтин,
Российский государственный университет нефти и газа им. И.М.
Губкина,
Московский государственный университет имени М.В. Ломоносова, Химический факультет, г. Москва
Новые катализаторы для гидроконверсии рапсового масла
РГУ нефти и газа имени И.М. Губкина разработаны новые катализаторы гидроконверсии рапсового масла, позволяющие получать ароматические углеводороды с выходом 40-60%. Сущность и новизна разработки – использование катализаторов на основе цеолитов структуры MFI и микромезопористых материалов, полученных ускоренным методом гидротермально-микроволнового синтеза. Микроволновое воздействие позволяет сократить продолжительность синтеза цеолитов и микромезопористых материалов, повысить их степени кристалличности. Значимость разработки обусловлена тем, что катализаторы, получаемые из синтезированных микроволновым методом цеолитов и микромезопористых материалов, превосходят аналоги, полученные традиционным гидротермальным методом.
чл.-корр. РАН А.Г. Дедов, академик И.И. Моисеев, д.х.н., проф.
А.С. Локтев,
Российский государственный университет нефти и газа им. И.М. Губкина, г.
Москва
Катализатор гидродехлорирования хлорбензола, полученный пиролизом пропитанных нитратом палладия древесных опилок
Малостадийным способом получены и охарактеризованы каталитические системы 0.5-7% Pd/C. Способ включает пропитку древесных опилок раствором нитрата палладия с последующим пиролизом в инертной атмосфере. Методами РФЭС и ПЭМ установлено, что без использования стадии восстановления палладий в этих системах присутствует в виде восстановленных нанометровых частиц с узким распределением по размеру, средний размер зависит от условий пиролиза и может составлять от 2,5 до 7 нм. Образующийся в результате пиролиза опилок углерод, по данным КР-спектроскопии, аналогичен активированным углям, а по данным низкотемпературной адсорбции азота, включает микропоры и имеет удельную поверхность от 1 до 240 м2/г, в зависимости от продолжительности пропитки и условий пиролиза. Обнаружена высокая активность всех полученных катализаторов в парофазном гидродехлорировании хлорбензола. Показано, что неполный пиролиз опилок препятствует образованию на поверхности палладиевых частиц карбида палладия, в результате неполностью пиролизованный катализатор высокоактивен в гидродехлорировании опасного экотоксиканта – гексахлорбензола до бензола в жидкой фазе.
Результаты работы опубликованы: Локтева Е.С., Голубина Е.В., Антонова М.В., Клоков С.В., Маслаков К.И., Егоров А.В., Лихолобов В.А. Катализатор гидродехлорирования хлорбензола, полученный пиролизом пропитанных нитратом палладия древесных опилок. Кинетика и катализ, 2015, том 56, № 6, с. 753-762.
д.х.н.
Е.С. Локтева
Московский государственный университет имени
М.В. Ломоносова, Химический факультет, г. Москва
Каталитическая активность систем на основе нанесённого четырёхъядерного карбонилрутената калия K2[Ru4(CO)13] в диспропорционировании циклогексадиенов и циклогексена
Установлено,
что при нанесении четырёхъядерного карбонилрутената калия
K2[Ru4(CO)13]
на уголь «Сибунит», SiO2,
д.х.н.
В.Б. Шур, С.М. Юнусов, З. Руммель, Е.С. Калюжная
Институт элементоорганических соединений им. А.Н. Несмеянова
РАН, г. Москва
Изучение возможности управления химическими свойствами наночастиц Au и Pt
Установлено, что благодаря разности работ выхода между материалами подложки и наночастиц происходит заряжение последних, что оказывает значительное влияние на их адсорбционные и реакционные свойства. В частности по-разному протекает взаимодействие водорода и кислорода на наночастицах золота, нанесенных на графит и окисленный кремний. В первом случае образуются молекулы воды, во втором — ОН-группы. Адсорбция воды на кристаллических наночастицах никеля, нанесенных на окисленные титан и алюминий (т.е. заряженных отрицательно), протекает диссоциативно с образованием на их поверхности ОН-групп, а на наночастицах, нанесенных на окисленный кремний и ВОПГ (т.е. заряженных положительно), проходит без диссоциации. Однако диссоциативная адсорбция водорода на кристаллических наночастицах при комнатной температуре наблюдается лишь в том случае, когда разность значений работ выхода между материалами наночастицы и подложки превышает по абсолютному значению 0.25 эВ (для подложек из окисленных титана и кремния, а также графита). Показано, что возможно управление химическими свойствами наночастиц Au и Pt, нанесенных на ВОПГ, путем создания на них зарядов за счет приложения электрических потенциалов различных полярности и величины.
Результаты работы опубликованы: Гришин М.В., Гатин А.К., Дохликова Н.В., Кирсанкин А.А., Кулак А.И., Николаев С.А., Шуб Б.Р. Адсорбция и взаимодействие водорода и кислорода на поверхности единичных кристаллических наночастиц золота. Кинетика и катализ. 2015. Т. 56. № 4. С. 539–546; Гатин А.К., Гришин М.В., Гуревич С.А., Дохликова Н.В., Кирсанкин А.А., Кожевин В.М., Локтева Е.С., Ростовщикова Т.Н., Сарвадий С.Ю., Шуб Б.Р., Явсин Д.А. Адсорбция водорода на наночастицах никеля с различной кристалличностью. Российские нанотехнологии. 2015. Т. 10. Вып.11-12. С.45-49.
д.х.н.
Б.Р. Шуб
Институт химической физики им. Н.Н. Семенова РАН, г.
Москва
Изучение закономерностей “состав-структура-свойство” в катализе триметаллическими сульфидами
Установлены взаимосвязи между степенью промотирования и геометрическими характеристиками нанесенных наноразмерных частиц триметаллических активных фаз NiCoMoS и KCoMoS и их каталитическими свойствами в реакциях гидрообессеривания дибензотиофена и гидроочистки смеси тиофена и гексена-1 (модельного бензина каталитического крекинга). Показано, что триметаллический катализатор NiCoMoS/Al2O3 со смешанной активной фазой NiCoMoS обеспечивает рекордно высокую частоту оборотов в реакции гидрообессеривания дибензотиофена по сравнению с опубликованными данными. Найдено, что высокий коэффициент ГДС/ГИД селективности KCoMoS/Al2O3 катализаторов в гидроочистки смеси тиофена и гексена-1 обусловлен не только морфологическими особенностями частиц активной фазы, но и формированием нового активного центра. Ведутся дальнейшие исследования и разработки новых триметаллических сульфидных катализаторов с пониженным содержанием металлов для селективной гидроочистки бензинов каталитического крекинга с сохранением октанового числа.
к.х.н. П.А. Никульшин, к.х.н. А.В. Можаев,
к.х.н. Д.И. Ишутенко, д.х.н. А.А. Пимерзин
Самарский государственный технический университет, г. Самара
Катализаторы для глубокой гидроочистки углеводородного сырья
Исследована роль сульфида кобальта на поверхности высокоэффективных катализаторов глубокой гидроочистки углеводородного сырья CoMoS/Al2O3. Показано, что частота оборотов СоМо центров возрастает в ~1.5 раза в гидрообессеривании дибензотиофена и гидродеазотировании хинолина, а также в 2 раза в гидрообессеривании 4,6-диметилдибензотиофена благодаря наличию источника активированного водорода — частиц Co9S8. С ростом температуры процесса эффект спилловера снижается.
Продолжаются исследования по изучению влияния состава Мо и W гетерополисоединений на состав и морфологию частиц (Ni)MoWS2 и каталитические свойства. Обнаружено синергетическое увеличение частоты оборотов в реакциях гидрирования нафталина и гидрообессеривания дибензотиофена на активных центрах катализатора, полученного на основе смешанной MoW-гетерополикислоты.
к.х.н.
П.А. Никульшин, к.х.н. Ал.А. Пимерзин, к.х.н. А.В. Можаев,
асп.
М.С. Куликова, д.х.н. Н.Н. Томина, д.х.н. А.А. Пимерзин
Самарский
государственный технический университет, г. Самара
Создание технологии синтеза палладий содержащих катализаторов на основе сверхсшитого полистирола для реакций кросс-сочетания
Разработана методика физико-химического исследования выбранных лабораторных образцов катализаторов реакции Сузуки с целью установления зависимости состав – каталитические характеристики. Проведено физико-химическое исследование лабораторных образцов палладий содержащих катализаторов реакции Сузуки методами низкотемпературной адсорбции азота, рентгенофотоэлектронной спектроскопии, ренгенофлуоресцентного анализа, просвечивающей электронной микроскопии, инфракрасной спектроскопии с преобразованием Фурье, термогарвиметрического анализа и дифференциальной сканирующей калориметрии.
На основании разработанной методики физико-химического исследования выбранных лабораторных образцов катализаторов реакции Сузуки показано, что физико-химическая характеризация синтезированных безлигандных катализаторов выбранными методами способна обеспечить понимание реакционной способности, что крайне важно для применения катализаторов с промышленной точки зрения.
Установлено, что все палладий содержащие катализаторы на основе СПС микро-мезопористые и обладают сходным характеристиками. Однако природа прекурсора и предварительное восстановление катализатора способны повлиять на процессы формирования кластеров и наночастиц палладия, не изменяя при этом структуры самой полимерной матрицы СПС. При этом восстановленные катализаторы стабильнее невосстановленных, что ранее было подтверждено результатами тестирования в лабораторных условиях в статическом режиме. Показано, что за наблюдаемую каталитическую активность в первую очередь отвечают малые кластеры Pd.
Результаты работы опубликованы: Nikoshvili L., Shimanskaya E., Bykov A., Yuranov I., Kiwi-Minsker L., Sulman E., Selective hydrogenation of 2-methyl-3-butyn-2-ol over Pd-nanoparticles stabilized in hypercrosslinked polystyrene: solvent effect, Catalysis Today, 2015, 241, Part B, p. 179-188; Nikoshvili L.Zh., Makarova A.S., Lyubimova N.A., Bykov A.V., Sidorov A.I., Tyamina I.Yu., Matveeva V.G., Sulman E.M., Kinetic study of selective hydrogenation of 2-methyl-3-butyn-2-ol over Pd-containing hypercrosslinked polystyrene, Catalysis Today, 2015, 256, P. 231-240; Gericke D., Ott D., Matveeva V.G., Sulman E., Aho A., Murzin D.Y., Roggan S., Danilova L., Hessel V., Loeb P., Kralisch D., Green Catalysis by Nanoparticulate Catalysts developed for Flow Processing? Case Study of Glucose Hydrogenation, RSC Adv. 2015, 5, p. 15898-15908; Sulman E., Sulman M., Tyamina I., Doluda V., Nikoshvili L., Lyubimova N., Sidorov A., Matveeva V., Adsorption Processes for the Synthesis of Catalytically Active Metal Nanoparticles in Polymeric Matrices, Chemical Engineering & Technology, 2015, 28 (4), p. 683-689; Berguerand C., Yarulin A., Cárdenas-Lizana F., Wärnå J., Sulman E., Murzin D.Yu., Kiwi-Minsker L., Chemoselective Liquid Phase Hydrogenation of 3-Nitrostyrene over Pt Nanoparticles: Synergy with ZnO Support, Ind. Eng. Chem. Res., 54 (35), 2015, p. 8659-8669; Sulman E.M., Grigorev M.E., Doluda V.Yu., Wärnå J., Matveeva V.G., Salmi T., Murzin D.Yu., Maltose hydrogenation over ruthenium nanoparticles impregnated in hypercrosslinked polystyrene, Chemical Engineering Journal, 282, 2015, P. 37-44; Заявка на получение патента РФ на изобретение № 2015114949 “Способ получения 4-метоксибифенила реакцией Сузуки-Мияура”, приоритет от 21 апреля 2015 г.
