
Конференция РФФИ
"Фундаментальная наука в
интересах развития критических технологий"
Владимир, 12-14 сентября, 2005 г.
О совещании Российских производителей катализаторов,
Владимир, 13 сентября 2005 г.
XVII симпозиум
"Современная химическая физика"
Туапсе, 18-25 сентября, 2005 г.
2-я Российская конференция
"Актуальные проблемы нефтехимии",
Уфа, 11-13 октября 2005 г.
За рубежом
"Фундаментальная наука в интересах развития критических технологий",
Владимир, 12-14 сентября, 2005 г.
Российский фонд фундаментальных исследований по инициативе ряда крупных ученых страны проводит цикл научно-деловых семинаров (конференций), основной целью которых является представление всем заинтересованным лицам наиболее значительных достижений фундаментальных исследований, полученных в ходе выполнения проектов Фонда и перспективных для промышленности.
Очередная такая конференция РФФИ "Фундаментальная наука в интересах развития критических технологий" с международным участием состоялась с 12 по 14 сентября 2005 года в городе Владимире. Конференцию организовал Институт катализа им. Г.К. Борескова СО РАН (Новосибирск) совместно с ЗАО НТЦ "Владипор" (Владимир), ЗАО "Мембраны" (Владимир) при поддержке Инновационного отдела РФФИ.
На конференции обсуждались наиболее перспективные достижения фундаментальной науки, имеющие важное значение для развития критических технологий федерального уровня и создания новых материалов в следующих областях:
Особое внимание на конференции уделялось опыту инновационной деятельности при освоении новой продукции.
Среди 130 участников конференции: 105 - ученые из академических и отраслевых научно-исследовательских организаций, ВУЗов; 25 - специалисты и представители промышленных предприятий. На конференции выступили с докладами специалисты из России, Беларуси, Украины, Казахстана, Азербайджана и Финляндии.
Научная программа конференции включала 10 пленарных лекций приглашенных докладчиков. Устные (34) и стендовые (58) доклады были представлены на секциях:
 В рамках конференции состоялся круглый стол "Проблемы и перспективы использования результатов фундаментальных исследований и семинар молодых ученых по направлению "Катализаторы и мембраны". Тезисы всех докладов Конференции "Фундаментальная наука в интересах развития критических технологий" были выпущены на компакт-диске.
В рамках конференции состоялся круглый стол "Проблемы и перспективы использования результатов фундаментальных исследований и семинар молодых ученых по направлению "Катализаторы и мембраны". Тезисы всех докладов Конференции "Фундаментальная наука в интересах развития критических технологий" были выпущены на компакт-диске.
Пленарную сессию конференции открыла лекция д.х.н. Б.С. Бальжинимаева "Стекловолокнистые катализаторы: от молекулярного уровня до промышленных испытаний" (Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск), которая была посвящена целенаправленной разработке и исследованию стекловолокнистых материалов с целью выявления и реализации их потенциальных свойств в катализе. Представлены результаты по масштабированию технологии их производства и пилотным испытаниям в процессах окисления диоксида серы, нейтрализации автомобильных выбросов дизельных двигателей и др.
Проблемам разработки новых мембранных гибридных процессов, сочетающих современные достижения мембранных методов и традиционных, для разделения жидких сред была посвящена лекция к.х.н. А.А. Поворова (ЗАО "Мембраны" г. Владимир). На сегодняшний момент в ЗАО "Мембраны" разработан ряд перспективных процессов, которые используют мембранные технологии для получения чистой питьевой воды и улучшения экологической обстановки.
Катализаторам нефтехимических и полимеризационных процессов посвящена лекция д.х.н. профессора П.Е. Матковского (Институт проблем химической физики РАН, г. Черноголовка, Московская обл.). В результате выполненных фундаментальных исследований и прикладных разработок в ИПХФ РАН разработана и запатентована серия различных катализаторов и около тридцати из них реализованы в коммерческих процессах.
О новых бескислородных предкерамических полимерах - нано-металлополикарбосиланах и наноразмерных наполнителях - уникальных материалах для повышения прочности и окислительной стойкости углеграфитов и стабилизаци высокопрочной керамики сообщил в пленарной лекции д.х.н. профессор П.А. Стороженко (ГНЦ РФ ФГУП ГНИИХТЭОС). В ГНИИХТЭОС впервые в мире разработаны методы плазмохимического промышленного получения и создана уникальная установка для производства ультрадисперсных и наноразмерных порошков металлов и сплавов, бора, нитридов, карбидов, оксидов металлов и др. соединений.
В пленарной лекции д.х.н. В.В. Гузеева (ФГУП "НИИ полимеров", г. Дзержинск Нижегородской обл.) рассмотрены структуры различных видов поливинилхлорида, полученного суспензионным, блочным (в массе), микросуспензионным и латексным способами, а также превращение исходной структуры при переработке. Благодаря фундаментальным исследованиям структуры макромолекул ПВХ, причин их нестабильности, синтезу и применению стабилизаторов, исследованиям свойств пластифицированных, жестких и наполненных композиций поливинилхлорид стал крупнотоннажным полимером.
В пленарной лекции "Природные гетерополициклические соединения, адресованные медицине" д.х.н. Э.Э. Шульц (Новосибирский институт органической химии им. Н.Н. Ворожцова СО РАН, г. Новосибирск) обобщены результаты исследований направленных трансформаций растительных алкалоидов в практически полезные соединения.
В настоящее время получение моторных топлив из синтез-газа (СО+Н2) рассматривается как перспективный путь развития топливной промышленности. Причина этого - большие ресурсы природного газа и эффективность получения на его основе альтернативных топлив, учитывая рост соотношения цен на нефть и на газ. Основное внимание исследователей привлекает создание новых перспективных катализаторов. В своей лекции чл.-корр. РАН А.Л. Лапидус (Институт органической химии им. Н.Д. Зелинского РАН, г. Москва) сообщает о перспективных кобальт-цеолитных катализаторах на основе новых типов высококремнистых цеолитов.
В XXI веке стратегическое значение имеют исследования, направленные на широкое использование каталитических экологически чистых технологий, сводящих до минимума объемы промышленных отходов или устраняющих причины их образования вовсе. Перспективам и достижениям в области экологического катализа была посвящена пленарная лекция д.х.н. В.Ф. Третьякова (Институт нефтехимического синтеза им. А.В. Топчиева РАН, г. Москва).
В рамках конференции состоялся Круглый стол РФФИ по теме "Проблемы и перспективы использования результатов фундаментальных исследований". О результатах и перспективах инновационно-ориентированных конкурсов РФФИ в своем выступлении рассказал И.П. Тихонов (Инновационный отдел РФФИ, г. Москва). Сообщение вызвало большое количество вопросов и интерес всех участников конференции.
Особое внимание участников привлекла лекция академика В.М. Бузника (Инновационно-технологический центр РАН, г. Черноголовка, Московская обл.), которая закрывала Круглый стол. В лекции было дано само определение "инновационная деятельность", проанализированы схемы инновационного продвижения разработок в рамках плановой и рыночных систем. Был представлен анализ общих проблем, стоящих перед исследователями, имеющими намерение заняться реализацией своих разработок через инновационные фонды. Были освещены вопросы поддержки малого инновационного предпринимательства со стороны государства и формирующихся в России инновационных инфраструктур.
Далее мы приводим краткую информацию о сделанных на конференции устных докладах.Устные доклады были представлены на четырех тематических секциях и секции молодых ученых.
Секция I. Катализаторы, мембраны и процессы на их основе
Перспективам использования методов плазмохимии для получения активных катализаторов реакции СО+О2 был посвящен устный доклад д.х.н. И.И. Михаленко (Российский университет дружбы народов, Москва).
В докладе д.х.н. В.С. Руднева (Институт химии Дальневосточного отделения РАН, Владивосток) были представлены результаты исследований формирования на алюминиевой основе тонкопленочных алюмоникелевых оксидных структур, данные по их составу, организации поверхности, а также каталитических свойствах в реакции окисления СО.
Проблемы гомогенного катализа при окислении насыщенных углеводородов - основных компонентов природного газа - под действием молекулярного кислорода и восстановителей осветил в своем докладе к.х.н. Е.Г. Чепайкин (Институт структурной макрокинетики и проблем материаловедения РАН, Черноголовка, Московская обл.).
Об особенностях механизма образования углеродных нанонитей, малые размеры и уникальная структура которых определяют их необычные механические и электронные свойства, рассказал в своем докладе д.х.н. В.В. Чесноков (Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск).
От группы авторов Н.А. Гайдай (Институт органической химии им. Н.Д. Зелинского РАН, г. Москва) представила доклад по гидрированию диоксида углерода на медных и никелевых катализаторах на основе углеродных носителей.
Перспективам использования в промышленно важных процессах новых никелевых катализаторов гидрирования, модифицированных гетерополисоединениями, был посвящен доклад к.х.н. М.Д. Навалихиной (Институт высоких температур РАН, г. Москва)
Новый способ получения метилэтилкетона каталитическим окислением н-бутиленов был представлен в докладе д.х.н. профессора К.И. Матвеева (Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск).
В Институте катализа им. Г.К. Борескова СО РАН разработан высокоактивный и селективный катализатор на принципиально новом керамометаллическом носителе, - об этом сообщил к.х.н. Н.А. Пахомов.
