22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы

Всероссийская научная конференция
«Пятый Байкальский материаловедческий форум» БМФ-2025
6-10 июля 2025 г.
побережье оз. Байкал в с. Горячинск
https://www.binm.ru/conf/2025_BMF5/
Основной организатор БМФ-2025 – одной из крупнейших материаловедческих конференций не только Сибирского региона, но и России – Байкальский институт природопользования СО РАН (г. Улан-Удэ), соорганизаторы – Бурятский государственный университет им. Доржи Банзарова (г. Улан-Удэ), Московский государственный университет им. М.В. Ломоносова (г. Москва), Федеральный исследовательский центр «Институт катализа имени Г.К. Борескова СО РАН» (г. Новосибирск), Уфимский университет науки и технологий (г. Уфа), а также Сибирское отделение РАН (г. Новосибирск).
Личное участие в работе конференции приняли более 350 человек, в том числе более 270 иногородних участников. Это представители науки, образования, производства из крупнейших научных центров европейской части России, Урала, Сибири и Дальнего Востока (Апатитов, Астрахани, Белгорода, Владивостока, Волгограда, Дубны, Екатеринбурга, Зеленограда, Иркутска, Казани, Кирова, Красноярска, Москвы, Нижнего Новгорода, Новосибирска, Оренбурга, Перми, Самары, Санкт-Петербурга, Саратова Снежинска, Солнечногорска, Сыктывкара, Ростова-на-Дону, Томска, Тюмени, Улан-Удэ, Уфы, Челябинска, Черноголовки, Якутска). Наиболее представительные делегации приехали из Москвы (85 человек), Новосибирска, Санкт-Петербурга, Екатеринбурга, Уфы (по 20–35 человек). Среди пленарных докладчиков – академики РАН Антипов Е.В (Москва), Бухтияров В.И. (Новосибирск), Гнеденков С.В. (Владивосток), Тананаев И.Г. (Апатиты), Ярославцев А.Б. (Москва), член-корреспондент РАН Синебрюхов С.Л. (Владивосток). С пленарными, ключевыми и секционными докладами на Форуме выступили около 65 профессоров и докторов наук. Более трети участников – молодые ученые (до 35 лет), аспиранты и студенты.
Цели Форума – осуществить анализ состояния научных исследований в области материаловедения и наметить стратегические направления их развития, способствовать преемственности поколений ученых-материаловедов и интеграции высшего образования, фундаментальной и прикладной науки. Его основные задачи – обсудить фундаментальные и прикладные проблемы материаловедения, представить последние достижения в данной области, выявить наиболее перспективные работы, в том числе, проводимые молодыми учеными, способствовать творческому общению специалистов и ученых, углублению связей между учеными Сибирского региона и центральной России, стимулировать распространение научных знаний и повышение престижа науки. Одна из ключевых проблем, обсуждаемых на Форуме – обеспечение технологической и экономической независимости России, пути решения вопросов импортозамещения.

Форум ставит перед собой амбициозные задачи: анализ современных тенденций в материаловедении, определение стратегических направлений развития отрасли, укрепление связей между наукой и производством. Особый акцент сделан на вопросах технологического прорыва, необходимого для обеспечения экономической независимости России.
Было сделано 11 пленарных, 27 ключевых, 4 спонсорских, более 90 секционных и около 80 постерных докладов. 88 докладов сделано в рамках конкурсной программы докладов студентов, аспирантов и молодых ученых. На пленарном заседании, посвященном подведению итогов работы конференции, всеми выступающими подчеркивались высокий уровень Форума, достижение его целей и необходимость проведения БМФ-2028.
Источник: Сайт Сибирского отделения Российской академии наук
https://www.sbras.ru/ru/news/53795
VII Всероссийская школа-конференция по катализу с международным участием
«Каталитический дизайн: от исследований на молекулярном уровне к практической реализации»
18-24 августа 2025 г.
г. Новосибирск, Россия
https://catdesign-7.tilda.ws
Институт катализа СО РАН в седьмой раз проводит Всероссийскую школу-конференцию с международным участием «Каталитический дизайн: от исследований на молекулярном уровне к практической реализации». Участники отметили роль Института в развитии каталитического дизайна и важность подготовки кадров.