д.х.н.,
проф. Э.М. Сульман
Тверской государственный технический
университет, Институт нано- и биотехнологий, г. Тверь
Полимерные нанокапсулы для реакций восстановления и кросс-сочетания Сузуки-Мияуры
Созданы полимерные нанокапсулы путем полимеризации предорганизованных виологен резорцинаренов, которые использованы для термоуправляемой инкапсуляции органических молекул и для иммобилизации на поверхности металлических наночастиц палладия, никеля, платины. Показано, что такие металлокластеры эффективно катализируют реакции восстановления и кросс-сочетания Сузуки-Мияуры, обеспечивая высокие выходы в мягких условиях: в водной среде, при комнатной температуре.
Результаты работы опубликованы: Sultanova, E.D., Salnikov,V.V., Mukhitova, R.Kh., Zuev, Yu.F., Osin, Yu.N., Zakharova, L.Ya, Ziganshina, A.Y., Konovalov, A.I. High catalytic activity of palladium nanoparticle clusters supported on a spherical polymer network. Chem. Commun., 2015, Vol. 51, p. 13317-13320; Sultanova, E.D., Krasnova, E.G., Kharlamov, S.V., Nasybullina, G.R., Yanilkin, V.V., Nizameev, I.R., Kadirov, M.K., Mukhitova, R.K., Zakharova, L.Y., Ziganshina, A.Y., Konovalov, A.I. Thermoresponsive Polymer Nanoparticles Based on Viologen Cavitands. ChemPlusChem, 2015, Vol. 80, p. 217-222.
академик А.И. Коновалов, Э.Д. Султанова, Т.Ю. Сергеева, Р.К. Мухитова,
к.х.н. А.Ю. Зиганшина,
к.х.н. Г.Р. Насыбуллина, д.х.н. В.В.
Янилкин, к.х.н. И.Р. Низамеев, д.х.н., доц. М.К. Кадиров, проф.
Л.Я. Захарова
Институт органической и физической химии им. А.Е.
Арбузова КазНЦ РАН, г. Казань
Электрохимическая активация гетерогенных никелевых нанокатализаторов
Впервые разработан метод электрохимической активации гетерогенных нанокатализаторов на основе комплексов никеля, иммобилизованных на поверхности силикатных наночастиц для реакций перфторалкилирования олефинов и других субстратов. Важными свойствами разработанных нанокатализаторов являются устойчивость во времени, сохранение активности после многократной регенерации как в апротонных, так и водно-органических средах.
Результаты работы опубликованы: Dudkina Yu.B., GryaznovaT.V., Osin Yu.N., Salnikov V.V., Davydov N.A., Fedorenko S.V., Mustafina A.R., Vicic D.A., Sinyashin O.G., Budnikova Yu.H. Nanoheterogeneous catalysis in electrochemically induced olefin perfluoroalkylation. Dalton Trans., 2015, Vol. 44, p. 8833–8838.
академик О.Г. Синяшин, к.х.н. Ю.Б. Дудкина,
к.х.н. Т.В. Грязнова,
к.х.н. С.В. Федоренко, д.х.н., доц. А.Р.
Mустафина, д.х.н. Ю.Г. Будникова
Институт органической и
физической химии им. А.Е. Арбузова КазНЦ РАН, г. Казань
Электрокаталитический метод селективного C-H фосфорилирования ароматических субстратов на основе комплексов Ni, Pd и Fe
Разработан простой эффективный электрокаталитический метод селективного C-H фосфорилирования ароматических субстратов с использованием комплексов солей никеля, палладия и железа, который отличается мягкими условиях и хорошим выходом.
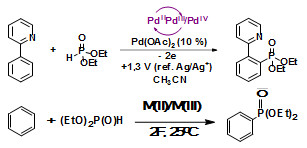
Результаты работы опубликованы: Будникова Ю.Г., Синяшин О.Г. Реакции фосфорилирования С-Н-связей ароматических соединений с участием металлов и их комплексов. Успехи химии, 2015, т. 84, № 9, с. 917–951; Хризанфоров М.Н., Стрекалова С.О., Грязнова Т.В., Хризанфорова В.В., Будникова Ю.Г. Новый метод окислительного металл-индуцированного фосфорилирования бензола. Изв. АН, Cер. хим., 2015, № 8, с. 1926-1932; Khrizanforov M.N., Arkhipova D.M., Shekurov R.P., Gerasimova T.P., Ermolaev V.V., Islamov D.R., Miluykov V.A., Kataeva O.N., Khrizanforova V.V., Sinyashin O.G., Budnikova Y.H.. Novel paste electrodes based on phosphonium salt room temperature ionic liquids for studying the redox properties of insoluble compounds. J. Solid State Electrochem., 2015, Vol. 19, p. 2883–2890; Gryaznova T., Dudkina Y., Khrizanforov M., Sinyashin O., Kataeva O., Budnikova Y. Electrochemical properties of diphosphonate-bridged palladacycles and their reactivity in arene phosphonation. J. Solid State Electrochem., 2015, Vol. 19, p. 2665–2672; Grayaznova T.V., Dudkina Y.B., Islamov D.R., Kataeva O.N., Sinyashin O.G., Vicic D.A., Budnikova Yu.Н. Pyridine-directed palladium-catalyzed electrochemical phosphonation of C(sp2)-H bond. J. Organomet. Chem., 2015, Vol. 785, p. 68-71; Дудкина Ю.Б., Грязнова Т.В., Катаева О.Н., Будникова Ю.Г., Синяшин О.Г. Электрохимическое С–Н-фосфорилирование 2-фенилпиридина в присутствии солей палладия. Изв. АН, Cер. хим., 2014, № 12, с. 2641-2647.
д.х.н. Ю.Г.
Будникова, к.х.н. Ю.Б. Дудкина, к.х.н. М.Н. Хризанфоров,
к.х.н.
В.В. Хризанфорова, к.х.н. Т.В. Грязнова, к.х.н. К.В. Холин
Институт органической и физической химии им. А.Е. Арбузова КазНЦ РАН, г. Казань
Катализаторы селективного гидрирования ацетилена
Получены катализаторы селективного гидрирования ацетилена в составе этан-этиленовой фракции методом статической пропитки оксида алюминия А-64 гексафторацетилацетонатом палладия, растворенного в среде в сверхкритическом состоянии, которые по своей активности и селективности по этилену вследствие своей более высокой дисперсности превосходят катализаторы, приготовленные традиционным методом водной пропитки.
Показано, что сверхкритическая регенерация, в отличие от традиционной высокотемпературной окислительной регенерации, может использоваться для катализаторов, обладающих высокой дисперсностью, и позволяет исключить увеличение среднего размера частиц палладия в составе катализатора.
В кумольном методе синтеза пропиленоксида переработка побочных продуктов эпоксидирования диметилфенилкарбинола и ацетофенона осуществляется путем их гидрирования в жидкой фазе на гетерогенных алюмопалладиевых катализаторах, модифицированных соединениями меди, соответственно, до изопропилбензола и этилбензола.
Процесс гидрирования проводили на стендовой установке при температуре 170-190°C давлении 1,5 МПа, объемной скорости подачи сырья 1 час - 1 и молярном соотношении водород: ДМФК = 4:1. В этих условиях конверсия ДМФК и АЦФ за один проход составляет 99,5 % мас. при селективности гидрирования ДМФК в ИПБ не ниже 98-99% мас.
д.х.н.,
проф. Х.Э. Харлампиди
Казанский национальный
исследовательский технологический университет, г. Казань
Новая методология каталитического синтеза алкенилфосфиновых производных
Впервые осуществлено каталитическое циклоалюминирование 1-алкинилфосфинов под действием 2-3 мольных эквивалентов Et3Al в присутствии каталитических количеств Cp2ZrCl2 с получением фосфорсодержащих алюминациклопент-2-енов с выходами 69 — 84% в зависимости от природы заместителя при ацетиленовой группе синтезированных фосфорсодержащих алюминациклопентенов. Последующим окислением алюминациклопентенов с помощью H2O2 или S8 селективно получены соответствующие фосфиноксиды или фосфинсульфиды. Взаимодействие фосфорсодержащих алюминийорганических соединений с PhPCl2 дало 4-замещенные 5-(дифенилфосфанил)-1-фенил-2,3-дигидро-1H-фосфолы с умеренным выходом. Разработанная методология открывает путь к синтезу новых типов Al/P-льюисовских пар и новых эффективных систем для полимеризации олефинов. Кроме того, фосфорсодержащие алюминациклопен-2-ены перспективны для получения лигандов на основе хиральных фосфоланов, содержащих несколько атомов фосфора.
чл.-корр. У.М. Джемилев, к.х.н. Р.Н. Кадикова, асп. З.Р. Саитова, д.х.н. И.Р. Рамазанов
Институт нефтехимии и катализа РАН, г. Уфа
Co(acac)2/dppe/Zn/ZnI2 — новая каталитическая система для селективного 6π+2π-циклоприсоединения алкинов и 1,2-диенов к 1,3,5,7-циклооктатетраену
Разработана эффективная многокомпонентная каталитическая система o(acac)2/dppe/Zn/ZnI2 для [6π+2π] циклоприсоединения терминальных алкинов и 1,2-диенов к 1,3,5,7-циклооктатетраену с получением ранее труднодоступных циклосодимеров — замещенных бицикло[4.2.2]дека-2,4,7-триенов и бицикло[4.2.2]дека-2,4,7,9-тетраенов с выходами 70-90%. Новая каталитическая система отличается высокой степенью воспроизводимости результатов, доступностью исходных компонентов и может быть использована для синтеза содимеров на примере субстратов, содержащих функциональные группы, что открывает перспективы синтеза ранее не описанных и уникальных каркасных соединений, представляющих интерес в качестве новых мономеров для получения полимерных материалов, высших адамантаноидов, основы для разработки современных противовирусных и противоопухолевых препаратов.
чл.-корр. У.М. Джемилев, к.х.н. Г.Н. Кадикова, д.х.н.
В.А. Дьяконов
Институт нефтехимии и катализа РАН, г. Уфа
Отечественный цеолитсодержащий катализатор алкилирования бензола этиленом в этилбензол, используемый в производстве стирола
Разработан эффективный способ приготовления отечественного катализатора алкилирования бензола этиленом в этилбензол — ключевой стадии процесса производства стирола. Способ заключается в синтезе высокодисперсного цеолита типа ZSM — 5 в H-форме с модулем, равным 30-35, и псевдобемита, в смешении полученных материалов и их формовки в гранулы с последующей термообработкой при 140-150 и 600-620°C в атмосфере воздуха. Катализатор по основным эксплуатационным характеристикам не уступает импортному аналогу, который применяется в настоящее время в промышленном производстве этилбензола в России (ОАО “Газпром нефтехим Салават”).