Как следует из доклада О.В. Барсукова (ООО "Компания КАТАХИМ", г. Москва), в каталитическом крекинге целесообразно применение бицеолитной каталитической системы, представляющей собой смесь катализаторов, содержащих отдельно цеолит типа Y и цеолит типа ZSM-5.
Перспективным для извлечения анионов Cr (VI) из промышленных стоков является метод электродиализа, позволяющий осуществлять непрерывное извлечение ионов из разбавленных растворов. Переносу Cr (VI) через неорганические композиционные мембраны был посвящен доклад к.х.н. Ю.С. Дзязько (Институт общей и неорганической химии им. В.И. Вернадского НАН Украины, г. Киев).
Секция II. Производство, переработка и исследование полимеров, эластомеров и каучуков
Чередующиеся сополимеры монооксида углерода (СО) с олефинами представляют собой новое поколение экологически чистых функциональных сополимеров, которые могут найти широкое применение в различных областях промышленности и сельского хозяйства. Об этом сообщил в своей лекции "Синтез и применение чередующихся сополимеров на основе окиси углерода и α-олефинов" д.х.н. Г.П. Белов (Институт проблем химической физики РАН, г. Черноголовка, Московская обл.).
Новым катионным латексам с полыми частицами был посвящен доклад к.х.н. О.В. Сорочинской (Санкт-Петербургский филиал Института катализа им. Г.К. Борескова СО РАН, г. Санкт-Петербург). Предложенным простейшим способом получения полых частиц с положительным зарядом путём перезарядки анионных латексов катионными ПАВ, автор предлагает решить проблему повышения термостабильности полых частиц.
Новому виду полимерных наноматериалов с заданными свойствами - поливинилхлориду с преобразованными поверхностными слоями - был посвящен доклад д.х.н. А.Я. Фридмана (Институт физической химии и электрохимии им. А.Н. Фрумкина РАН, г. Москва).
Д.х.н. В.Е. Платонов (Новосибирский институт органической химии им. Н.Н. Ворожцова, г. Новосибирск) сообщил о новом методе получения широкого круга полифторированных хлор- и бромсодержащих ароматических соединений ряда бензола, индана, пиридина путем сопиролиза доступных полифторарентиолов и их аналогов с хлором и бромом в проточной системе при 400-5000С и атмосферном давлении. Предложенный метод обеспечивает высокие выходы и высокую чистоту сырых продуктов. Он может послужить основой при создании укрупненной установки для производства полифторхлор- и - бромаренов.
Интересные результаты по модификации поверхности ультрафильтрационных мембран из полиакрилонитрила и полисульфона представил к.х.н. В.П. Касперчик (ГНУ Институт физико-органической химии НАН Беларуси, г. Минск, Республика Беларусь)
Новые бифункциональные катализаторы и технология процесса полимеризации прошли успешные опытно-промышленные и промышленные испытания на ОПЗ ИНХП НАН Азербайджана, АО "ЕЗСК" (г.Ефремов, Россия), в Научно-исследовательском центре фирмы "Петким" (г.Измит, Турция) в процессах получения низко- и высокомолекулярного 1,4-цис- и 1,4-цис+1,2-полибутадиенов, и готовы к широкому промышленному внедрению, - сообщил д.х.н. Ф.А. Насиров (Институт нефтехимических процессов Национальной академии наук Азербайджана, Баку, Азербайджан).
Секция III. Химические продукты для медицины, сельского хозяйства, бытового и специального назначения
Доклад д.т.н. Б.С. Федорова (Институт проблем химической физики РАН, г. Черноголовка, Московская обл.) был посвящен синтезу неизвестных ранее комплексных соединений платины, подавляющих развитие метастазов злокачественных опухолей. В настоящее время синтезирован широкий спектр метаболически активных соединений - генераторов NO, которые при их использовании в качестве хемосенсибилизаторов в химиотерапии опухолей способны усиливать противоопухолевый эффект этих цитостатиков.
О методе получения эпибрассинолида - нового класса фитогормонов, стимулирующих прорастание семян, рост и развитие растений, корнеобразование, повышающих устойчивость к стрессовым условиям произрастания, снижающих потребность в химических средствах защиты растений, доложил аспирант М.А. Филатов (Московский государственный университет им. М.В. Ломоносова, г. Москва).
Высокой противомикробной, ноотропной, анальгетической и противовоспалительной активностью обладает ряд соединений, полученных новым методом синтеза конденсированных систем из гетероциклов, - сообщил в своем докладе д.х.н.
В.Л. Гейн (Пермская государственная фармацевтическая академия, г. Пермь).
Как сообщил в своем докладе д.х.н. В.А. Бабкин (Иркутский институт химии им. А.Е. Фаворского СО РАН, Иркутск), в Иркутском институте химии им. А.Е. Фаворского СО РАН совместно с Институтом цитологии и генетики СО РАН для сельского хозяйства разработан препарат на основе экстрактивных веществ из древесины лиственницы - Лариксин. Препарат прошел Государственную регистрацию. Он содержит в своем составе дигидрокверцетин (около 70 %) и смолистые вещества древесины лиственницы. Лариксин обладает свойствами биологического регулятора роста растений, является индуктором иммунитета к грибковым заболеваниям, ускоряет развитие вегетативных органов.
Доклад к.х.н. Н.К. Кобяковой (ФГУП "НИИ полимеров", г. Дзержинск Нижегородская обл.) был посвящен разработке технологии получения нового четвертичного аммониевого соединения - Алкацетама. Уже организован выпуск современного высокоэффективного композиционного дезинфицирующего средства на его основе с использованием преимущественно отечественной сырьевой базы, что позволит ограничить закупки дезинфектантов за рубежом.
В докладе д.т.н. З.П. Пай (Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск) были освещены современные подходы к разработке экологически и экономически приемлемых каталитических способов получения ряда промышленно важных продуктов тоннажного органического производства целевых эпоксидов или моно- и дикарбоновых кислот, а также методов тонкого органического синтеза с использованием продуктов возобновляемого природного сырья.
Как следует из доклада к.х.н. А.М. Бескопыльного (Волгоградский филиал Института катализа СО РАН, г. Волгоград) существующую в настоящее время проблему недостаточной оснащенности опытно-промышленной базы РАН, не позволяющей быстро осуществлять наработку опытных партий химической продукции и получать исходные данные для проектирования конкретного промышленного производства, можно решить с помощью комплексов модульных установок "Уфа-1" и "Уфа-2". Возможности оборудования комплексов "Уфа - 1" и "Уфа - 2" позволяют выполнять практически все операции химико-технологических процессов. Блочный принцип коммутации оборудования позволяет быстро создавать законченные технологические линии производства конкретного продукта или масштабирования технологического процесса синтеза в жидкой и газовой фазе, ректификации, фильтрации, сушки, в т.ч. во взвешенном слое, процессов с использованием обратного осмоса, центрифугирования, тонкого и плёночного испарения, дробления и фасовки твёрдых продуктов.
В докладе "Разработка научных основ получения высокотехнологичных ингредиентов для пищевой и фармацевтической промышленности методом сверхкритической флюидной экстракции" Н.В. Гришатова (ЗАО "БИОЦЕНТР", г. Нижний Новгород) сообщает о разработке технологии получения биологически активных веществ из побегов сосны путем экстракции двуокисью углерода, находящейся в сверхкритическом состоянии.
Проблеме противоязвенных препаратов, а именно синтезу импортзаменяющих висмутсодержащих лекарственных субстанций был посвящен доклад д.х.н. Ю.М. Юхина (Институт химии твердого тела и механохимии СО РАН, Новосибирск). Разработан способ получения висмут-калий-аммоний-цитрата, лекарственной субстанции для препаратов типа "Де-нол", а также предложен способ синтеза висмутсодержащего цитрата, лекарственной субстанции для препарата типа "Пилорид".
В докладе к.ф.-м.н. Н.В. Булиной (Институт физики им. Л.В. Киренского СО РАН, г. Красноярск) были приведены результаты применения науглероженного сорбента Al2О3 для разделения фуллеренов, применяемых для получения новых материалов и прекурсоров, использующихся для синтеза противовирусных и противораковых препаратов, для получения металлов и сплавов с новыми свойствами, для изготовления энергоемких аккумуляторных батарей.
К.х.н. Душкин А.В. (Институт химии твердого тела и механохимии СО РАН, Новосибирск) выступил с докладом на тему "Механохимическая технология получения быстрорастворимых лекарственных средств и биологически активных добавок". Суть технологического процесса заключается в механической обработке порошкообразной смеси исходных веществ ударно-истирающими воздействиями в специальных мельницах. В результате формируются композитные частицы порошка с развитой поверхностью контакта твердых фаз. Уникальной особенностью таких материалов является быстрая реакция нейтрализации при гидратации (растворении) с образованием раствора соли фармацевтического вещества. Аналогичным образом получаются твердые дисперсные системы малорастворимых лекарственных веществ и хорошо растворимых наполнителей, их твердые растворы и межмолекулярные комплексы.
Способность ланолина при растирании с водой поглощать более 150 % воды не теряя мазевых характеристик делает его идеальной мазевой основой в фармацевтической промышленности для получения мягких лекарственных форм, где активные вещества внедряются в виде растворов, и косметике для изготовления кремов, помад и других продуктов. Унифицированной технологии переработки шерстного жира в ланолин был посвящен доклад д.х.н., профессора С.Р. Конуспаева (Казахский национальный аграрный университет, г. Алматы, Республика Казахстан). Полученный ланолин соответствует фармакопейной статье на ланолин безводный, получен сертификат соответствия. Технология отработана на лабораторном реакторе объемом 1 литр и опытном реакторе объемом 60 литров.