Мероприятие проводится в первую очередь для студентов и молодых ученых, чтобы познакомить их с современными подходами к созданию и исследованию катализаторов как с фундаментальной, так и с прикладной точки зрения. Лекции от ведущих специалистов охватывают тематику от исследований катализаторов на атомно-молекулярном уровне и основ их приготовления до моделирования новых систем и решений задач защиты окружающей среды.
География в этом году включает Барнаул, Красноярск, Новосибирск, наукоград Кольцово, Москву, Санкт-Петербург, Омск, Тверь, Томск, Тулу, Уфу. На школе выступит гость из Мексики – профессор Национального политехнического института Хорхе Анчейта, который давно сотрудничает с учеными Института катализа.
От Борескова к кадрам для «СКИФ»
Директор ФИЦ «ИК СО РАН», академик РАН Валерий Бухтияров отметил, что базу для каталитического дизайна заложил еще директор-организатор института Георгий Боресков: «Когда создавался институт, Георгий Константинович Боресков отстаивал идею, что катализ – это в первую очередь прикладная наука, и время показало, что он был абсолютно прав. Изучение именно актов каталитического взаимодействия на молекулярном уровне позволило открыть структуру активных центров сначала в гомогенных, а затем в гетерогенных катализаторах. Создание катализаторов происходит гораздо более эффективно, если мы много знаем о процессе на атомно-молекулярном уровне».

По мнению руководителя Отдела физико-химических исследований на атомно-молекулярном уровне ИК СО РАН, члена-корреспондента РАН Олега Мартьянова, в основу конференции заложены идеи, которые были формулированы при создании Института катализа – это комплексный подход к изучению проблем катализа.
«Нужно использовать не один, пусть даже уникальный физический метод, а комплекс методов, которые бы дополняли друг друга на разных пространственных и временных масштабах. Боресков большое внимание уделял исследованию механизма каталитических реакций и уже в 1960-х годах, следуя идее о взаимном влиянии катализатора и реакционной среды, реализовал экспериментальные исследования в режиме «in situ», т.е. исследования непосредственно в ходе проведения каталитического процесса. Кстати, одна из первых лабораторий института как раз называлась "Лаборатория физических методов исследования", – рассказал он.

Ученый секретарь ИК СО РАН, к.х.н. Юрий Дубинин подчеркнул, что событие имеет большое значение для молодых специалистов:
«Конференция изначально была нацелена на молодежь, и, в принципе, все доклады на ней делают молодые ученые. Для них это в первую очередь возможность рассказать о своей работе в комфортной среде и узнать об исследованиях коллег. На мероприятии выступают эксперты, которые подают информацию интересно и понятно. А еще участие в школе помогает расширить кругозор».

По словам главного научного сотрудника Центра коллективного пользования «СКИФ» д.ф.-м.н. Яна Зубавичуса, конференция актуальна в плане подготовки кадров для центра, который начнет свою работу в скором времени.
«Эта конференция – хорошая традиция ИК СО РАН. Популяризация каталитической науки среди молодежи, особенно в контексте скорого ввода в эксплуатацию «СКИФ», и подготовка кадров – одно из магистральных направлений. Нужно информировать, искать новых пользователей, придумывать яркие интересные задачи. Счет будущих пользователей для ЦКП идет на тысячу, а в области катализа понадобится несколько сотен специалистов».
Источник: Сайт Института катализа СО РАН
https://catalysis.ru/news/detail.php?ID=41541
Развитие концепции об «активных центрах в катализе»: Мишель Будар
чл.-корр. РАН, проф. В.А. Лихолобов
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Как отмечалось в предыдущем материале “К 100-летию введения концепции «активных центрах в катализе»”, значительный шаг в подходах к изучению явления катализа произошел в 1925 году, когда Хью Стотт Тейлор в своей статье [H. S. Taylor/ A Theory of the Catalytic Surface/ Proc. R. Soc. Lond. A (1925) 108, 105-111; doi: 10.1098/rspa.1925.0061] аргументированно выдвинул модель каталитически активных поверхностей, основанную на их неоднородности (дефектности) и наличии на них участков, сложенных атомами с уменьшенным числом «соседей», с высокой реакционной способностью и динамичностью, и поэтому обладающих повышенной каталитической активностью в осуществлении химических реакций (рис. 1).