д.х.н. Б.И. Кутепов, к.х.н. О.С. Травкина, к.х.н.
И.Н. Павлова, к.х.н. А.Н. Хазипова
Институт нефтехимии и катализа РАН, г. Уфа
Бестемплатный синтез при переменном pH мезопористых титаносиликатов — катализаторов для окислительных превращений фенолов
С целью разработки бестемплатных способов синтеза мезопористых металлосиликатов с узким распределением пор для реакций жидкофазного окисления объемных органических молекул водными растворами пероксида водорода изучены закономерности золь-гель синтеза титаносиликатов с использованием тетраэтоксисилана и тетраэтоксититана. Показано, что рН среды в ходе синтеза заметно влияет на пористую структуру образующихся титаносиликатов. Установлено, что внедрение атомов титана в кремнеземную матрицу с образованием каталитически активных связей Ti-O-Si происходит в кислых средах (рН < 3), тогда как мезопористая структура формируется только в щелочной среде. В результате проведенных исследований разработан способ золь-гель синтеза при переменном pH мезопористых титаносиликатов с узким распределением пор (2-7 нм), каталитически активных в реакциях селективного окисления алкилфенолов водными растворами пероксида водорода.
д.х.н. Б.И. Кутепов, д.х.н. Н.Г. Григорьева,
к.х.н.
Р.Р. Талипова, к.х.н. И.Н. Павлова
Институт нефтехимии и катализа
РАН, г. Уфа
Первый пример синтеза N-арил(гетарил)-тетраоксазаспиро-алканов в качестве потенциальных антималярийных и антигельминтных соединений
Разработан новый, эффективный метод синтеза пентаоксаспироалканов циклоконденсацией 1,1-бис(гидроперокси)-циклоалканов с формальдегидом (~ 20°C, THF, 95%) с участием Sm(NO3)3•6H2O в качестве катализатора. Каталитическими превращениями пентаоксаспироалканов с ариламинами получены ранее неизвестные N-арилтетраоксазаспироалканы с выходом 90-99%. Новые полициклические пероксидные соединения интересны в качестве потенциальных антималярийных, антигельминтных и противораковых лекарственных средств.
к.х.н. Н.Н. Махмудиярова, асп. Л.В. Кулешова, д.х.н. А.Г. Ибрагимов
Институт нефтехимии и катализа РАН, г. Уфа
Метод повышения фоточувствительности диоксида титана
Разработан эффективный метод повышения фоточувствительности TiO2 посредством конъюгации наночастиц CdS. Композиты CdS/TiO2 получены золь-гель методом при использовании в качестве среды стабильного коллоидного раствора наночастиц CdS. Проведена оценка фотокаталитических свойств композитов CdS/TiO2 в видимой области спектра с использованием двух реакций окисления: этанола до ацетальдегида (λ > 420 нм) и гидрохинона до хинона (λ = 440 — 460 нм).
Установлено, что при парциальном окислении этанола синтезированные композиты CdS/TiO2 проявляют высокую активность и стабильность, их квантовая эффективность зависит от мольного соотношения CdS:TiO2 и составляет ~ 2% (0.81 ммоль/ч). Показано, что степень превращения гидрохинона не зависит принципиальным образом от структуры CdS/TiO2 и составляет ~ 60%.
Результаты работы опубликованы: Pestov A.V., Kuznetsov V.A., Mekhaev A.V., Gorbunova T.I., Saloutin V.I., Smirnov S.V., Vichuzhanin D.I., Matafonov P.P. Designing new adhesive materials based on epoxy oligomers filled with organic compounds // Polymer Science, Series D. Glues and Sealing Materials. 2015. V. 8. N 2. P. 149 — 152.
д.х.н., проф. В.И. Салоутин, к.х.н. Т.И.Горбунова
Институт органического синтеза им. И.Я. Постовского
УрОРАН, г. Екатеринбург
Разработка новых подходов к фиксации молекул дибензо-краун-эфира на полимерной или неорганической подложке
С целью создания доступных краун-эфир-содержащих сорбентов разрабатываются подходы к фиксации молекул дибензо-краун-эфира на полимерной или неорганической подложке. Разработан новый метод синтеза дибензо-краун-эфиров, отличающийся от известных использованием гетерогенного катализатора — оксида наноразмерного металла. За счет повышения хемоселективности процесса выходы продуктов повышаются, снижается продолжительность процесса, отпадает необходимость в использовании инертного газа, упрощаются процессы выделения промежуточных и целевых продуктов реакции. Метод позволяет использовать вместо дорогих дитозилатов олигоэтиленгликолей их галогенпроизводные. Запатентованы процессы получения дибензо-краун-эфиров, а также полупродуктов их получения. Разработаны методы синтеза замещенных дибензо-краун-эфиров, содержащих хлорметильные группы, удобные для химической иммобилизации краун-эфиров на полимерную или неорганическую подложку.
Результаты работы опубликованы: Maksimovskikh A.I., Fedorova O.V., Rusinov G.L., Charushin V.N. Sorbents based on crown-ethers in the processes of separation of rare earth ions. Abstracts of the IX International Conference of Young Scientists on Chemistry “Mendeleev-2015”, St. Petersburg, 2015, p. 82.
академик В.Н. Чарушин, к.х.н. Г.Л. Русинов,
к.х.н. О.В. Федорова, к.х.н. И.Г. Овчинникова, А.И.Максимовских
Институт органического синтеза им. И.Я. Постовского УрОРАН, г. Екатеринбург
Исследование механизма межмолекулярного переноса водорода при превращении углеводородов на Pt-содержащих катализаторах с различной кислотностью носителя
Изучены закономерности превращения смесей 1-гексена и циклогексана на цеолитном (HрзэY + ZSM-5) и Pt-Al-силикатном катализаторах. Установлено, что применение Pt-содержащих катализаторов позволяет снизить температуру процесса до 300°C, снизить содержание непредельных соединений в 3.6 раза и увеличить выход жидких продуктов на 5 мас. % по сравнению с цеолитсодержащим катализатором. Результаты важны для улучшения показателей (снижение содержания сернистых и непредельных соединений) разрабатываемого в Институте процесса облагораживания низкосортных бензинов без использования молекулярного водорода.
д.х.н.,
проф. А.С. Белый, к.т.н. В. П. Доронин
Институт проблем переработки углеводородов
СО РАН, г. Омск
Синтез и исследование морфологических типов углерода, образующихся при каталитической конверсии метана на массивном металлическом катализаторе, нагретом электрическим током
Изучено влияние термической подготовки массивного полиметаллического катализатора (фехраль), нагретого электрическим током, на синтез новых морфологических форм углерода при каталитической конверсии метана. Установлено, что при нагреве катализатора переменным током в среде азота и гелия образующееся углеродное волокно состоит из овальных образований, представляющих собой концентрические слои углерода, вложенные друг в друга. В среде аргона образуются углеродные волокна другого строения. Результаты важны для разработки технологий создания композиционных материалов специального назначения.
чл.-корр.
РАН В.А. Лихолобов, Е.А. Райская
Институт проблем переработки
углеводородов СО РАН, г. Омск
Катализатор для переработки тяжелого нефтяного сырья
Разработана каталитическая система для безводородного крекинга тяжелого углеводородного сырья, состоящая из мезопористого алюмосиликата (SiO2/Al2O3 = 20) с диаметром пор до 50Å и газофазного наноразмерного порошка никеля (НР-Ni) со средним размером частиц 20 нм. Наличие мезопористой структуры в синтезированном алюмосиликате обеспечивает доступность его активных центров, расположенных в объёме, для крупных молекул нефтяного сырья (смол и асфальтенов), где они подвергаются деструкции. Алюмосиликатный катализатор и частицы металла способствуют протеканию реакций диспропорционирования и перераспределения водорода, вследствие чего увеличивается количество легких фракций и снижается газо- и коксообразование.
д.х.н. А.В. Восмериков, д.х.н. А.К. Головко
Институт химии нефти СО РАН, г. Томск,
д.х.н. Б.И. Кутепов
Институт
нефтехимии и катализа РАН, г. Уфа
Изучение процесса деполимеризации биомассы древесины осины в сверхкритическом этаноле
Установлены закономерности процесса деполимеризации биомассы древесины осины в сверхкритическом этаноле в присутствии катализаторов на основе высококремнеземных цеолитов. В оптимальных условиях процесса достигнуто существенное (в 2,6 раза) увеличение выхода легкокипящих жидких продуктов на катализаторе с соотношением SiO2/Al2O3 = 30, имеющем наиболее высокую концентрацию высокотемпературных кислотных центров. В составе легкокипящей фракции увеличивается содержание продуктов превращения карбогидратов древесины: 5-гидроксиметилфурфурола более чем на порядок, фурфурола в 3,5 раза, этилового эфира левулиновой кислоты в 3,4 раза по сравнению с фракцией жидких продуктов, полученной без катализатора.
Предложен новый подход к синтезу биологически активных сульфатов тритерпеновых соединений бетулина и его производных, основанный на использовании зеленого сульфатирующего агента — сульфаминовой кислоты и основного катализатора мочевины. Разработаны новые экологически безопасные способы синтеза сульфатов бетулина, бетулиновой кислоты, ацетата и пропионата бетулина в виде аммониевых, натриевых и калиевых солей, не использующие агрессивные сульфатирующие агенты и токсичные растворители.
д.х.н. Б.Н. Кузнецов
Институт химии и химической технологии СО РАН, г.
Красноярск
Исследование особенностей поведения в гидротермальных автоклавных условиях исходных и отработанных катализаторов нефтепереработки
Эффективность работы катализаторов определяется стабильностью основных характеристик, а именно, химического, структурного и фазового состава, наличием примесей и т.д. Поэтому оперативный мониторинг состояния катализаторов нефтепереработки приобретает особое значение. Изменение состава и физико-химических характеристик объектов, в том числе содержащих металлы платиновой группы (МПГ), удобно исследовать с применением автоклавных технологий. Поскольку МПГ и их соединения в большинстве процессов являются кинетически инертными, применение окислительного кислотного автоклавного вскрытия при повышенных температурах позволяет обеспечить надежный экспрессный метод химического анализа.
Показана эффективность применения автоклавного вскрытия катализаторов нефтепереработки с целью определения их химического состава. Исследованы особенности поведения в гидротермальных автоклавных условиях исходных и отработанных катализаторов в окислительных и восстановительных средах. Установлена взаимосвязь степени растворения платины, входящей в состав катализаторов риформинга, с ее дисперсностью и проведена оценка изменения состояния платины в катализаторах в процессе эксплуатации.
д.т.н. Н.Н. Довженко
Сибирский Федеральный университет, Институт нефти и газа, г. Красноярск
Исследование каталитической реакции Сузуки-Мияуры в присутствии комплексов Pd с эндогенными анионами галогенов
Использование комбинации УФ-спектроскопии и кинетических исследований (operando-исследования) позволило выяснить роли основания и эндогенных анионов галогенов в реакции Сузуки-Мияуры, которая на сегодняшний день наиболее интенсивно исследуется среди реакций кросс-сочетания, катализируемых соединениями палладия. Установлена положительная роль основания, заключающаяся в образовании его соединений с палладием, участвующих в ключевой стадии каталитического цикла. Показано, что устойчивость комплексов палладия с эндогенными анионами галогенов является важнейшим фактором каталитической активности. Результаты operando-исследования реальной каталитической реакции Сузуки-Мияуры с арилиодидами позволили установить, что значительная часть палладия (catalyst resting state) находится в форме галогенидных ацидокомплексов типа [PdX4]2- за пределами основного каталитического цикла реакции.