Секция IV. Энергосбережение и экология
В докладе к.х.н. А.С. Лермонтова (Институт нефтехимического синтеза им. А.В. Топчиева РАН, г. Москва) предлагается использовать этиловый спирт для получения углеводородов бензинового ряда. Метод каталитической конверсии этанола с получением углеводородов бензинового ряда позволяет использовать получаемые продукты в качестве топлива в обычных двигателях внутреннего сгорания, при полном отсутствии в их составе серы, а также вовлекать отходы лесной, деревоперерабатывающей, целлюлозно-бумажной промышленности, полеводства и животноводства в процесс получения биоэтанола - возобновляемого сырья топливного назначения.
В ИФОХ НАН Беларуси разработаны новые ионообменные волокна ФИБАН, способные в бисульфитной форме поглощать формальдегид из водных растворов и полностью восстанавливать свои сорбционные свойства после регенерации. При этом регенерирующий раствор не содержит ни формальдегида, ни каких-либо других веществ, токсичных для микроорганизмов, - сообщила в своем докладе к.х.н. Г.В. Медяк (Институт физико-органической химии Национальной академии наук Беларуси, Минск, Республика Беларусь).
Доклад д.т.н. С. Прейса (Технический Университет, г. Лаппеенранта, Финляндия) был посвящен исследованиям процесса фотокаталитического окисления паров несимметричного диметилгидразина в воздухе в проточных реакторах в неустановившемся и стационарном режимах реакции, а также в реакторе периодического действия.
Доклад к.т.н. В.Б. Карпухина (Институт физической химии РАН, г. Москва) был посвящен новому спиральному фильтрующему элементу (СФЭ) для очистки газовых выбросов от твердых примесей. Его отличительной особенностью является способность увеличивать площадь проходного сечения фильтрующей перегородки при подаче потока жидкости или газа в направлении, обратном тому, при котором производилось фильтрование. В результате - СФЭ регенерируется значительно эффективнее других материалов и, как показывают результаты исследований, позволяют осуществлять абсолютную очистку газовых потоков от твердых радиоактивных частиц, включая окислы трансурановых элементов.
Д.х.н. В.К. Яцимирский (Киевский национальный университет имени Тараса Шевченко, г. Киев, Украина) в докладе "Окисление CO на WO3 и MoO3 с добавками Pt(Pd) и Cs+, cформированных при восстановлении H2" сообщает о разработке нового подхода к получению композитных катализаторов окисления СО.
Исследованию процессов образования оксида азота(I) при контактном окислении аммиака на Pt-Rh-Pd-Ru катализаторах был посвящен доклад к.т.н. В.П. Колесникова (Невинномысский технологический институт ГОУ ВПО "СевКавГТУ", Невинномысск). Изучены условия, при которых минимизируется выход N2O и определены условия повышенного его продуцирования.
Секция молодых ученых
Наряду с основными секциями конференции с целью привлечения внимания к наиболее важным вопросам фундаментальных исследований в области критических технологий, была организована секция молодых ученых. По результатом заслушанных докладов председателем секции д.х.н. М.Ю. Синёвым были отмечены два доклада, вызвавших наибольший интерес: доклад Н.В. Крысинской (Российский химико-технологический университет им. Д.И. Менделеева, Москва), посвященный обработке жидких радиоактивных отходов методом испарения через мембрану, и доклад П.В. Бердниковой (Институт катализа им. Г.К. Борескова СО РАН, Новосибирск) о перспективных катализаторах окисления органических соединений - пероксополиоксовольфроматах, применяемых в сочетании с четвертичными аммониевыми катионами для проведения реакций в двухфазных растворах.
На закрытии конференции было принято коллегиальное решение, в котором участники обратили внимание РФФИ на ряд проблем, а Оргкомитету конференции поручили донести принятые решения до РФФИ.
РЕШЕНИЕ КОНФЕРЕНЦИИ
- В целом конференция РФФИ "Фундаментальная наука в интересах развития критических технологий" была содержательной и полезной для оценки новых тенденций в области мембран, катализаторов, химии для медицины, новых материалов, обмена мнениями между участниками из академических институтов и представителями промышленности, а также установления взаимовыгодных контактов.
- На состоявшейся конференции было представлено большое количество докладов с фундаментальными исследованиями, которые получили финансовую поддержку в форме грантов РФФИ, Президента РФ, Президиума РАН, программы "Ведущие научные школы России"; междисциплинарных интеграционных проектов СО РАН и др. фондов. Такая активность участников приветствовалась при формировании научной программы прошедшей конференции. Рекомендовать и далее участникам еще более активно участвовать не только в освоенных, но и в новых конкурсах, таких, например, как конкурс инновационно-ориентированных проектов РФФИ.
- На конференции был представлен ряд научно-прикладных разработок, имеющих высокую степень готовности к их опытно-промышленному и промышленному освоению - все то, что собственно и соответствовало изначально обозначенной цели ее проведения. Рекомендовать организаторам последующих конференций при отборе докладов выделять доклады, отражающие опыт инновационной деятельности при освоении новой продукции.
Участники конференции считают необходимым:
З.П. Пай, Л.Я. Старцева, А.С. Носков
Институт катализа им. Г.К. Борескова СО РАН,
г. Новосибирск

Фото участников Конференции
Владимир, 13 сентября 2005 г.
В сентябре 2005 года в старинном городе Владимире состоялось совещание российских производителей катализаторов, организованное Минпромэнерго, Институтом катализа им. Г.К. Борескова, ОАО "ВНИИ НП" и рядом др. организаций.
С докладами на совещании выступили зам. начальника отдела промышленной политики в химическом производстве России И.И. Куликов, директор Института катализа СО РАН академик В.Н. Пармон, директор "ВНИИ НП" профессор В.М.Капустин, зам. директора Института катализа профессор А.С.Носков, представители ряда отраслевых институтов и катализаторных производств. Большая организационная работа была проведена Н.П. Алфимовой и В.П. Шмачковой (ИК СО РАН) и представителями хозяев совещания П.В. Дубягой, И.Н. Скворцовой (ЗАО НТЦ "Владипор", ЗАО "Мембраны", Владимир) и др.
"Сибнефть - Омский НПЗ" представлял главный специалист-технолог В.И. Горденко, ЭНПО "Неорганика" - зам. директора С.Н.Соловьев, "АЛВИГО" - главный инженер А.П.Митронов и главный специалист Л.М.Родин, "ВНИИНП" представлял зам. ген. директора В.А.Хавкин, ОАО "Отечественный катализатор " - ген. директор В.Г. Пак, Новомосковский институт азотной промышленности представляли директор по науке О.П.Фирсов и профессор Е.З. Голосман.
В подготовленных предложениях организаторов совещания и выступлениях участников было отмечено, что два года назад до Совета Безопасности РФ была доведена информация о критической зависимости ряда отраслей промышленности от поставок зарубежных адсорбентов и катализаторов. Эти проблемы требуют особого внимания со стороны государства, так как серьезно угрожают экономической и стратегической безопасности страны. В связи с реорганизацией структуры правительства РФ практическая реализация уже выработанных предложений не получила развития. Большую остроту проблема обеспечения катализаторами базовых отраслей экономики России приобретает в связи с вступлением России в ВТО.
В представленных докладах В.Н. Пармона, И.И. Куликова и др. отмечалось, что с применением катализаторов производится до 15 % ВВП России (в США эта доля составляет до 30 %). Общий объем рынка катализаторов в мире составляет около 20 млрд долларов и с их применением производится продукции на сумму свыше 2000 млрд долларов. Темпы роста производства катализаторов в мире превышают рост экономики в целом. Известно, что применение катализаторов - наиболее эффективный и экономичный способ в решении проблем экологии и рационального использования ресурсов. Российский рынок оценивается в 540 млн долларов, однако их производство постоянно снижается и отрасль находится в стагнации. Доля каталитических процессов в ряде важнейших отраслей промышленности и в том числе в стратегических достигает 90 %. Достигнутый уровень отечественных научно-технических разработок в области создания ряда катализаторов соответствует, а в ряде случаев и превышает общемировой.
Сейчас в РФ 47 крупных и мелких предприятий занимаются производством катализаторов. Среди производимых 400 промышленных катализаторов в России почти три десятка марок выпускает катализаторное производство Новомосковского института азотной промышленности (НИАП). Как представляется, хорошим, а может быть в какой-то степени хрестоматийным примером, является разработка НИАП катализаторов метанирования HKM-I, HKM-2, HKM-4A. Проведены глубокие фундаментальные исследования совместно с ИОХ им. Зелинского РАН (профессор Якерсон В.И. и др.). Благодаря тщательной изученности, длительным пилотным испытаниям на реальных газах эти катализаторы являются одними из лучших в мире. Подтверждением этого является фантастический срок их службы - 15-16 лет и то , что, несмотря на экспансию импортных катализаторов, доля отечественных в процессе метанирования и в настоящее время по данным академика В.Н. Пармона составляет практически 100 %. И это в то время, как доля многих отечественных катализаторов в ряде химических и нефтехимических процессов не превышает 10-50 %.