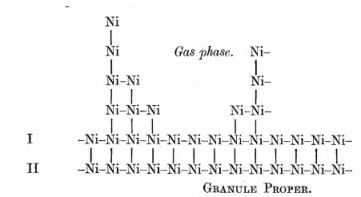
Рисунок 1
(является рис. 1 в выше отмеченной статье Х.С. Тэйлора):
Вид поверхности (взгляд вдоль поверхности) частицы
металлического никеля («черни») с «активными центрами»,
образованными атомами Ni с уменьшенным числом «соседей»;
динамичными являются атомы с минимальным числом соседей.
Эта модель Х. Тэйлора сильно отличалась от предложенной ранее И. Лэнгмюром в 1918 году “шахматной модели” [I. Langmuir/ The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum/ Journal of the American Chemical Society (1918), 40(9), 1361–1403; doi:10.1021/ja02242a004] поверхностей с равномерным распределением статичных участков, образованных одинаковыми, или отличающимися по химической природе, атомами, занимающих позиции, диктуемые кристаллической решёткой твёрдого тела (рис. 2).

Рисунок 2
(является рис. 1 в выше отмеченной статье И. Лэнгмюра):
Вид поверхности (взгляд перпендикулярно к поверхности)
кристаллита («чешуйки») слюды (mica – алюмосиликат мусковит)
с регулярным, формируемым находящимися в объёме
кристаллита катионами Al и Si, расположением на ней
атомов кислорода и водорода (от ОН-групп).
Эти атомы формируют поверхность таким образом,
что возникающие элементарные пространства (углубления)
между атомами окружены разной комбинацией атомов кислорода и водорода,
определяющей адсорбционные и каталитические свойства этой поверхности.
С одной стороны, точка зрения Х.С. Тейлора заинтересовала многих ученых и исследователей, но с другой – подверглась и критике, особенно в части термина «активированной диссоциативной адсорбции на дефектах (активных центрах) поверхности». Интересно, что в этом плане И. Лэнгмюр, с которым Х. Тэйлор был знаком ещё с 1913 года в период своей работы у М. Боденштейна в г. Ганновере, занимал в возникающих дискуссиях позицию «наблюдателя», переключив всё своё внимание на работы «по физике и химии молекул на поверхности раздела фаз» (за что он получил в 1932 г. Нобелевскую премию). Этой части истории о сопоставлениях «плоско-однородной» и «неоднородно-дефектной» моделей каталитической поверхности Х.С. Тэйлор коснулся в своих докладах 1940-го года [National Research Council. 1940. Twelfth Report of the Committee on Catalysis, National Research Council/ Chapter III: Activated Adsorption (by H.S. Taylor), p. 28-39; Chapter IV: Active centers (by H.S. Taylor), p. 40-51; Washington, DC: The National Academies Press. https://doi.org/10.17226/21586], в которых он проводил мысль, что, по-видимому, эти модели относятся к разным предельным «видам» поверхности катализатора: первого «вида» – к поверхности, сложенной протяжёнными плоскостями (характерной для монокристаллов, слюд, металлической фольги и т.п.), а второго «вида» – к поверхности частиц очень мелких размеров (характерной, например, для частиц металлических черней, коллоидов и т.п.), имеющих пористость. Интересно, что приблизительно об этом же и в том же 1940-м году проводил свои рассуждения И. Лэнгмюр в обзоре [Langmuir, I. (1940). Monolayers on solids. Journal of the Chemical Society (Resumed), 511-543; doi:10.1039/jr9400000511], в заключительной части которого (стр. 541-543, где обсуждаются данные, полученные в работах Х.С. Тэйлора) в конце стр. 543 написано: «….при адсорбции на порошкообразных или пористых подложках вряд ли можно было бы ожидать найти условия, соответствующие тем, которые мы постулировали в нашей теории адсорбции на плоских решетках. Однако было обнаружено, что гиперболическая изотерма, первоначально полученная для плоских поверхностей, часто хорошо применима и к порошкообразным подложкам… и, поэтому… постулаты, лежащие в основе этой теории, никоим образом не противоречат активированной адсорбции. На самом деле, именно в случае активированной адсорбции, в отличие от ван-дер-ваальсовой адсорбции, мы должны обнаружить сильные силы (strong forces), которые связывают атомы или молекулы с определенными местами поверхности».