Предложены методы установления механизмов сложных каталитических процессов, характеризующихся существенной нестационарностью активного катализатора, базирующихся не на традиционных измерениях каталитической активности, а на измерениях дифференциальной селективности реакций с использованием естественной многомаршрутности, возникающей в результате конкуренции различных изотопомеров субтрата за активный катализатор. Показана перспективность operando-исследований катализатора, использующих информацию о его дифференциальной селективности, а не активности.
Результаты работы опубликованы: R. Cano, A. F. Schmidt, G. P. McGlacken. Direct arylation and heterogeneous catalysis; ever the twain shall meet. Chemical Science. 2015, V. 6, N 10, p. 5338-5346; А.А. Курохтина, Е.В. Ларина, А.Ф. Шмидт. Исследование кинетического изотопного эффекта на естественном содержании изотопов для различения механизмов гомогенного и гетерогенного катализа в реакциях Хека и Сузуки. Кинетика и катализ. 2016, Т. 57, № 1, с. 34-41.
д.х.н., проф. А.Ф. Шмидт
Иркутский государственный университет, г. Иркутск
Синтез циглеровских катализаторов гидрирования на основе комплексов Co и Ni
Найден оптимальный способ синтеза циглеровских катализаторов гидрирования ненасыщенных соединений на основе комплексов кобальта и никеля и предложены схемы формирования и функционирования активных в катализе наночастиц. Показано, что в циглеровских катализаторах гидрирования алюминийорганические соединения выполняют функции восстановителя Ni(II)→Ni(I)→Ni(0), стабилизатора и ингибитора, причем последние две функции тесно связаны. Независимо от деталей структуры поверхностных соединений триэтилалюминия с никелем, триэтилалюминий будет оказывать отравляющее влияние на гидрогенизационную активность. Формирующиеся в процессах катализа поверхностные гидриды и алкильные соединения никеля также взаимодействуют с триэтилалюминием по типу мостиковых комплексов, стабилизируя наночастицы никеля и ингибируя их каталитические свойства. Введение в каталитическую систему протонодонорных соединений (H2O, ROH, acacH), которые взаимодействуют с AlEt3, устраняет ингибирующий эффект и приводит к резкому возрастанию частоты (TOF) и числа (TON) оборотов катализаторов циглеровского типа. Регулирование содержания протонодонорных соединений позволяет повысить стабильность нанокластеров в условиях гидрогенизационного катализа. Полученные результаты позволяют более осознано подходить к формированию катализаторов при их использовании в конкретных каталитических процессах, а также наметить способы дальнейшего повышения количественных характеристик (TOF и TON) катализаторов циглеровского типа в гидрировании.
Результаты работы опубликованы: F.K. Schmidt, Y.Y. Titova, S.S. Kolesnikov, L.B. Belykh. Nanosized nickel Ziegler-type hydrogenation catalysts: The role of organoaluminum and proton-donating compounds in their formation and optimum catalysis. J. Appl. Catal. A. General. 2015, V. 499, p. 177 — 187; Ф.К. Шмидт, Ю.Ю. Титова, Л.Б. Белых. Функции алюминийорганических и протонодонорных соединений в процессах формирования и функционирования наноразмерных никелевых катализаторов гидрирования циглеровского типа. Кинетика и катализ, 2015, Т. 56, № 5, с. 574-583; Пат. 2565673 РФ, МПК B01J 21/02 B01J 23/755 B01J 31/12 C07C 5/02. Никелевый катализатор гидрирования. Ф.К. Шмидт, Ю.Ю. Титова, Л.Б. Белых; заявитель и патентообладатель Иркут. гос. ун-т. — № 2014149223/04; заявл. 05.12.2014; опубл. 20.10.2015, Бюл. № 29, 11 c.
д.х.н. Ф.К. Шмидт
Иркутский государственный университет, г. Иркутск
Новая модификация суперосновной каталитической системы — суспензия KOH/DMSO
Разработана новая модификация суперосновной каталитической системы — суспензия KOH/DMSO, представляющая собой динамичный континуум наночастиц различного размера и молекулярных агрегатов. Система меняет свои каталитические свойства в широких пределах в зависимости от температуры и концентрации компонентов. Новая каталитическая система впервые позволила осуществить многоуровневую селективную каскадную СН-функционализацию алифатических и циклоалифатических кетонов ацетиленом. Таким образом, впервые был реализован эффективный однореакторный синтез полициклических мостиковых ацеталей с енольной функцией (метилен-6,8-диоксабицикло[3.2.1]октанов) — реакционноспособных строительных блоков органической и медицинской химии, производных феромонов насекомых, гормонов млекопитающих, токсинов морских организмов. Подготовлена статья для журнала JOC.
Найден эффективный органический катализатор Et3N/MeCN, обеспечивающий в условиях микроволновой активации протекание двух конкурирующих каскадных реакций при взаимодействии цианопропаргиловых спиртов с карбоновыми кислотами — образование 3(2Н)-фуранонов и 2,3-дигидрофуранов. Выход и соотношение продуктов в основном зависит от концентрации катализатора и для 2,3-дигидрофуранов достигает максимума при концентрации Et3N 5 мол%. Обе конкурирующие реакции протекают через один и тот же интермедиат (цианометиленкетоэфир), который циклизуются либо внутримолекулярно в 3(2Н)-фураноны, либо межмолекулярно со второй молекулой цианопропаргилового спирта, образуя 2,3-дигидрофураны. При этом катализатор на первой стадии каскадного процесса активирует тройную связь, способствуя образованию цвиттериона, а на завершающей стадии играет роль депротонирующего агента. Подготовлена статья для журнала Synthesis.
академик Б.А. Трофимов
Иркутский институт химии СО РАН им. А.Е. Фаворского СО РАН, г. Иркутск
Новый экспериментальный метод получения этанола с неравновесной поляризацией ядерных спинов для новых приложений ядерного магнитного резонанса
Продемонстрирован новый экспериментальный метод получения этанола с неравновесной поляризацией ядерных спинов для новых приложений ядерного магнитного резонанса. Метод основан на гетерогенном каталитическом гидрировании паров винилацетата параводородом с последующим гидролизом полученного в реакции этилацетата в водной среде с образованием этанола и ацетата натрия. Важным достоинством предложенного простого подхода является возможность получения этанола с неравновесной поляризацией ядерных спинов в водном растворе, который не содержит катализатора и органического растворителя. Реализованный метод является важным шагом вперед на пути получения гиперполяризованных биосовместимых контрастных агентов, весьма перспективных для развития новых диагностических подходов с использованием магнитно-резонансной томографии (МРТ) и спектроскопии (МРС).
Результаты работы опубликованы в журнале издательства Nature: O.G. Salnikov, K.V. Kovtunov, I.V. Koptyug. Production of catalyst-free hyperpolarised ethanol aqueous solution via heterogeneous hydrogenation with parahydrogen, Scientific Reports, 5, 13930 (2015).
д.х.н. И.В. Коптюг, к.х.н. К.В. Ковтунов, О.Г. Сальников,
Международный томографический центр СО РАН, г. Новосибирск
Корреляция Бренстеда-Эванса-Поляни в окислительном катализе
На основе обширного массива экспериментальных данных по кинетике реакций каталитического окисления проведен анализ корреляций Бренстеда-Эванса-Поляни (БЭП). Показано, что энергия активации реакций глубокого окисления, Е, определяется двумя термодинамическими характеристиками: теплотой реакции, QR, и теплотой адсорбции кислорода, QO2, которые образуют универсальный Бренстедовский параметр, Quni, с Бренстедовским коэффициентом β = 0.5. Впервые получена универсальная форма уравнения БЭП, которая позволяет в рамках единой зависимости описать скорости реакций глубокого окисления различных классов органических веществ. Это создает фундаментальную основу для разработки эффективных катализаторов процессов глубокого окисления.
Результаты работы опубликованы: G.I. Panov, M.V. Parfenov, V.N. Parmon. The Brønsted-Evans-Polanyi Correlations in Oxidation Catalysis. Catalysis Reviews, 2015, Vol. 57, No. 4, p. 436-477.
д.х.н., проф. Г.И. Панов, к.х.н. М.В. Парфенов,
академик В.Н. Пармон
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Подходы к сжиганию углеводородного сырья с высоким содержанием сернистых соединений в кипящем слое катализатора глубокого окисления
Разработаны подходы к сжиганию углеводородного сырья с высоким содержанием сернистых соединений в кипящем слое катализатора глубокого окисления. Суть разработки заключается в сжигании сырья в неизотермическом слое катализатора, когда сочетаются полное окисление топлива при 700-750°C и при более низких температурах (500°C) с эффективным превращением SO2 в SO3 для последующего связывания кальцитом в виде CaSO4. Степень улавливания SO2 увеличивается с 40-45% (в изотермическом слое) до 95% в неизотермическом слое. Низкие температуры процесса позволяют снизить выбросы оксидов азота до норм ПДК и предотвратить разложение твердого CaSO4, который уносится из реактора пневмотранспортом и сепарируется в циклоне. Проведено моделирование процесса в кипящем слое и определены основные кинетические параметры связывания серы. Разработка ориентирована на экологически безопасную утилизацию высокосернистых отходов, включая нефтешламы, кислые гудроны, отходы нефтепереработки, природные битумы, иловые осадки коммунальных очистных сооружений.
д.х.н. В.А. Яковлев
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Окисление третичных аминов пероксидом водорода в условиях межфазного катализа с применением бифункциональных металлокомплексных катализаторов
Проведены исследования в области тонкого органического синтеза по изучению возможности окисления третичных аминов пероксидом водорода в условиях межфазного катализа с применением бифункциональных металлокомплексных катализаторов на основе оксо- и пероксовольфрамовых соединений.
Проведен скрининг катализаторов в реакции окисления N-фосфонометилиминодиуксусной кислоты (ФИДУК) 32% раствором пероксида водорода с получением её N-оксида — важного полупродукта в синтезе биологически активных соединений, например, глифосата. Каталитические системы были охарактеризованы методом ИК спектроскопии. Показано, что проведение реакции окисления ФИДУК пероксидом водорода до её N-оксида в двухфазной системе с использованием тетраядерных пероксополиоксокомплексов вольфрама Q3{PO4[WO(O2)2]4}, где Q3 — органический катион, позволяет осуществлять процесс в одну стадию, в мягких условиях (Т ~ 100°C, Р — атм.) с достижением высоких конверсии субстрата (99%) и селективности по целевому продукту (97%), снимая проблему отделения катализатора (см. Табл.).