Можно привести и другие примеры - разработка ИК СО РАН и ИППУ СО РАН, Омск, носителя сибунита, марганцевых катализаторов очистки газов и др. К сожалению, многие новые разработки промышленных катализаторов основаны на базе каталитических систем, созданных 10-15 лет тому назад, что обусловлено низким финансированием, устаревшим оборудованием, оттоком научных кадров, разрушением значительного числа НИИ и в первую очередь отраслевых институтов. Вместе с тем по данным основателя фирмы Хальдор Топсе разработка катализаторов мирового уровня может быть осуществлена фирмами с числом квалифицированных научных сотрудников не менее 50 и с использованием оборудования стоимостью не менее 20 млн долларов. Сколько российских научных организаций могут соответствовать этим рекомендациям? И все же есть эффективные разработки катализаторов и их внедрение, осуществляемое ИК СО РАН, Институтом проблем переработки углеводородов СО РАН,Омск, ВНИИ НП, ФГУП "Синтез", ИНХС РАН, ИОХ им. Зелинского, НИАП и др.
Конечно, для создания конкурентоспособных отечественных катализаторов необходима концентрация научно-технических исследований ряда научных коллективов и, безусловно, выделение государственных заказов на разработку перспективных катализаторов. Необходимо формирование госрезерва катализаторов для стратегически важных процессов. На совещании рассматривалось предложение создания холдинговой структуры для проведения единой маркетинговой и научно-технической политики по производству, использованию и разработке отечественных катализаторов и адсорбентов, не уступающих по своим характеристикам импортным.
Представляется целесообразным на первом этапе создать ассоциацию отечественных производителей катализаторов (в форме некоммерческого партнерства) с целью объединения усилий по взаимодействию с органами государственной власти и выполнению комплексных крупномасштабных проектов по разработке перспективных катализаторов.
Участники совещания договорились провести следующее рабочее совещание отечественных производителей катализаторов в 2006 г. в Новосибирске или Новомосковске, или в Ярославле. Место встречи и дата проведения совещания будут уточнены.
Е.З. Голосман
Д.х.н., проф.
НИАП, г. Новомосковск
"Современная химическая физика ",
Туапсе, 18-25 сентября, 2005г.
Химическая физика давно преодолела узкие рамки классической науки и превратилась в крупную научную культуру, интегрирующую физику, химию и биологию. Предмет ее интереса - электронно-атомно-молекулярные процессы, в которых происходят преобразования электронных оболочек и перестройки ядерных каркасов. Именно эти процессы определяют физику и химию полупроводников, лазерную физику и химию, физику и химию ферментов, нейрофизиологию и нейрохимию, физику и химию поверхности, наноэлектронику и нанохимию - все те научные направления, которые сегодня определяют технологический прогресс цивилизации.
С 18 по 29 сентября 2005 года в пансионате МГУ "Буревестник" (пос. Вишневка, Лазаревский район, г. Сочи) проходил ежегодный XVII Всероссийский Симпозиум "Современная химическая физика" . Симпозиум был организован совместно Институтом химической физики им. Н.Н. Семенова Российской академии наук и Московским государственным университетом им. М.В.Ломоносова при поддержке Президиума Российской академии наук и Российского фонда фундаментальных исследований. В работе Симпозиума приняли участие более 250 ученых из 25 городов России, а также их коллеги из Белоруссии, Украины и Армении. На Симпозиуме было представлено 5 пленарных лекций, более 30 устных докладов и около 200 стендовых. Традиционно в работе Симпозиума приняли участие молодые ученые, аспиранты и студенты. В этом году их было около 60 человек.
XVII симпозиум затронул многие интересные темы, но наиболее обстоятельно, с анализом современного состояния и оценкой перспектив, были обсуждены направления, где имеются крупные прорывы, где наиболее ярко обнаруживается новизна.
Магнитно-полевые эффекты в деформации немагнитных кристаллов (NaСl, LiF, PbS) демонстрируют значительную и пока необъяснимую зависимость предела текучести кристаллов от магнитного поля (А.Е. Смирнов, Институт кристаллогафии РАН). Микротвердость кристаллов гидрофталата калия также увеличивается на 12-15 % при выдержке кристаллов в магнитном поле 0,2-1,3 Тл в течение 5 мин. (М. Колдаева, Институт кристаллографии РАН). Кристаллы гидрофталатов аммония и цезия, напротив, снижают микротвердость на 10-15 % после выдержки их в магнитном поле. Подвижность дислокаций в кремнии, легированном фосфором, также сильно зависит от магнитного поля (А. Скворцов, Ульяновский гос. университет).
Все эти эффекты возникают на уровне взаимодействия движущихся дислокаций со стопорами. Общая идея сводится к тому, что при встрече дислокации с примесным атомом (стопором) локальная область их контакта становится спин-селективным нанореактором, в котором электронные переносы рождают ион-радикальные пары со спиновыми состояниями синглет или триплет. Магнитное поле, как считается, влияет на спиновые переходы между этими состояниями и снимает спиновые запреты на расщепление или сцепление дислокаций со стопором. Эта идея кажется правдоподобной лишь на первый поверхностный взгляд. В месте контакта дислокации со стопором имеются сильные электрические поля. Энергия их намного больше, чем расщепление между синглетом и триплетом в спин-селективном нанореакторе. Загадочно, как малые энергии спиновых состояний могут влиять на систему дислокация + стопор. Это обстоятельство не составляет загадки в химических реакциях, где состояния реагентов и продуктов не тождественны и где есть спиновые запреты. В системе дислокация + стопор начальные и конечные состояния тождественны и не видно никаких причин для спиновых запретов и, следовательно, для магнитно-полевых эффектов. Можно принять, что синглет-триплетное расщепление велико и сравнимо по энергии с энергией механических напряжений. Но, во-первых, физически это маловероятно; во-вторых, при этом условии не должны наблюдаться магнитные эффекты. Очевидно, что наблюдаемая совокупность магнитно-полевых эффектов по-прежнему составляет загадку для понимания. Решение ее открыло бы путь к управлению прочностью и механикой твердых тел с помощью магнитных полей.
Физика и химия одиночных молекул - новое направление, пролог молекулярной электроники - были представлены в блестящих работах школы проф. Б.Р. Шуба (ИХФ РАН) в режиме туннельной спектроскопии. Разработаны методы детектирования одиночных молекул, нанотруб, кластеров, измерены их энергетические спектры, индентифицированы электронные состояния. Даны прекрасные методы определения размеров кластеров по их спектральным расщеплениям, которые отличаются высокой точностью измерения размера. Новые методы туннельной спектроскопии надежно обоснованы теоретически и широко используются в анализе состояния поверхностей (в том числе в практической химии энергетически насыщенных систем).
Другое направление основано на захвате отдельных молекул каплями сверхтекучего гелия. Далее в этих каплях спектроскопически детектируются состояния молекул, их димеров, тримеров, комплексов их с другими молекулами. Так как молекулы и их комплексы находятся в жидкости и при сверхнизких температурах, это обеспечивает высокое спектроскопическое разрешение и высокие метрологические характеристики молекул и их комплексов. Особый интерес представляет проект изотопного разделения в микрокаплях сверхтекучего гелия. Идея его очень красива: изотопные молекулы имеют слегка смещенные спектры и потому при облучении нанокапли гелия с изотопно различающимися молекулами приобретают разную энергию и испаряют, теряют разное число атомов гелия. Далее эти микрокапли отклоняются пучком атомов криптона или неона, причем капли, несущие разные изотопные молекулы, отклоняются по-разному из-за различия в их массах (т.е. числе оставшихся атомов гелия в микрокапле). Другими словами, изотопный эффект измеряется не по массе изотопа, а по массе гелиевых микрокапель (Г.Н. Макаров, Институт спектроскопии РАН, Троицк).
Химическая энергетика представлена в нескольких направлениях. Во-первых, разрабатываются и широко используются в практике новые режимы сверхадиабатического и фильтрационного горения: экономические и низкозатратные процессы обжига кирпича и керамики, кинетика горения углерода и угля, фронтальная газификация углерода, массоперенос в горении металло-оксидных систем, извлечение редких металлов в режиме горения, газификация полимеров, коксовых остатков и т.д. (Буров Ю.М., Беккер А.В., Волкова Н.Н., Гудкова И.Ю., Розенберг А.С., Салгановский Е.А., Салгановская М.В., Лемперт Д.Б. - ИПХФ РАН, Черноголовка).
Во-вторых, создаются новые системы преобразования энергии реакций на атомно-молекулярном уровне в энергию излучения: хемилюминесценция в твердфазной реакции XeF2 с гидрооксидом урана, создание люминесцирующих систем в реакциях лантаноидов и уранила с персульфатом натрия, люминесценция в окислительно-восстановительных реакциях (Хазимуллина Л.Н., Масягутова - ИОХ УНЦ, Уфа; Хурсан С.Л. - Башкирский гос. университет, Уфа; Назаров В.Б. - ИПХФ РАН, Черноголовка; Стояновский В.О. - Институт катализа СО РАН, Новосибирск).
В-третьих, активно обсуждался новый эффект - преобразование энергии, выделяющейся в ходе гетерогенной реакции на границе металл-газ в электрический ток. Обоснована возможность создания источника тока нового типа. На примере рекомбинации атомов водорода на поверхности пленки золота, толщиной 10 нм, нанесенной на поверхность кристаллов кремния, показана возможность создания высоковозбужденных колебательных состояний Н2,передающих энергию электронам металла. Эти горячие электроны стимулируют ток в кристалле кремния, на который нанесен металл (Харламов В.Ф. - Орловский гос. технический университет, Орел).
В-четвертых, обсуждалось состояние науки о создании жидкофазных лазеров с солнечной накачкой. Тема эта очень актуальна, однако существенного прогресса в этой области пока не достигнуто (Серегин А.А. - ГНЦ РФ ФЭИ, Обнинск).
Кроме того, были широко представлены и другие, традиционные направления - химическая структура, реакционная способность, кинетика, изотопия, катализ, фотохимия, комплексообразование и т.д. Следует отметить большой интерес участников к лекциям Городецкого В.В. "Исследование механизма окисления СО кислородом и оксидом азота на Pd" , Плахутина Б.Н. "Фундаментальные энергетические параметры для атомной d оболочки" , Чернозатонского Л.А. "Нанотрубные структуры: от исследований к технологиям" и Гордона Е.Б. "Низкотемпературные химические реакции при сверхвысоких давлениях" .

Высказано единодушное решение всех участников симпозиума - провести новый симпозиум в следующем, 2006г.
академик А.Л. Бучаченко
профессор. Б.Р. Шуб
"Актуальные проблемы нефтехимии "
Уфа, 11-13 октября 2005 г.
2-я Российская конференция "Актуальные проблемы нефтехимии" (с международным участием) в определенном смысле является наследницей проводившихся в 70-х-90-х г.г. прошлого века Нефтехимических симпозиумов социалистических стран. После некоторого перерыва проведение нефтехимических конференций было возобновлено в 2002 г. Тогда совместными усилиями Объединенного Научного совета РАН по химии нефти, газа и ископаемого твердого топлива, Института Нефтехимического синтеза им. А.В.Топчиева РАН, Химического факультета МГУ им. М.В.Ломоносова в Подмосковье была организована 1-я конференция, которая привлекла огромное внимание нефтехимиков и специалистов смежных областей. Вторую конференцию было решено провести в Уфе, столице Башкортостана, который является флагманом Российской нефтехимии, поскольку в нем сосредоточены крупнейшие нефтехимические предприятия - "Салаватнефтеоргсинтез " , ЗАО "Каучук" и т.д. Проведение конференции в Уфе сделало возможным активное участие в ней многочисленных нефтехимиков из Башкирии и Татарстана.
Несомненная важность и актуальность проведения исследований в области нефтехимии диктуется тем, что наша страна является одним из основных мировых производителей нефти, газа и угля, доходы от их продажи являются фундаментом бюджета страны. Углубление переработки нефти на отечественных предприятиях является стратегической задачей. Возрастающие требования к экологической чистоте нефтехимических процессов и других процессов использования углеводородного сырья также могут быть выполнены с участием нефтехимии. Как сказано в приветствии Президента Республики Башкортостан М.Г. Рахимова, направленном в адрес участников конференции, "В условиях сокращения добычи и ухудшения качества нефти при одновременном ужесточении экологических требований к нефтепродуктам проблемы нефтепереработки и нефтехимии становятся особенно значимыми. Это прежде всего - глубокая очистка нефтяного сырья от серы, увеличение эффективности каталитических процессов нефтепереработки с вовлечением тяжелых нефтяных остатков и квалифицированное использование полученных нефтепродуктов".
2-я Российская конференция "Актуальные проблемы нефтехимии" вызвала значительный интерес по всей России и за рубежом. В ней приняли участие более 300 нефтехимиков и специалистов смежных областей из многих регионов России, в том числе нефтедобывающих и нефтеперерабатывающих районов. Кроме того, в конференции приняли участие специалисты из Украины, Белоруссии, Казахстана, Азербайджана. Знаменательно, что почти половина участников конференции работают в Башкортостане и Татарстане, старейших и наиболее значительных нефтеперерабатывающих регионах Европейской части России, то есть одна из целей конференции - обеспечение широкого и активного участия этих регионов в научных обменах была выполнена.
Основную работу по организации конференции взяли на свои плечи три института - Институт нефтехимического синтеза им. А.В.Топчиева РАН (Москва, директор академик Платэ Н.А.), Институт органической химии УНЦ РАН (Уфа, директор академик Юнусов М.С.), и Институт нефти и химии РАН (Уфа, директор член-корреспондент РАН Джемилев У.М.). Главным координатором был Объединенный научный совет РАН по химии нефти, газа и ископаемого твердого топлива (председатель член-корреспондент РАН Хаджиев С.Н., ученый секретарь Соболева Т.В.).
Конференция включала пленарные и ключевые лекции ведущих ученых, устные доклады и стендовые сессии. Кроме того, в рамках конференции был проведен круглый стол по проблемам ожижения газа. Всего на конференции было представлено 189 докладов более 500 авторов.
Знаменательно, что с ключевыми и пленарными докладами выступили крупнейшие ученые нашей страны, причем программа была составлена так, чтобы показать участникам конференции не только обзор достижений в нефтехимической науке, но и на стыке нефтехимии и других наук, для того чтобы открыть молодым ученым перспективные направления работы на будущее. Так, в докладе Н.А. Платэ и С.Н. Хаджиева (ИНХС РАН) были показаны перспективы применения нанотехнологий в нефтепереработке и нефтехимии. Как подчеркивалось в докладе, объемы финансирования разработок в области нанотехнологий растут опережающими темпами во всем мире, и особенно в США. Приведены примеры размерных эффектов для многочисленных каталитических реакций. Показаны возможности использования нанотехнологий в процессах глубокой переработки нефти и мазутов, в ожижении углей. Представлена блок-схема нефтехимического завода будущего, способного к переработке многовариантного сырья, начиная от нефти и природного газа, и заканчивая тяжелыми нефтяными остатками, ископаемым твердым топливом и растительной биомассой.
В докладе проф. С.Д. Варфоломеева (МГУ им. М.В. Ломоносова, ИПБФ РАН) проведен анализ сегодняшнего состояния и перспектив применения ферментативного катализа в нефтехимии. Обсуждены проблемы переработки возобновляемого сырья, которые пока еще не столь актуальны в нашей стране, но будут чрезвычайно важны в будущем. Эти процессы входят в зону так называемой Зеленой химии, или химии в интересах устойчивого развития, поскольку позволяют обеспечить человечество на длительную перспективу экологичными возобновляемыми источниками энергии и химических продуктов в конечном счете только за счет энергии Солнца. Именно солнечная энергия обеспечивает рост биомассы, образующей главный источник возобновляемого сырья, в том числе и для нефтехимии. На настоящий момент, как следует из лекции С.Д. Варфоломеева, разработан уже целый ряд процессов, позволяющих получать из СО2 при помощи ферментативного катализа не только этанол, но и ряд базовых нефтехимических продуктов, например, биоразлагаемых полимеров. Обсуждается проведение с использованием ферментативного катализа "невозможных" химических реакций, в которых окислителем служит вода, например, для окисления этанола в ацетальдегид. Эти реакции становятся возможными за счет эффективного биокаталитического поглощения водорода.
В лекции У.М. Джемилева (ИНХК РАН, Уфа) также рассмотрены подходы, которые позволяют заменить нефтяное сырьё возобновляемым - путем активации малых молекул, получаемых, например, при переработке биомассы. В качестве стратегических проблем нефтехимии в докладе упомянуты (а) решение проблемы получения, хранения и транспортирования дешевого водорода; (б) использование возобновляемого природного сырья; (в) разработка гомогенных, гетерогенных и ферментоподобных катализаторов, обладающих высокой селективностью и длительным сроком службы; (г) увеличение доли углеводорoдного сырья в нефтехимическом синтезе. В докладе У.М. Джемилева предложен ряд сопряженных химических реакций, которые позволяют на основе этанола (которого можно получить до 180 кг из тонны деловой древесины) и хлоруглеводородов получать целый набор важных нефтехимических продуктов - кетоны, спирты, фенол, алифатические и ароматические сложные эфиры и т.д. при 200 С на молибденовых, ванадиевых, марганцевых катализаторах. Представлен новый способ бета-этилирования альфа-олефинов, который позволяет с высокой эффективностью с использованием катализаторов на основе циркония получать ценные мономеры для реакций полимеризации. В качестве перспективных технологий будущего названы реакции окисления метана закисью азота, формальдегидом, а также окислительного сочетания с использованием серы. Кроме того, в докладе обсуждаются и другие способы квалифицированного использования извлекаемой из сернистых нефтей элементарной серы.