Таблица (Q = [C5H5N(CH2)15Me]+, Т = 70°C, τреак.= 1.5 ч)
| № пп | Катализатор (Cat) | [Cat]×103М | Конверсия ФИДУК, % | Селективность по N-оксид-ФИДУК,% |
| 1 | Q3[PW12O40],(I) | 0.1 | 8 | 80 |
| 2 | Q2[W2O3(O2)4],(II) | 0.2 | 64 | 90 |
| 3 | Q3{PO4[WO(O2)2]4},(IV) | 0.1 | 99 | 97 |
| 4 | Na2WO4(III) | 0.4 | 94 | 95 |
| 5 | — | 0 | 1-2 | 0 |
Результаты работы опубликованы в журнале Catalysis Communications, 2015, Vol. 71, P. 102-105
академик В.Н. Пармон, д.т.н. З.П. Пай,
к.х.н.
Т.Б. Хлебникова, Д.Ю. Ющенко
Институт катализа им. Г.К. Борескова
СО РАН, г. Новосибирск
Способ получения алифатических карбоновых кислот
Проведены исследования с целью разработки новых способов получения импортозамещающих продуктов — алифатических карбоновых кислот. Исследования приводили на примере реакций окисления α-алкенов (октен-1, децен-1, додецен-1) пероксидом водорода в двухфазной системе с использованием бифункциональных катализаторов на основе пероксовольфраматных комплексов. На выбор α-алкенов в качестве субстратов повлияло то, что они являются дешевыми крупнотоннажными продуктами нефтехимии, получаемые в результате высоктемпературной олигомеризации этилена.
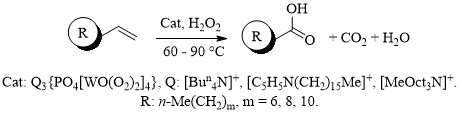
Результаты работы защищены патентом РФ № 2554000, опубл. 20.06.2015, Бюл. № 17. — 10 с.
д.т.н. З.П. Пай, к.х.н. Л.В. Малышева,
П.В. Бердникова, П.В. Оленева
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Раздельное и совместное метанирование СО и СО2; паровая конверсия диметоксиметана (ПК ДММ) на бифункциональных катализаторах
С помощью кинетических методов и ИК спектроскопии in situ исследовано протекание реакций раздельного и совместного метанирования СО и СО2 в избытке водорода на катализаторах, приготовленных из нитрата никеля (Ni/CeO2) и хлорида никеля (Ni(Cl)/CeO2). Установлено, что активация СО и Н2 протекает на поверхности Ni, а активация СО2 — на поверхности носителя. Доказано, что введение хлора в катализатор модифицирует поверхность СеО2, что резко тормозит активацию СО2 и приводит к высокой селективности метанирования СО в присутствии СО2.
Исследованы закономерности протекания реакции паровой конверсии диметоксиметана (ПК ДММ) на бифункциональных катализаторах CuO-CeO2/γ-Al2O3 и CuO-ZnO/γ-Al2O3. Показано, что эти катализаторы весьма эффективны в ПК ДММ и обеспечивают при P = 1 атм и Т ~ 300°C полную конверсию ДММ, низкое содержание CO (< 1 об.%) в реформате и производительность по водороду ~15 л H2/(ч·г). При помощи физико-химических методов и каталитических экспериментов установлено, что реакция протекает по последовательной кинетической схеме через стадию гидратации ДММ на Бренстедовских кислотных центрах γ-Al2O3 и стадию ПК метанола/формальдегида на нанесенных частицах, состоящих из смешанных CuO-CeO2 и CuO-ZnO оксидов. Смешанные CuO-CeO2 и CuO-ZnO оксиды представляют собой частицы, нанесенные на γ-Al2O3 размером 50-100 нм.
д.х.н., проф. В.А. Собянин
Институт катализа им.
Г.К. Борескова СО РАН, г. Новосибирск
Разработка технологии получения препарата осельтамивир и метода получения гамма-бутиролактона
Разработана технология получения препарата осельтамивир, являющегося эффективным средством борьбы против вируса птичьего гриппа Н5N1. Использование на заключительной стадии процесса новых золотосодержащих катализаторов, промотированных палладием, разработанных в ИК СО РАН (В.И. Бухтияров), позволяет успешно гидрировать азидную группу, не затрагивая двойную связь. Разработанная технология предполагает многократное повторное использование катализатора, что значительно снижает себестоимость процесса.
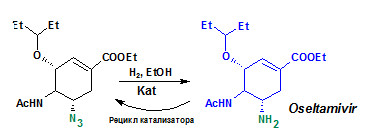
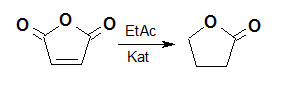
Разработан метод получения гамма-бутиролактона жидкофазным гидрированием малеинового ангидрида с использованием палладий содержащих катализаторов. Разработанный метод позволяет получать продукт с высоким выходом и содержанием основного вещества более 99 %.
д.х.н., проф. С.В. Сысолятин
Институт проблем химико-энергетических
технологий СО РАН, г. Бийск
Изучение кинетики ферментативного гидролиза лигноцеллюлозного материала
Установлено, что кинетика ферментативного гидролиза в ацетатном буфере лигноцеллюлозного материала (ЛЦМ) мискантуса (М) и ЛЦМ плодовых оболочек овса (ПОО) при различных концентрациях субстрата может быть описана математической моделью на основе модифицированного уравнения Михаэлиса-Ментен; показано, что при увеличении концентрации субстрата от 33,3 до 120,0 г/л выход редуцирующих веществ (РВ) снижается от 98 до 67% для ЛЦМ М и до 55% для ЛЦМ ПОО, что обусловлено субстратным ингибированием. Установлено, что в изученном диапазоне концентраций субстрата начальная скорость ферментолиза для ЛЦМ ПОО выше на 1 г/(л·ч), чем для ЛЦМ М. Рекомендована концентрация субстрата не менее 60 г/л для последующего успешного синтеза биоэтанола.
Результаты работы опубликованы: Будаева В.В., Скиба Е.А., Байбакова О.В., Макарова Е.И., Орлов С.Е., Кухленко А.А., Удоратина Е.В., Щербакова Т.П., Кучин А.В., Сакович Г.В. Катализ в промышленности, 2015, Т. 15, № 5, с. С. 60-66. DOI: 10.18412/1816-0387-2015-5-60-66; Макарова Е.И. Биоконверсия непищевого целлюлозосодержащего сырья: энергетических растений и отходов АПК… на соискание ученой степени кандидата технических наук: 03.01.06 / ГНУ ВНИТИБП (Московская область, Щелково). Бийск, 2015, 161 с.; Патент РФ № 2533921, С13К1/02. Способ предварительной обработки целлюлозосодержащего сырья для ферментативного гидролиза. Будаева В.В., Макарова Е.И., Скиба Е.А., Золотухин В.Н., Сакович Г.В. № 2013140699/20; Заявл. 03.09.2013; Опубл. 27.11.2014.
к.х.н. В.В. Будаева
Институт проблем химико-энергетических технологий СО РАН, г. Бийск
Разработка и усовершенствование промышленных катализаторов и технологий
Получение сверхвысокомолекулярного полиэтилена с особой морфологией, способного к переработке из твердой фазы с достижением высоких прочностных и модульных характеристик
Впервые в РФ по программе государственного задания V.44.2.6 «Исследование механизма действия постметаллоценовых катализаторов на основе комплексов хлоридов титана различного строения, в первую очередь функциональных самоиммобилизующихся и биядерных систем при полимеризации этилена и сополимеризации этилена α-олефинами» разработана новая технология получения реакторных порошков сверхвысокомолекулярного полиэтилена (СВМПЭ), способного к переработке из твердой фазы с получением лент или волокон со сверхпрочными и сверхмодульными свойствами. Указанная разработка для РФ представляет государственную значимость, поскольку в РФ до настоящего времени не организовано производство СВМПЭ. Технология защищена тремя патентами РФ (№ 2459835 С2, № 2552636 С2, № 2561921 С1, № 2545182), четвёртый патент в данный момент находится на рассмотрении. Реализация новой разработки с решением проблемы переработки СВМПЭ в изделия со сверхпрочными (3,6-3,9 ГПа) и сверхмодульными (130-140 ГПа) свойствами из твёрдой фазы может обеспечить потребности РФ в данном материале.
чл.-корр. РАН С.С. Иванчев
Санкт-Петербургский филиал Института катализа им. Г.К. Борескова СО РАН, г. Санкт-Петербург
Разработка комплексных технологий по переработке матричной нефти
Исследованы физико-химические характеристики матричной нефти Оренбургского нефтегазоконденсатного месторождения и ее фракций, а также кинетическая устойчивость матричной нефти с различным содержанием высокомолекулярных соединений в тяжелой части. Выявлено значительное содержание редких металлов в тяжелой части матричной нефти. Проведенные исследования позволили использовать тяжелый остаток матричной нефти в качестве нового типа сырья для процесса гидроконверсии. Разработан оптимальный вариант технологического процесса глубокой переработки тяжелой части матричной нефти в низкокипящие фракции способом прямой каталитической гидрогенизации без предварительной подготовки сырья на формирующихся в реакционной среде нанокатализаторах с попутным выделением имеющихся в сырье ценных металлов. Показано, что процесс для данного вида сырья целесообразно проводить при температуре 420 — 450°C, давлении 7 — 10 МПа и со специально разработанной каталитической системой в количестве менее 0,01% с учетом рециркуляции и регенерации.
Работа выполнена ОАО «ВНИПИнефть» совместно с ИНХС имени А.В. Топчиева, ИПФХ, ЦВМТ и РГУ нефти и газа имени И.М. Губкина).
д.т.н., проф. В.М. Капустин
ОАО «ВНИПИнефть», Москва
Проектирование опытно-промышленной установки гидроконверсии тяжелого высокосернистого сырья мощностью 50 тыс. т/год
Осуществляется разработка проектной и рабочей документации для строительства опытно-промышленной установки гидроконверсии в рамках реализации комплекса ОАО «ТАНЕКО». Реализация проекта позволит перейти на новый уровень проведения испытания суспендированных катализаторов на основе парамолибдата аммония на производстве. Эта установка является первой в своем классе, использующей данный вид катализатора и данную технологию в промышленности. Проектируемая установка обеспечивает необходимую гибкость для эксплуатации в широком диапазоне уровней давления (от 7 до 14,5 МПа) с различными концентрациями наноразмерного катализатора. Катализатор может испытываться в реакторах противоточного смешения или идеального вытеснения, что позволит совершенствовать состав и свойства катализатора. Проведение процесса при 470°C позволит достигать степени конверсии свыше 90% масс.
Работа проводится ОАО «ВНИПИнефть» совместно с ИНХС имени А.В. Топчиева.
д.т.н., проф. В.М. Капустин
ОАО «ВНИПИнефть», Москва
Производство и промышленное применение катализаторов для синтеза Фишера-Тропша
Успешно построена и запущена в эксплуатацию катализаторная фабрика ООО «ИНФРА» в г. Троицке. Технология производства катализатора S1 для синтеза Фишера-Тропша успешно масштабирована силами ТИСНУМ и внедрена в промышленном масштабе. Изготовлена первая промышленная партия катализатора на экспорт.