Пленарная лекция И.И. Моисеева (РГУНГ, Москва) была посвящена обзору последних достижений в области селективного окисления. В своей, как всегда, блестящей лекции И.И. Моисеев провел анализ важнейших процессов селективного окисления с учетом влияния этих процессов на окружающую среду. Селективность при этом оценивается как отношение выхода целевого продукта к сумме всех продуктов, но вводится, в соответствии с определением П. Шелдона, фактор окружающей среды, равный отношению суммарного выхода побочных продуктов к выходу целевого продукта. В соответствии с этим определением фактор окружающей среды при получении ацетальдегида из угля многократно превышает единицу, а при каталитическом окислении этилена составляет приблизительно 1. Этот процесс в настоящее время дает до 3 млн. т в год ацетальдегида, его научные основы были разработаны под руководством И.И. Моисеева еще в 60-х годах прошлого века. Дальнейшее окисление ацетальдегида до уксусной кислоты с селективностью до 98 % можно осуществить в присутствии кобальт-марганцевого катализатора при невысоких температурах. В лекции представлены способы, которым позволят значительно повысить селективность реакций, например, такие, как переход из газовой в жидкую фазу; использование стоп-реагентов, совершенствование катализатора, предотвращение прямого контакта кислорода с окисляемым субстратом, использование различных вариантов каталитических реакторов - мембранных, псевдоожиженного слоя, и многие другие. В заключение были очерчены задачи, актуальные в настоящее время в области селективного окисления.
Лекция И.И. Моисеева настолько хорошо построена и так легко воспринимается аудиторией, что ее можно рекомендовать как публичную лекцию, для того чтобы продемонстрировать неспециалистам уникальные возможности каталитической химии для решения важнейших научных и практических задач.
Несколько лекций были непосредственно посвящены каталитическим процессам в нефтехимии, которые в настоящее время, как видно и из приведенного выше материала, составляют фундамент нефтехимии. Доклад В.Н. Пармона (ИК СО РАН) был посвящен анализу использования гетерогенных катализаторов для нефтехимии и нефтепереработки. В качестве основных проблем, которые связаны с применением катализа в области нефтехимии, с учетом специфики российских условий, названы следующие: необходимость углубления переработки нефти; быстрый рост потребления высокооктанового бензина; большой дефицит ароматических соединений; избыток легких фракций предельных углеводородов (С3-С4). С упором на эти стратегические пункты развития, в докладе представлены разработанные в Институте катализа им. Г.К. Борескова процессы "БИФОРМИНГ", "БИМТ" и "БИЦИКЛАР", новые микросферические катализаторы крекинга, биметаллические катализаторы риформинга (позволяющие значительно улучшить октановые числа получаемых бензинов), а также композитные селективные сорбенты воды. Необходимо особенно отметить, что внедрение, например, новых катализаторов крекинга только на Омском НПЗ привело к повышению выхода бензина за 1995 г. на 250 тыс. т. Что касается новых процессов, то одной из важных отличительных особенностей является энергосбережение.
В докладе И.И. Ивановой (Химический факультет МГУ им. М.В. Ломоносова) каталитические свойства цеолитов были рассмотрены в аспекте повышения экологической безопасности нефтехимических процессов, которая базируется на высокой селективности и производительности цеолитных катализаторов.
Другой новый класс катализаторов малотоннажных нефтехимических процессов на основе каликсаренов и других сложных молекул был описан в докладе Э.А. Караханова (Химический факультет МГУ им. М.В. Ломоносова). Как следует из всех перечисленных докладов, будущие успехи нефтехимии связаны с увеличением селективности катализаторов, что приводит к снижению до минимума количества образующихся отходов, а также потребляемой энергии для рециклирования и вторичной переработки сырья и отходов, которое необходимо в случае реакций низкой селективности.
Еще один ключевой доклад был сделан вице-президентом ОАО АК Сибур д.х.н. В.Л. Байбурским. В своем докладе на примере фирмы "Сибур" В.Л. Байбурский определил направления и указал способы, которыми научные разработки могут быть представлены вниманию фирм и получено финансирование для технологической проработки и внедрения. Этот вопрос чрезвычайно остро стоит даже в нефтехимии, где, казалось бы, все научные разработки ведутся в тех направлениях, которые востребованы промышленностью, и особенно важен для других областей химии, не столь однозначно ориентированных на производство. Кроме того, был очерчен круг задач, которые в первую очередь будут востребованы крупными фирмами, специализирующимися в области нефтепереработки, такими как Сибур.
В связи с большим количеством заявок на устные доклады работу конференции пришлось разбить на секции. Всего их было шесть, они перечислены ниже, хотя работа конференции была организована так, что пленарные и ключевые лекции могли прослушать все участники конференции, а затем параллельно работало не более двух из перечисленных ниже секций. Таким образом, практически все желающие смогли посетить интересные для них доклады.
Итак, работали следующие секции:
Что касается последней секции, первоначально эту тематику планировали обсудить в рамках круглого стола, однако работающие в этом направлении участники конференции попросили оргкомитет провести заседание в более удобной для них форме, что и было сделано.
Первоначально конференцию планировали провести в течение 3 дней - с 11 по 13 октября, однако в связи с большим количеством заявок все три дня были заняты пленарными и секционными заседаниями, а также стендовыми сессиями, так что запланированный в рамках конференции круглый стол по проблемам ожижения газа (процессы "Gas to liquid") было решено провести 14 октября. На круглый стол, который организовал представитель фирмы "Юкос" В.Н. Кастерин, были специально приглашены представители фирм "Зюд-Хеми АГ" А. Башинский, "UOP" В. Рыбкин и "Дэви Процесс Текнолоджиз, GB" С. Холоуэй, которые выступили с докладами. Круглый стол вызвал большой интерес среди участников конференции, поскольку снова, как и в докладе В.Л. Байбурского, обсуждались проблемы, связанные с промышленной реализацией научных разработок. В своем выступлении представитель фирмы "Дэви Процесс Текнолоджиз, GB" С. Холоуэй рассказал о своем опыте в этой области. Он критически оценил состояние инжиниринговых исследований в Российской науке и посоветовал внимательнее отнестись к зарубежному опыту.
Рассмотрим несколько подробнее, какие новые направления исследований были представлены на конференции.
Ряд докладов был посвящен разработке новых методов и процессов получения высших олефинов, которые служат основой для получения полиолефиновых масел, высших моно- и полиалкилароматических углеводородов. В настоящее время доля продуктов, получаемых из высших олефинов, растет опережающими темпами, поэтому эта задача является весьма актуальной. В докладе П.Е. Матковского и др. (ИПХФ РАН) приведен обзор разрабатываемых в ИПХФ РАН новых селективных процессов получения из этилена индивидуальных альфа-олефинов С4, С6, С8 в присутствии комплексов одновалентного хрома и титана. Ряд других олефинов получен методом этенолиза растительных масел, метатезиса гексена-1, октена-1 и их смесей. Приведены также примеры использования цирконоценов для получения высших олефинов. Гомо- и сополимеризация широкого круга соединений, включающих бутадиен, метилметакрилат, пропиленкосид, капролактон в присутствии комплексов кобальта и неодима изучена группой ученых ИНХС (Нехаева Л.А., Фролов В.М. и др.). Другая группа изучила особенности полимеризации диенов в присутствии титан-магниевых катализаторов, причем оказалось, что при транс-1,4-полимеризации изопрена образуется уникальный термопластичный материал. При сополимеризации бутадиена с изопреном возможно, меняя соотношения исходных мономеров, регулировать кристалличность статистических сополимеров (О.В. Сметанников, Е.А. Мушина и др.). В Омском Институте проблем переработки углеводородов удалось найти замену нестойким кремнийфосфатным системам олигомеризации бутенов, используя модифицированный сульфатной серой и оксидом бора оксид алюминия, причем показатели процесса оказались не хуже, чем для применяемых в настоящее время катализаторов (И.А. Басова и др.). В Институте нефтехимии и катализа (Уфа) также ведутся работы по олигомеризации, например, стирола и альфа-метилстирола в присутствии цеолитов или фосфатных катализаторов.
Ряд докладов был посвящен изучению процессов алкилирования. Ученые химического факультета МГУ им. М.В. Ломоносова разработали новые катализаторы алкилирования бензола додеценом-1: микро-мезопористые, которые превосходят по свойствам как микропористый морденит, так и мезопористый МСМ-41 (С.Е. Тимошин, Е.Е. Князева и др.), а также мезопористые на основе диоксида циркония (А.В. Сунгуров, С.В. Лысенко), которые способны проводить алкилирование не только с высокой скоростью, но также при повышенной селективности по целевому продукту
2-фенилдодекану.
Активно изучаются в России и за рубежом процессы глубокой переработки нефтяного сырья. Так, в Екатеринбурге (ИОХ УРО РАН) разработаны научные основы термического сольволиза макромолекулярного органического сырья в нефтяных остатках, позволяющего получать продукты топливного направления (Е.И. Андрейков и др.). Гидрогенизационная переработка нефти, в том числе и высокосернистой, под небольшим давлением позволяет исключить стадии подготовки сырья и предварительного фракционирования и осуществить переработку сырья глубиной 90-92 % с хорошими выходами автомобильного бензина и дизельного топлива (А.А. Кричко и др., совместная разработка ФГУП ИГИ, ООО Углеродтопхимтехнология и ОАО Тулаинжнефтегаз).
Предварительная обработка нефти комбинацией ультразвукового и СВЧ облучения, как было показано, приводит к повышению выхода светлых фракций, и попутно предотвращает микробное загрязнение почв (С.А. Карпов и др., РГУНГ).
Закономерности окислительного каталитического крекинга мазутов изучены в работах ученых из Казахстана (Р.Х. Ибрашева и др.). В этом случае подаваемый в реактор кислород воздуха инициирует симметричную деструкцию высокомолекулярных углеводородов с образованием фракции легкого газойля, а также изомеризацию и циклизацию углеводородов бензиновой фракции до аренов.
В работе И.В. Билеры и Ю.А. Колбановского (ИНХС РАН) для получения низших олефинов использована реакция оксипиролиза, причем было показано, что с увеличением молекулярного веса исходных алканов степень превращения растет.
Разработке новых катализаторов нефтехимических процессов, методов их модификации с целью повышения стабильности, активности, селективности, посвящена большая группа исследований. Здесь можно выделить крупное направление, связанное с использованием цеолитных и подобных микро- и мезопористых систем. Работы в этом направлении активно ведутся на Химическом факультете МГУ (под руководством И.И. Ивановой), в Новосибирском Институте катализа им. Г.К. Борескова, в Омском филиале указанного института, в Томске (УНТР ООО Томскнефтехим и Томский госуниверситет), в ФГУП ЦНИИЛКА, а также в ИНХС РАН. Например, в работе А.В. Смирнова изучено инкапсулирование сульфидов металлов в цеолит, что позволило получить эффективные в ароматизации пропана и превращении газового конденсата катализаторы на основе цинка.
В то же время наблюдается тенденция к использованию природных алюмосиликатов в качестве катализаторов нефтехимических процессов, частичной или полной замены сложных катализаторов на более простые, на основе модифицированных оксидов алюминия, пилларированных глин. Эти направления активно развиваются в работах Казахстанских ученых.
Поскольку разведанные запасы нефти включают большое количество сернистых нефтей, значительное внимание привлекает разработка процессов обессеривания или переработки сырья с повышенным содержанием серы. Так, в докладе Р.Р. Мухаметовой и др. (ГУП "Институт нефтехимпереработки Республики Башкортостан" ) описаны способы селективной очистки окисленных масляных фракций из сернистых и высокосернистых нефтей. При этом предварительное окисление пероксидом водорода позволяет снизить содержание общей серы в рафинатах после очистки, а также снизить кратность разбавления сырья без потери выхода.
В докладе А.М. Мазгарова (ОАО "ВНИИУС" , Казань) был представлен разработанный татарскими учеными одностадийный процесс очистки легкого углеводородного сырья от сероводорода, меркаптанов, карбонилсульфида и сероуглерода ДМД-2М. Как подчеркивается в докладе, этот процесс является удачным примером доведения научной разработки до промышленного применения.
В совместной работе В.В. Смирнова (Химический факультет МГУ) и М.В. Цодикова (ИНХС РАН) представлены катализаторы селективного окисления сероводорода и меркаптанов - металл-оксидные и металлокомплексные системы, иммобилизованные на слоистом активированном кремнеземе. Продолжительность работы таких катализаторов в 2-3 раза выше, чем аналогичных катализаторов на традиционных носителях - кремнеземе и оксиде алюминия. Выявлены причины стабилизации оксидных систем в новых катализаторах.
Интересный подход продемонстрирован в докладе башкирских ученых (Н.К. Ляпина и др., ИОХ УНЦ РАН, Уфа). В работе предлагается квалифицированное использование сераорганических соединений, входящих в состав нефтей, в качестве сырья для нефтехимических процессов, например, меркаптаны можно использовать для синтеза кето- и аминосульфидов и их полифункциональных производных. Тиоциклоалканы, тиаарены и их производные перспективны в гидро- и цветной металлургии, в производстве полимеров и растворителей.
Разработка методов получения нефтехимических продуктов на основе природного газа активно проводится в ГУНГ, ИК СО РАН, ИОХ им. Н.Д. Зелинского РАН, ИНХС РАН. На конференции были представлены результаты исследования процесса окислительной димеризации метана с получением этилена (А.Г. Дедов, А.С. Локтев и др., ГУНГ); ароматизации пропан-бутановой фракции на промотированных цеолитах типа пентасил (те же ученые); ароматизации метана в бензол в неокислительных условиях на цеолите ZSM5, модифицированном введением никеля и молибдена (А.В. Восьмериков и др., ИХН СО РАН, Томск, совместно с ИФПМ СО РАН, Томск и ИК СО РАН). Изучено влияние проницаемости водорода через пористые мембранные системы в превращениях метанола на мембранных катализаторах.
Близко связаны между собой тематически еще две секции конференции - анализ качества нефтепродуктов и экологические проблемы нефтехимии и нефтепереработки. Без разработки способов надежного определения содержания экотоксикантов в нефтехимических продуктах невозможно решить проблемы загрязнения. В докладе А.Г. Дедова (ГУНГ) была показана возможность замены плазменных атомно-абсорбционных анализаторов на аналогичные приборы с электротермической атомизацией, которые значительно проще в эксплуатации, причем показана удовлетворительная воспроизводимость результатов, полученных на этих приборах, при анализе содержания металлов, в том числе свинца, в бензинах. Несколько докладов посвящено использованию сорбентов на основе углерода (в том числе буроугольных) для ликвидации разливов нефти (И.Н. Маликов и др., Шахтинский институт (филиал) Южно-Российского гостехуниверситета, Шахты), или очистки сточных вод предприятий нефтехимии и нефтепереработки (Г.С. Головин и др., ФГУП ИГИ).
К сожалению, недостаток времени и огромное количество заявок на доклады не позволили организаторам провести намеченный предварительно круглый стол по проблемам образования в нефтехимии. Однако эта тематика была отчасти отражена в ряде докладов.
Проведенный анализ представленных на конференции докладов показывает, что в России и странах бывшего СССР активно развиваются все направления нефтехимической науки. Здесь не были упомянуты еще многие сделанные на конференции доклады, посвященные разработке методов получения отдельных малотоннажных нефтехимических продуктов, способов модификации катализаторов нефтехимических процессов и нетрадиционных способов обработки нефтехимического сырья с целью углубления переработки и повышения ее экономических показателей. Отрадно было видеть, что в конференции приняли участие молодые ученые - до половины зала на заседаниях было представлено учеными до 35 лет. Для молодых ученых, особенно из отдаленных районов нашей страны, на конференции представилась возможность активных плодотворных дискуссий с крупнейшими учеными - нефтехимиками: Н.А. Платэ, И.И. Моисеевым, В.В. Луниным, М.С. Юнусовым, У.М. Джемилевым, С.Н. Хаджиевым, В.Н. Пармоном, С.Д. Варфоломеевым, Э.А. Карахановым, О.П. Паренаго и многими другими.
Безусловно, конференцию " Актуальные проблемы нефтехимии " можно считать успешной.
Секретариат конференции
Any shape you want, you get
Rods, cubes, stars, and hexagons: These are just a few of the shapes into which gold nanoparticles can be coaxed into forming uniformly and in high yield. University of South Carolina, Columbia, chemists Tapan K. Sau and Catherine J. Murphy control nanoparticle shape by systematically varying the parameters of a solution-based seed-mediated procedure they developed for producing nanocrystals. The procedure involves preparing gold seed particles and then adding an amount of the seed solution to a solution containing cetyltrimethylammonium bromide, chloroauric acid (HAuCl4), ascorbic acid, and sometimes a small amount of silver nitrate [J. Am. Chem. Soc., 126, 8648 (2004)]. The procedure is note-worthy not only for its high yield but also for its simplicity, requiring only aqueous solutions, room temperature, and one surfactant instead of various additives to control particle size and shape.
HTTP://WWW.CEN-ONLINE.ORG
Ñ & EN / JULY 12, 2004
Binary catalyst aids synthesis of polycarbonates
One of the most promising green polymerization processes is the alternating copolymerization of CO2 and epoxides to make polycarbonates. Several research groups have been exploring ongoing problems in polycarbonate synthesis related to catalyst efficiency, reaction conditions, and control of polymer structure and molecular-weight distribution. In the latest effort, Xiao-Bing Lu and Yi Wang at Dalian University of Technology, in China, report a novel binary catalyst system that affords an efficient conversion of CO2 and propylene oxide to poly-(propylene carbonate) with high selectivity and stereoregular control under mild reaction conditions [Angew. Chem. Int. Ed., 43, 3574 (2004)]. The researchers used a chiral cobalt-(III) salen complex in conjunction with a quaternary ammonium salt, optimizing the catalyst activity by changing a substituent group on the cobalt catalyst and changing the quaternary ammonium anion. They achieved two- to threetimes higher catalyst turnover frequency at significantly lower CO2 pressure than previously reported by others using a cobalt catalyst alone.
HTTP://WWW.CEN-ONLINE.ORG
Ñ & EN / JULY 12, 2004
Simpler strategy for direct C-C coupling
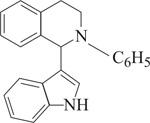 A new type of selective C-C bond-forming reaction has been devised in which C-H bonds of two molecules cross-couple together in the simplest way possible to construct complex organic molecules of pharmañeutical interest. The"crossdehydrogenative coupling
(CDC) reaction, developed by Zhiping Li and Chao-Jun Li of McGill University, in Montreal, eliminates the need to first functionalize molecules before they can be coupled together and avoids the need for secondary elimination or hydrogenation reactions. Overall, the strategy makes synthetic schemes shorter and more efficient, the researchers note. They recently reported CDC reactions between sp3 and sp carbon atoms and between sp3 and sp3 carbon atoms, and now report examples of sp3-sp2 coupling (J. Am. Chem. Soc. 2005, 127, 6968). In the indolyl tetrahydroisoquinoline derivative shown, the red bond comes from a CDC reaction between the sp3 C-H bond of a quinoline and an aryl sp2 C-H bond of an indole. CDC reactions are carried out under mild conditions and are catalyzed by relatively inexpensive copper bromide.