Также были проведены успешные исследования влияния теплопроводящих добавок в композитные катализаторы Фишера-Тропша на функционирование этих катализаторов. Установлено, что по достижении теплопроводности свыше 4 Вт/м/К наступает качественное изменение в составе продуктов и в производительности гранулированных катализаторов. По результатам исследования опубликована большая статья: Asalieva E. Gryaznov K., Kulchakovskaya E., Ermolaev I., Sineva L., Mordkovich V. Fischer-Tropsch synthesis on cobalt-based catalysts with different thermally conductive additives. Applied Catalysis A: General,2015, Vol. 505, p. 260-266.
Также разработаны исходные данные для строительства первого завода по получению синтетической нефти из природного газа с применением катализатора S1 в интересах компании «ИНФРА».
д.х.н. В.З. Мордкович
Технологический институт сверхтвердых и новых углеродных материалов, Троицк, г. Москва
Катализатор и процесс синтеза дивинила из этанола; катализатор дегидрирования пропана в пропилен
Создана опытная установка, на которой будут уточнены параметры промышленного производства дивинила из этанола, что позволит предложить процесс для промышленной реализации. Также разработана технология производства катализатора для промышленности.
Разработана промышленная технология производства катализатора дегидрирования пропана, обладающего высокой механической прочностью. На лицензионной основе технология предложена для реализации в Иране совместно с технологией дегидрирования пропана в пропилен. В настоящее время ведется проектирование промышленной установки.
д.т.н., проф. Г.Р. Котельников
ОАО НИИ «Ярсинтез», г. Ярославль
Воздействие водных модифицированных растворов поташа на никелевые катализаторы метанирования
В рамках изучения проблемы разрушения катализаторов под воздействием абсорбентов, применяющихся для поглощения диоксида углерода из конвертированного газа, исследовано влияние водного раствора поташа на механическую прочность и срок службы Ni-Al катализатора НИАП-07-01 (НКМ-1). Были сделаны следующие выводы:
Проведенные предварительные исследования воздействия водного раствора поташа на основные свойства нового никелевого цементсодержащего катализатора метанирования марки НИАП-07-07 (НКМ-7), в котором алюминаты кальция выполняют роль гидравлического связующего, показали его высокую устойчивость. Продувка влажным насыщенным паром или промывка горячим конденсатом полностью восстанавливают его работоспособность.
Исследования по воздействию на катализатор метанирования марки НИАП-07-07 (НКМ-7) водного раствора поташа и других видов абсорбентов продолжаются.
д.х.н., проф. Е.З. Голосман, к.т.н. В.Н. Ефремов
ООО “НИАП-КАТАЛИЗАТОР”, г. Новомосковск
Исследование потенциала катализаторов на алюмокальциевой основе в получении азотсодержащих органических продуктов
Предложен новый способ получения N-алкилпиперидинов на Ni-Al-Ca-содержащем катализаторе, который позволяет повысить выход целевых продуктов в оптимальных условиях более чем на 29,0% в сравнении с прототипом, а производительность более чем в 1,1-1,5 раза.
На Cu-Zn-алюмокальциевых катализаторах в найденных оптимальных условиях впервые был достигнут выход высших алифатических нитрилов (96,0-98,3%), который более чем на 10,0% выше в сравнении с лучшими зарубежными аналогами. По производительности по бензонитрилу эти контакты превышают известные аналоги в 1,14-1,20 раза. Сu-Al-Ca- и Сu-Mg-Al-Ca-cодержащие композиции имеют каталитический потенциал в синтезе алифатических нитрилов из алифатических спиртов и аммиака на уровне Сu-Zn-Al-Ca оксидных образцов. Расширение ассортимента катализаторов для данного процесса позволяет более гибко управлять процессом получения алифатических нитрилов.
В модельной реакции получения дибутиламина гидроаминированием н-бутанола аммиаком при нагрузке по спирту 0,4 ч-1, молярном соотношении BuOH:NH3:H2 = 1:2,0:6,0 в пределах температур 200-240°C исследован каталитический потенциал ряда новых Cu-, Cu-Zn-, Cu-Mg-, Ni- содержащих образцов на алюмокальциевой основе.
Выявлено, что наивысшие показатели эффективности демонстрирует медьалюмокальциевый контакт: при 220С конверсия бутанола достигает 98%, а селективность по целевому дибутиламину до 88,0%. Лучшие из новых катализаторов в оптимальных условиях не уступают в селективности по данному амину зарубежным аналогам.
Результаты работы защищены тремя патентами: Пат. 99072 Украина, МПК С07D 295/023 (2006.1), C07D 295/03 (2006.1), C07D 295/02 (2006.1), C07D 211/06 (2006.1) Способ получения N-алкилпиперидинов / Белов В.В., Марков В.И., Сова С.Б., Волощенко Д.В. (Украина), Голосман Е.З., Ефремов В.Н., Круглова М.А., Трошина В.А. (Россия) ; - № а 2011 09918; заявл. 10.08.2011; опубл. 10.04.12, Бюл. № 13. — 4 с.; Пат. 92433 Украина, МПК7 С 07 С 253/24 (2006.1). Способ получения алифатических нитрилов / Белов В.В., Марков В.И., Сова С.Б., Ященко Т.М., Оненко К.А. (Украина), Голосман Е.З., Круглова М.А., Нечуговский А.И., Трошина В.А. (Россия) ; - № а 2009 13264 ; заявл. 21.12.09 ; опубл. 25.10.10, Бюл. № 20. - 4 с.; Пат. 92476 Украина, МПК С07С 253/24 (2006.01) Способ получения алифатических нитрилов /Белов В.В., Марков В.И., Сова С.Б., Порохня О.В. (Украина), Голосман Е.З., Нечуговский А.И., Кашинская Е.В. (Россия); - № u 2013 08269; заявл. 01.07.2013 ; опубл. 26.08.2014, Бюл. №16. - 4 с.
к.х.н. В.В. Белов
ГВУЗ “Украинский государственный
химико-технологический университет”, г. Днепропетровск
д.х.н.,
проф. Е.З. Голосман
ООО “НИАП-КАТАЛИЗАТОР”, г.
Новомосковск
“Катализ для переработки возобновляемого сырья: топливо, энергия, химические продукты” (CRS-3)
6-11 сентября 2015 г., Катания, Сицилия, Италия

3-я Международная конференция “Катализ для переработки возобновляемого сырья: топливо, энергия, химические продукты” (Catalysis for Renewable Sources: Fuel, Energy, Chemicals, CRS-3) прошла 6-11 сентября 2015 года в городе Катания (Сицилия, Италия). Необходимость проведения встреч по данной тематике показали предыдущие конференции CRS-1 (2010 г., Санкт-Петербург, Россия) и CRS-2 (2013 г., Лунд, Швеция), которые продемонстрировали высокий интерес к этому чрезвычайно актуальному направлению в науке и технологии. Конференция в Швеции положила начало традиции посещения объектов по переработке возобновляемого сырья. Выбор места проведения конференции CRS-3 был обоснован интенсивным развитием биохимических технологий в Италии и базировался на желании продолжить традицию посещения объектов переработки растительного сырья. В научной программе конференций CRS особое внимание уделяется всестороннему рассмотрению проблем, связанных с исследованием в области получения ценных биопродуктов, альтернативных и возобновляемых источников энергии с применением каталитических подходов.
В работе Третьей Международной конференции “Катализ для переработки возобновляемого сырья: топливо, энергия, химические продукты”, которая проводилась как сателлит 12-го Европейского конгресса по катализу EuropaCat-2015, приняли участие около 100 специалистов в области каталитической переработки возобновляемого сырья из 25 стран мира. В конференциях CRS традиционно участвуют представители ведущих мировых научных школ, а также известных международных компаний, осуществляющих исследования в области биокатализа. Конференция CRS-3 была организована совместно с Университетом Мессины (Сицилия, Италия). Сопредседателями конференции выступили профессор Габриеле Ченти, руководитель отдела катализа Университета Мессины, и академик Валентин Николаевич Пармон, научный руководитель Института катализа им. Г.К. Борескова СО РАН. Научная программа конференции включала 6 пленарных лекций, 6 ключевых лекций, две презентации, 46 устных и около 30 стендовых докладов.
Тематика устной и стендовой сессий конференции отразила современные тенденции развития этой быстро развивающейся области знаний:
Катализ в лесохимии для производства ценных химических продуктов
Использование биомассы в нефтехимии
Каталитические процессы для производства биотоплива
Био-фото-электрокаталитическая конверсия возобновляемых источников энергии
Пленарную сессию открыла известный специалист в области биохимических технологий из Университета Мессины (Италия) профессор Сиглинда Перафонер с лекцией “Новые возможности объединения заводов био- и солнечной энергетики”. В своем выступлении проф. Перафонер представила анализ перспектив массового использования биохимических, а также других альтернативных источников энергии. Было отмечено, что использование биомассы как химического сырья, переработка диоксида углерода, использование возобновляемых источников энергии – все это элементы, характеризующие новый этап развития химических и энергетических технологий с низкими выбросами углекислого газа. Важным элементом в этом направлении является также интеграция производств био- и солнечной энергетики, что позволит сократить выбросы парниковых газов и в то же время улучшить экономику всего процесса получения энергии.
Пленарную сессию продолжил профессор Марк Вениаминович Цодиков (Институт нефтехимического синтеза им.
А.В. Топчиева РАН, Москва, Россия), выступив с лекцией “Перспективные каталитические реакции для получения топлив и химических продуктов на
основе биооксигенатов”.Были представлены данные об исследованиях селективной конверсии биосубстратов в алканы, олефины, ароматические
углеводороды и нафтеновые компоненты бензина и дизельных фракций, а также н-бутанола и пентанола-2. Также были продемонстрированы результаты
тестирования коммерческих и оригинальных катализаторов на основе алюминия и цеолитных носителей, содержащих наноразмерные биметаллические
активные компоненты I, II, V-VIII групп.
<
Профессор Гай Марин (Университет Гента, Бельгия) выступил с докладом “Катализ для возобновляемых ресурсов: конверсия биоспирта”. Доклад был посвящен использованию катализа для кислотной конверсии биоспиртов, которая представляет большой интерес для научных кругов и промышленности, включает в себя сложные каталитические реакции и предлагает способы производства возобновляемых химических веществ и топлив. Олефины, полученные путем дегидратации биоспиртов, могут рассматриваться в качестве строительных блоков для некоторых базовых продуктов, таких как биотоплива и полимеры. Тем не менее, развитие энергоэффективного процесса в значительной степени зависит от селективности и активности катализатора и требует глубокого понимания каталитического механизма реакции. Это представление основано на фундаментальных механистических аспектах каталитической конверсии биоспиртов. Значение имеет как структура цеолита, так и модель и строение химического реактора, все это влияет на скорость реакции и конечный продукт.