A new type of selective C-C bond-forming reaction has been devised in which C-H bonds of two molecules cross-couple together in the simplest way possible to construct complex organic molecules of pharmañeutical interest. The"crossdehydrogenative coupling
(CDC) reaction, developed by Zhiping Li and Chao-Jun Li of McGill University, in Montreal, eliminates the need to first functionalize molecules before they can be coupled together and avoids the need for secondary elimination or hydrogenation reactions. Overall, the strategy makes synthetic schemes shorter and more efficient, the researchers note. They recently reported CDC reactions between sp3 and sp carbon atoms and between sp3 and sp3 carbon atoms, and now report examples of sp3-sp2 coupling (J. Am. Chem. Soc. 2005, 127, 6968). In the indolyl tetrahydroisoquinoline derivative shown, the red bond comes from a CDC reaction between the sp3 C-H bond of a quinoline and an aryl sp2 C-H bond of an indole. CDC reactions are carried out under mild conditions and are catalyzed by relatively inexpensive copper bromide.
HTTP://WWW.CEN-ONLINE.ORG
Ñ & EN / MAY 23, 2005
Oxaziridines catalyze C-H oxidation selectively
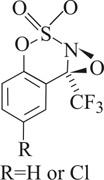 The oxaziridines shown catalyze the regioselective hydroxylation of unactivated C-H bonds, according to a new study (J. Am. Chem. Soc. 2005, 127, 15391). Alkane hydroxylation remains a formidable challenge in reaction design, write Justin Du Bois and Benjamin H. Brodsky of Stanford University. Certain oxaziridines are known to convert alkanes to alcohols stoichiometrically. Brodsky and Du Bois now report novel N-alkoxysulfonyl oxaziridines that can be used to perform such hydroxylations catalytically. The oxaziridines were generated in situ by treating the corresponding imine precursors with hydrogen peroxide and a suitable cocatalyst. The researchers find that the oxaziridine system performs regioselective and stereospecific oxidations of a variety of aliphatic substrates, including alkenes and alcohols. Guided by computational modeling, they are now refining the catalyst to improve turnover rate and substrate scope.
The oxaziridines shown catalyze the regioselective hydroxylation of unactivated C-H bonds, according to a new study (J. Am. Chem. Soc. 2005, 127, 15391). Alkane hydroxylation remains a formidable challenge in reaction design, write Justin Du Bois and Benjamin H. Brodsky of Stanford University. Certain oxaziridines are known to convert alkanes to alcohols stoichiometrically. Brodsky and Du Bois now report novel N-alkoxysulfonyl oxaziridines that can be used to perform such hydroxylations catalytically. The oxaziridines were generated in situ by treating the corresponding imine precursors with hydrogen peroxide and a suitable cocatalyst. The researchers find that the oxaziridine system performs regioselective and stereospecific oxidations of a variety of aliphatic substrates, including alkenes and alcohols. Guided by computational modeling, they are now refining the catalyst to improve turnover rate and substrate scope.
HTTP://WWW.CEN-ONLINE.ORG
Ñ & EN / OCTOBER 31, 2005
Chemical steps to biomass fuel
Integrated biorefineries have been proposed as a way to make gasoline, diesel fuel, and chemical feedstocks directly from carbohydrates such as sugars, starch, and cellulose derived from dedicated crops, agricultural waste, and urban yard debris. Chemical engineers George W. Huber, James A. Dumesic, and coworkers at the University of Wisconsin, Madison, who have been developing lab-scale chemical processes that could be important in biorefineries, now report chemistry to produce C7 to C15 alkanes from biomass (Science 2005, 308,1446). The team designed a multistep aqueous-phase process that begins with carbonyl-containing intermediates, such as hydroxymethylfurfural, that can be made from glucose. The intermediates first undergo an aldol cross-condensation with acetone or an aldol self-condensation using a solid base catalyst to make larger compounds, followed by a dehydration-hydrogenation process that uses a bifunctional metal-acid catalyst to form the linear alkanes. The alkanes can be phase-separated from the water. Biorefinery production of alkanes to make sulfur-free gasoline and diesel could be more energy-efficient and less costly than producing ethanol or hydrogen from biomass.
HTTP://WWW.CEN-ONLINE.ORG
Ñ & EN / JUNE 6, 2005
Engelhard buys catalyst line
Engelhard has expanded its catalysts business with the acquisition of the synthesis gas catalyst unit of Nanjing Chemical Industry, a subsidiary of Sinopec. Though Engelhard won't say how much it paid for the business, a spokesman says the purchase includes manufactoring and R&D facilities in Nanjing that employ more than 600 people. Engelhard manufactures the cobalt catalyst for Sasol's Fischer-Tropsch slurry bed reactor, used to convert natural gas to synthetic diesel and petrochemical feedstock. The firm says the Nanjing Chemical business will extend its gas-to-liquids catalyst operation into the market for syngas used to make ammonia, hydrogen, methanol, and other products.
HTTP://WWW.CEN-ONLINE.ORG
Ñ & EN / JUNE 6, 2005
Nanocluster catalyst lives longer
Chinese chemists report a rhodium nanocluster catalyst that demonstrates "unprecedented " lifetime and activity in benzene hydrogenation under forcing conditions (J. Am. Chem. Soc. 2005, 127, 9694). The rhodium nanoclusters, which tend to coalesce into bulk metal on their own, are stabilized by the novel combination of a pyrrolidone-substituted, ionic-liquidlike copolymer dissolved in an imidazolium ionic liquid. The total turnovers for the catalyst - a measure of catalytic lifetime - exceeded 20,000 over five runs, which is more than five times the previous record for benzene hydrogenation by a nanocluster catalyst. Yuan Kou and coworkers at Peking University suggest that the high stability and activity of the rhodium catalyst are due to the combined stabilizing influences of the ionic liquid and the pyrrolidone-substituted copolymer. The stabilized rhodium nanoclusters, each roughly 3 nm across, were synthesized by hydrogenation of a mixture of RhCl3. 3H2O and the copolymer dissolved in the ionic liquid.
HTTP://WWW.CEN-ONLINE.ORG
Ñ & EN / JUNE 27, 2005
Catalyst caught on a tape
Chemist regard tape made of DuPont's Teflon as an indispensable sealer for lab equipment, but according to researchers in Germany, Teflon tape may be just as useful inside a reaction flack. John A. Gladysz and Long V. Dinh of the University of Erlangen-Nuremberg have discovered that Teflon tape is surprisingly effective at introducing and recovering homogeneous fluorous catalysts from a reaction mixture (Angew. Chem. Int. Ed. 2005, 44, 4095). Thermomorphic fluorous catalysts dissolve in organic solvents only at elevated temperatures, so chemists usually have to heat their reaction mixtures to get the catalyst into solution and then cool and decant the mixture to recover the catalyst. Gladysz and Dinh found that if they added Teflon tape to a ketone hydrosilylation reaction featuring a fluorous rhodium catalyst, the reaction could proceed with much less catalyst. Upon cooling, the catalyst clung to the tape so that the chemists could fish it out of the mixture. The researchers speculate that their findings could lead to industrial-scale reactors or reactor components that use Teflon to release and recapture certain fluorous catalysts.
HTTP://WWW.CEN-ONLINE.ORG
Ñ & EN / JUNE 27, 2005
Enzyme mimic raises questions
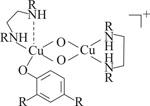 A model complex that mimics the active site of tyrosinase hydroxylates phenol substrates by a mechanism different from the one the enzyme is thought to use, according to a new study (Science 2005, 308, 1980). Liviu M. Mirica, Daniel Stack, and coworkers at Stanford University say their findings raise the possibility that tyrosinase may use an alternative mechanism, too. Tyrosinase - which plays a key role in melanin formation - relies on a pair of copper ions to activate O2, which then hydroxylates a CH group of a phenol. The CuO2Cu active species has long been assumed to contain an intact O2 molecule, with each oxygen atom bound to both Cu(II) ions. In the model complex, however, hydroxylation is performed by a bis(oxo)dicopper(III) species (Shown, R=tert-butyl) formed then the substrate binds. The O-O bond has already been broken in this species, which has detected spectroscopically at -120 oC and confirmed computationally. It remains to be seen whether tyrosinase uses such a species, Stack notes.
A model complex that mimics the active site of tyrosinase hydroxylates phenol substrates by a mechanism different from the one the enzyme is thought to use, according to a new study (Science 2005, 308, 1980). Liviu M. Mirica, Daniel Stack, and coworkers at Stanford University say their findings raise the possibility that tyrosinase may use an alternative mechanism, too. Tyrosinase - which plays a key role in melanin formation - relies on a pair of copper ions to activate O2, which then hydroxylates a CH group of a phenol. The CuO2Cu active species has long been assumed to contain an intact O2 molecule, with each oxygen atom bound to both Cu(II) ions. In the model complex, however, hydroxylation is performed by a bis(oxo)dicopper(III) species (Shown, R=tert-butyl) formed then the substrate binds. The O-O bond has already been broken in this species, which has detected spectroscopically at -120 oC and confirmed computationally. It remains to be seen whether tyrosinase uses such a species, Stack notes.
HTTP://WWW.CEN-ONLINE.ORG
Ñ & EN / JUNE 27, 2005