Профессор Донато Аранда (Университет Рио де Жанейро, Бразилия) выступил с лекцией “Получение биодизеля и углеводородов из биомассы водорослей”. Он отметил, что рост энергопотребления повышает уровень загрязнения окружающей среды, и именно это делает необходимым развитие “чистых” источников энергии, которые позволят стабилизировать ситуацию и существенно замедлить темпы загрязнения. Поэтому исследование было сосредоточено на разработке новых возобновляемых источников сырья для производства топлив, которые смогут заменить нефть. В качестве такого источника был предложен биодизель, в частности, использование биомассы микроводорослей, которая может стать экологичной заменой нефтепродуктам. Возобновляемые углеводороды, полученные из липидов, являются хорошей альтернативой дизельным топливам. В процессе получения таких топлив, включающем реакции гидролиза, риформинга глицерина, гидрирования жирных кислот и их декарбоксилирования, хорошо себя показали палладиевые катализаторы. В водно-липидной системе они обеспечивали 100%-ный выход углеводородов из морских водорослей. Процесс имеет дополнительное преимущество: ни на одном этапе сушка не является необходимым условием предварительной обработки водорослей. Они могут быть использованы непосредственно, так как содержащаяся в них вода принимает участие в реакциях гидролиза и риформинга.
В лекции “Фундаментальное понимание искусственного фотосинтеза при производстве солнечной энергии” профессора Кэн Ли (Институт химической физики, г. Далянь, Академия наук Китая) были представлены научно-технические достижения в области солнечной энергетики при использовании искусственного фотосинтеза, а именно фотокатализа и фотоэлектрокатализа. Проделанная работа открывает новые возможности для сборки полупроводниковых кристаллов на основе системы искусственного фотосинтеза с использованием двойных катализаторов на разных гранях.
Завершил пленарную сессию профессор Дмитрий Юрьевич Мурзин (Университет Або, Турку, Финляндия), представив доклад “Катализ в синтезе биомассы, получение продуктов с физиологическими свойствами”. Он отметил, что в последние годы много усилий было посвящено валоризации целлюлозы, гемицеллюлозы и лигнина, присутствующих в древесине и являющихся типичным непродовольственным видом биомассы, способным выступать в качестве источника топлива. Кроме того, биомасса лигноцеллюлозы содержит ряд экстрактивных веществ: терпены и терпеноиды, жиры и воски, фенольные соединения и другие субстраты (алканы и водорастворимые соединения, такие как моно- и дисахариды). Были рассмотрены примеры, демонстрирующие потенциал природных продуктов в качестве источника сырья для тонкой химии, пищевой и фармацевтической промышленности. Так, натуральные продукты и препараты используются для лечения 87% всех заболеваний человека, действуя как антибактериальные и противораковые средства, антикоагулянты, противопаразитарные препараты и иммунодепрессанты. В свою очередь, каталитические методы позволяют повысить качество конечной продукции.
Ключевые лекции привлекли не меньшее внимание аудитории. Так, профессор Симони Менегетти (Федеральный Университет Алагоаса, Бразилия) представила доклад “Катализаторы для производства химических веществ из возобновляемых источников”. Промышленное производство химических веществ из возобновляемых источников является задачей, которая совмещает в себе концепции зеленой химии и устойчивого развития, и в этом контексте важную роль играет химия масел. В лакокрасочной промышленности алкидные смолы, синтезированные из полиолов, двухосновных кислот и жирных кислот, обладают хорошими эксплуатационными свойствами, которые делают их часто используемым материалом в этой области; это хороший пример полимера, полученного из возобновляемого сырья. В ходе конференции также были представлены ключевые лекции “Синтез производных О-изопропилидена из сахаров с использованием модифицированных сульфокислотой катализаторов на основе оксида кремния” (профессор Парасураман Селвам, Индийский Технологический Институт Мадраса, Индия), “Эффективная каталитическая деполимеризация лигнина в сверхкритическом этаноле” (профессор Эмиль Хенсен, Технологический Университет Эйдховена, Нидерланды), “Физический” катализ химических реакций под влиянием внешних электрических полей. HDTV и СВЧ в катализе” (профессор Сергей Дмитриевич Варфоломеев, Институт биохимической физики им. Эмануэля, Москва, Россия), “Судьба ископаемого и био углерода в FCC переработке” (профессор Клод Миродатос, Институт катализа и защиты окружающей среды Лиона, Франция), “Нанокомпозитные катализаторы трансформации биотоплива в синтез-газ: проектирование, механизм реакции и реализация” (профессор Владислав Александрович Садыков, Институт катализа им. Г.К. Борескова СО РАН).
Презентационный доклад о развитии работ в области каталитической переработки возобновляемого сырья в Институте катализа представил академик Валентин Николаевич Пармон (Институт катализа им. Г.К. Борескова СО РАН).
На конференции были представлены устные и стендовые доклады, многие из которых вызвали активное обсуждение. Тезисы всех представленных на конференции лекций и докладов можно найти по адресу:
http://www.catalysis.ru/resources/institute/Publishing/Report/2015/ABSTRACTS_CRS-3-2015.pdf.

Заключительной и весьма значимой частью программы конференции CRS-3 стала поездка на объект – завод по производству электроэнергии путем сжигания древесного сырья в кипящем слое.

Предварительно доктор Сержио Гуцци, представитель руководства предприятия, сделал презентационный доклад на конференции по приглашению организаторов. Затем участники конференции посетили предприятие по производству электроэнергии из древесины, расположенное в провинции Энна (остров Сицилия). Они имели возможность ознакомиться с особенностями технологии, увидеть действующие реакторы и аппараты, услышать пояснения специалистов предприятия. Поразительная активность участников, задававших массу вопросов местным специалистам, продемонстрировала большой интерес к биоэнергетике. Поиск возможностей посещения таких объектов является непростой задачей, как с организационной точки зрения, так и с юридической. Но участники высоко оценивают результаты подобных встреч со специалистами ведущих в мире производств, возможность приобрести новые контакты для дальнейшей работы, оценить уровень развития технологий в разных странах.

Статьи по материалам отобранных Оргкомитетом докладов опубликованы в журнале Catalysis for Sustainable Energy (de Gruyter Open access).
На закрытии участниками было отмечено, что отличительной чертой конференции CRS является особенная атмосфера, созданная в том числе и ее организаторами. Невероятная активность делегатов в обсуждении докладов, их участие в интересных дискуссиях, желание получить позитивный и полезный результат придают конференции определенный заряд. Аудитория также высоко оценила профессиональное использование организаторами возможностей, предоставленных конференц-центром отеля Шератон, в котором проходила конференция и проживали ее участники. В частности, использование телекоммуникаций и видеопроекций позволило оперативно получать информацию о программе текущей научной сессии, обеспечило доступ к любым необходимым сведениям, мотивировало четкое соблюдение регламента. В своем заключительном слове директор Конференц-центра отеля Шератон выразил благодарность Оргкомитету за четкую, грамотную и профессиональную работу, пригласил обращаться по поводу проведения следующих конференций.
Участниками было принято решение провести конференцию CRS-4 в 2017 году, обсудив возможность сделать ее сателлитом очередного Европейского конгресса по катализу EuropaCat-2017, который будет проходить в Италии.

Материал подготовили
Т.В. Замулина, В.А. Яковлев, С.С. Логунова, С.А. Хромова
(ИК СО РАН, Новосибирск)
When it comes to making an environmentally friendly polymer, chemists tend to think that starting with a renewably sourced monomer is all it takes. But according to Eugene Y.-X. Chen of Colorado State University, a biobased monomer is only one of three criteria needed to build such a material: A green synthesis and product recyclability are important too.
To emphasize that point, Chen and postdoctoral researcher Miao Hong have developed a new synthetic process to create what they believe is a truly sustainable polymer. They polymerize a biobased monomer using a metal-free organocatalyst to make a fully recyclable material. Chen and Hong described their approach in a pair of presentations during a Division of Polymer Chemistry session Sunday at the American Chemical Society national meeting in San Diego.

Chen and Hong created what they call truly sustainable polymers by using a biobased monomer in an organocatalyst polymerization process to make recyclable polymers.
The Colorado State researchers started out by designing the first practical ring-opening polymerization of γ-butyrolactone, a sugar-derived compound that is used as a solvent and building block in fine and specialty chemical applications. Although ring-opening polymerization of cyclic lactones is a common way to make biodegradable polyesters, γ-butyrolactone is an exception. Chemists had labeled the compound nonpolymerizable, Chen noted, because the ring is too stable except under extreme pressure and moderate temperature.
Nevertheless, after probing an array of thermodynamic, kinetic, and processing conditions, Chen and Hong discovered lanthanum an d yttrium catalysts that polymerize γ-butyrolactone via a coordination-insertion mechanism. The team figured out how to control the reaction equilibrium and vary the amount of catalyst to produce high-molecular-weight linear and cyclic polyesters. They also discovered that heating the bulk materials above 200°C completely recycles the polymers back to the γ-butyrolactone monomer (Nat. Chem. 2015, DOI: 10.1038/nchem.2391).
But that still wasnt good enough, Chen said. The team took the polymerization to the next level by discovering an organocatalyst to sidestep toxicity and availability issues associated with metal catalysts. The researchers again probed an array of reaction conditions to zero in on a polyaminophosphazine catalyst with alcohol initiators. The phosphazine is a superstrong base capable of deprotonating the γ-butyrolactone ring to generate the active species that propagates the polymerization (Angew. Chem. Int. Ed. 2016, DOI: 10.1002/anie.201601092).
This is a real tour-de-force study that combines mechanistic understanding, sustainability, and practical importance, commented Cornell Universitys Geoffrey W. Coates, whose group develops catalysts for preparing biodegradable polymers. These new phosphazene initiators, coupled with polymer recycling, constitute an exciting advance in the development of new biodegradable polyesters.
Chen and Hong convincingly demonstrate that what was thought to be an impossible hurdle can be overcome by a smart selection of appropriate catalysts and reaction conditions, added Jean-Franois Carpentier, a polymerization catalysis expert at the University of Rennes 1. Although the catalytic rate and other improvements are needed to make the process cost effective, and the health and safety of the organocatalyst must be considered, the achievement will surely launch new interest in this field, Carpentier noted. The ability to start with biomass-derived cyclic lactones creates a nice playground for future investigations and developments.
Waste material from the energy crop elephant grass yields flavor and fragrance compounds
Fast-growing grasses and trees such as switchgrass and poplar offer a sustainable alternative to petroleum as a source of fuels and chemicals. But to make fuels from plant sugars in cellulose and hemicellulose, producers generally have to remove the surrounding lignin, a hardy phenolic polymer that helps support the plants structure. Instead of wasting this abundant resource, scientists have been seeking ways to make useful chemicals from it. Now a new study shows that an inexpensive nickel-based catalyst can transform 68% of lignin from the grass Miscanthus, or elephant grass, into valuable aromatic compounds, while preserving plant sugars for subsequent conversion to fuel (ACS Sustainable Chem. Eng. 2016, DOI: 10.1021/acssuschemeng.5b01776).
Converting lignin to useful chemicals is challenging because the polymer is highly heterogeneous and recalcitrant to degradation. Recently, however, scientists have found that some precious metals can catalyze the breakdown of lignin into valuable aromatic monomers used in flavor and fragrance chemistry. Mahdi M. Abu-Omar of Purdue University and his colleagues used a palladium-zinc catalyst to convert 50% of lignin in poplar into two phenolic products (Green Chem. 2015, DOI: 10.1039/C4GC01911C). In 2014, Abu-Omar founded the company Spero Energy to develop the groups technology. But to make the process less expensive and more environmentally friendly, they sought a cheaper, more earth-abundant catalyst. Nickel, just above palladium on the periodic table, was the obvious choice.
In the current study, the researchers adapted their previous method to use a nickel catalyst supported with activated carbon. In a reactor, they combined 1 g of milled Miscanthus with 45 ml methanol, and introduced the catalyst in a microporous stainless steel cage to prevent solid reaction products from recombining with the catalyst. Under high pressure, they heated the reactor to 225°C, which breaks the lignin polymer into shorter, soluble units that contact the catalyst through the cage. The nickel catalyst then helps deoxygenate these oligomers into aromatic products.
Using this method, the team turned 68% of the lignin from Miscanthus into phenolic products, including dihydroeugenol, propylsyringol, and ferulic acid methyl esters, which can be used to make vanillin. The reaction also left behind a solid, carbohydrate residue, the researchers found. With an iron chloride catalyst, the team then converted this to furfural and levulinic acid with up to 86% yield. These products can be used to make biofuels or other chemicals.
R. Tom Baker, a catalysis researcher at the University of Ottawa, calls the study a landmark paper, noting that a simple two-step process converts more than half the mass of the grass feedstock into value-added chemicals. Although the biobased chemicals industry is currently facing major economic challenges from unreasonably inexpensive petroleum, he says, this exciting work shows that technical advances towards viable biorefineries are well underway.
Energy: Nanoparticle-based photocatalyst system evolves hydrogen gas from water with 100% efficiency
A solar-powered nanoscale system can evolve hydrogen from water with perfect efficiency. A cadmium sulfide quantum rod (yellow) absorbs photons, activating electrons (negative charges) that are transferred to a platinum catalyst (purple) to produce hydrogen gas (small purple spheres), and leaving behind holes (positive charges) that are localized to an embedded quantum dot (green).
A major goal in renewable energy research is to harvest the energy of the sun to convert water into hydrogen gas, a storable fuel. Now, with a nanoparticle-based system, researchers have set a record for part of the process, reporting 100% efficiency for the half-reaction that evolves hydrogen (Nano Lett. 2016, DOI: 10.1021/acs.nanolett.5b04813).
To make such water-splitting systems, researchers must find the right materials to absorb light and catalyze the splitting of water into hydrogen and oxygen. The two half-reactions in this processthe reduction of protons to hydrogen, and the oxidation of water to oxygenmust be isolated from each other so their products dont react and explode. Completing the cycle in an efficient, stable, safe fashion with earth-abundant elements is an ongoing challenge, says chemist Nathan S. Lewis of Caltech, who was not involved in this study.
Until recently, the efficiency of the reduction step had maxed out at 60%. One challenge is that electrons and positive charges formed in the light absorption process can rapidly recombine, reducing the number of electrons available for the hydrogen production reaction. To overcome this problem, several years ago, Lilac Amirav of TechnionIsrael Institute of Technology and her colleagues designed a nanoparticle-based system (J. Phys. Chem. Lett. 2010, DOI: 10.1021/jz100075c) that would physically separate the charges formed during photocatalysis.
The teams system comprises a light-harvesting cadmium sulfide quantum rod with a quantum dot made of cadmium selenide embedded near one end. A platinum tip on the opposite end catalyzes hydrogen production. When placed in water in a gas-tight reaction cell and exposed to visible light, the quantum rod absorbs photons, releasing electrons. The rod then transfers electrons to the platinum tip, reducing protons to form hydrogen, and leaving behind positively charged vacancies called holes at the cadmium selenide dot.
When the team initially tested the system in water, however, it had only 20% efficiency for hydrogen production. To improve performance, the researchers tested a variety of reaction conditions. In the new study, they achieved success by increasing the pH and adding isopropyl alcohol. Under these conditions, the holes oxidize hydroxide anions, which are more abundant at the high pH, to yield hydroxyl radicals. The alcohol then donates electrons to the radicals, preventing charge recombination and keeping more electrons available to evolve hydrogen.
When the team measured hydrogen production with this updated setup, they achieved up to 100% efficiency. The results shatter the previous benchmarks for all systems, Amirav says.
Lewis says the teams achievement is a step toward a stable, efficient system for solar fuels production. He notes, however, that this work addresses only one of the necessary half-reactions.
Adapting the work to a full water splitting system may be challenging, says Amirav, because cadmium sulfide corrodes under long exposure to light. However, she has recently shown that adding another catalyst, such as iridium oxide or ruthenium, could improve its photochemical stability (J. Mater. Chem. A 2015, DOI: 10.1039/C4TA06164K; Angew. Chem., Int. Ed. 2015, DOI: 10.1002/anie.201411461).
Imaging: Technique could help improve catalyst performance and make reactions such as water splitting viable

REACTIVITY MAP
By using a fluorescence microscopy method with nanometer resolution, researchers pinpointed catalytic hot spots on a TiO2 nanorod (white outline) where positive-charge-mediated reactions (left) and electron-mediated reactions (center) occur. Red regions are the most active. Knowing the hot-spot locations, they then selectively modified those areas with a cocatalyst (bumps in SEM image, right).
Solid chunks of matter catalyze most of todays industrial-scale chemical processes. If scientists knew exactly where reactions occur on these catalytic solids, they could customize them to improve the performance of catalysts already in use and help bring new ones to market.
By devising a fluorescence microscopy technique with single-molecule resolution, researchers at Cornell University have created a method for pinpointing the sites of catalytic reactions with nanoscale resolution. The scientists used the method to generate surface maps of microscopic catalytic hot spots and then boosted the activity of those spots by decorating them with a cocatalyst (Nature 2016, DOI: 10.1038/nature16534).
The team, which was led by Cornells Justin B. Sambur and Peng Chen, applied the method to TiO2 nanorods used in light-induced water-splitting reactions. Solar-driven water splitting has been studied intensely for decades because it could provide a nearly limitless supply of clean-burning hydrogen if a suitable catalyst can be found.
When light shines on a TiO2 anode in a photoelectrochemical cell, short-lived excited electrons and positive charges form on the catalysts surface. The positive charges, called holes, can oxidize water, evolving O2 and forming hydrogen ions (H+). Then the electrons can reduce the ions to form H2. Often, however, electrons and holes quickly recombine, quenching the excitation before the catalytic reaction can occur.
Depositing a cocatalyst on the anode could help enhance the oxygen evolution step of water splitting. But knowing exactly where to put this oxygen evolution catalyst (OEC) is a challenge: Putting it in the wrong place can hinder light absorption, weakening the anodes performance.
To find the answer, the Cornell team scanned nearly 40 TiO2 nanorods for catalytic hot spots by using two types of probe reactionsan electron-induced reduction and a hole-induced oxidation. Both reactions convert a nonfluorescent organic molecule thats been added to the water-splitting cell to a fluorescent one. The molecule lights up immediately, identifying where on the nanorod it was converted. From those measurements, the team made catalytic activity maps and used them along with the microscopy method to selectively deposit tiny quantities of a cobalt borate OEC on the nanorod hot spots. This deposition improved the nanorods water-splitting abilities.
An imaging technique that uses cleverly chosen fluorescent reporter molecules to examine the fate of photogenerated electrons and holes in a semiconductor at the nanometer scale is a breakthrough, says Bruce A. Parkinson, a photocatalysis specialist at the University of Wyoming.
Not only did the Cornell scientists devise a sensitive mapping method, they made several unexpected observations. For example, most of the nanorods contain just a couple of reactive hot spots and large, relatively unreactive areas, even though the composition and structure of the rods are fairly uniform. Oxidations and reductions also tend to occur in nearly the same spots. And even though adding a cocatalyst to a hot spot makes it hotter as expected, adding the OEC to a cold spot on the TiO2 surface causes an improvement in catalytic activity thats greater than the improvement at the hot spot.
Many of the insights obtained in this study are somewhat counterintuitive, Wyomings Parkinson says. Researchers working in this field will have to take them into account in future studies of photodriven catalytic reactions, he adds.
Chemical & Engineering News
|
April 25-30, 2016 |
|
|
26-29 апреля 2016 г. |
|
|
May 2-6, 2016 |
|
|
May 4-6, 2016 |
http://www.chemia.uj.edu.pl/nauka-i-badania/ |
|
May 8-11, 2016 |
|
|
11-13 мая 2016 г. |
|
|
15-20 мая 2016 г. |
|
|
May 18-20, 2016 |
|
|
20-21 мая 2016 г. |
+7 987 666 7547 |
|
May 22-26, 2016 |
|
|
May 23-27, 2016 |
|
|
23-26 мая 2016 г. |
|
|
24-26 мая 2016 г. |
|
|
24-27 мая 2016 г. |
|
|
May 29 – June 4, 2016 |
|
|
1-4 июня 2016 г. |
|
|
5-15 июня 2016 г. |
|
|
June 13-17, 2016 |
|
|
June 21-22, 2016 |
|
|
23-27 июня 2016 г. |
|
|
June 27-30, 2016 |
|
|
27 июня – 1 июля 2016 г. |
|
|
June 30 – July 1, 2016 |
|
|
June 30 – July 2, 2016 |
http://www.icc2016china.com/uploadfiles/img/file/ |
|
June 30 – July 2, 2016 |
http://www.icc2016china.com/uploadfiles/img/file/ |
|
June 30 – July 2, 2016 |
http://www.icc2016china.com/uploadfiles/img/file/ |
|
June 30 – July 2, 2016 |
http://www.icc2016china.com/uploadfiles/img/file/ |
|
June 30 – July 3, 2016 |
|
|
June 30 – July 3, 2016 |
|
|
3-7 июля 2016 г. |
|
|
July 3-8, 2016 |
|
|
July 4-6, 2016 |
http://www.rsc.org/ConferencesAndEvents/RSC |
|
July 9-11, 2016 |
|
|
July 9-11, 2016 |
|
|
July 9-11, 2016 |
|
|
July 10-12, 2016 |
|
|
July 10-14, 2016 |
|
|
July 10-15, 2016 |
|
|
14-15 июля 2016 г. |
|
|
July 18-20, 2016 |
|
|
July 20-22, 2016 |
|
|
July 25-29, 2016 |
|
|
August 28 – September 2, 2016 |
|
|
September 4-7, 2016 |
|
|
September 4-8, 2016 |
|
|
4-8 сентября 2016 г. |
|
|
September 5-8, 2016 |
|
|
6-9 сентября 2016 г. |
|
|
12-15 сентября 2016 г. |
|
|
13-16 сентября 2016 г. |
|
|
19-20 сентября 2016 г. |
|
|
September 19-22, 2016 |
|
|
September 19-23, 2016 |
|
|
19-23 сентября 2016 г. |
|
|
19-30 сентября 2016 г. |
|
|
20-23 сентября 2016 г. |
|
|
26-30 сентября 2016 г. |
|
|
October 2-6, 2016 |
|
|
3-7 октября 2016 г. |
|
|
4-5 октября 2016 г. |
|
|
4-7 октября 2016 г. |
|
|
October 12-14, 2016 |
|
|
18-19 октября 2016 г. |
|
|
18-21 октября 2016 г. |
|
|
October 23-26, 2016 |
|
|
November 11-13, 2016 |
|
|
17-18 ноября 2016 г. |
(812) 718 27 52 |
|
November 27 – December 2, 2016 |
|
|
2017 |
|
|
March 2-3, 2017 |
|
|
August 27 – September 1, 2017 |
|