Эти публичные дискуссии привели Х.С. Тэйлора к заключению о необходимости интенсификации исследований о влиянии процессов измельчения, т.е. диспергирования, металлических частиц на их каталитические свойства. Проведению таких исследований способствовало то, что к 1930 году уже стало возможным экспериментально различать типы взаимодействий между молекулами в газовой фазе и поверхностью катализатора, различать во многих случаях неспецифическую, физическую адсорбцию газов (которая, как считалось, имеет малое значение в явлении катализа) и, с другой стороны – хемосорбцию, требующую для образования адсорбированного комплекса преодоление активационного барьера. Кроме того, метод оценки общей площади поверхности твёрдых материалов, который появился в 1938 году в результате исследований С. Брюнауэра, П. Эммета и Е. Теллера [Brunauer, S., Emmett, P.H., & Teller, E. (1938). Adsorption of Gases in Multimolecular Layers. Journal of the American Chemical Society, 60(2), 309–319. doi:10.1021/ja01269a023] по физической адсорбции таких газов как азот и бутан, при температурах, близких к их соответствующим точкам кипения, предоставил очень мощный инструмент, с помощью которого можно было пересмотреть всю проблему «активных центров» или неоднородности поверхности катализаторов. Интересно отметить, что Paul H. Emmet был в «химическом дереве» Х.С. Тэйлора его «внуком по науке», поскольку подготовил свою диссертационную работу в 1925 году под руководством Artur F. Benton, бывшего в своё время аспирантом Х.С. Тэйлора, защитившего диссертацию в 1922 году и впоследствии занимавшегося вопросами адсорбции и растворения газов металлами, например [Benton, A. F. (1932). Adsorption and solution of gases by metals. Transactions of the Faraday Society, 28, 202. doi:10.1039/tf9322800202]. Однако, экспериментальную реализацию этой «задумки» Х.С. Тэйлор смог в полном объёме начать только через 7 лет, из-за вспыхнувшей Второй мировой войны и участия в исследованиях по задачам Манхэттенского проекта.
И вот, в 1946 году профессор Х.С. Тэйлор, являясь заведующим кафедрой химии Принстонского университета, начал формировать молодой исследовательский коллектив для изучения особенностей катализа высокодисперсными металлическими частицами. Следуя уже накопившемуся опыту, такая группа должна была состоять из 8-10 магистров, выбранных по конкурсу порядка 10-ти человек на место (опираясь на результаты экзаменационной сессии, собеседования с обсуждением собственноручно написанной «спецификации исследовательских способностей», а также внешних рекомендаций) с задачей выбрать претендентов, отличающихся умом, трудолюбием, настойчивостью и не имеющих при возникновении сложностей из-за дефицита знаний свойств “to grist for the mill” /«лить воду на мельницу» (по-видимому, играть на руку)/ экзаменатору [Hugh S. Taylor /Princeton University (Chemical education )/ Journal of Chemical Education, Volume 24, Issue 10, October 1947, p. 470-513; doi.org/10.1021/ed024p470]. Как результат, среди претендентов, прошедших этот конкурс, комиссией был особо выделен магистр из Лёвенского университета, Бельгия (University of Louvain) Мишель Будар (Michel Boudart ).
Став аспирантом у Х.С. Тэйлора, Мишель Будар инициативно и с творческим энтузиазмом (за что он был номинирован на стипендии Belgian American Educational Foundation Fellowship, 1948, и Procter Fellowship, 1949) выполнил поставленные ему задачи по сопоставлению каталитических свойств ряда металлов (медь, серебро, золото), взятых в виде фольг и коллоидов, в реакции H/D-обмена и разложения муравьиной кислоты до СО2 и Н2 и уже в 1950-м году получил степень PhD. В ходе своих ранних исследований М. Будар обнаружил ряд неизвестных ранее эффектов, объяснение которым он дал, базируясь на концепции «активных центров Тэйлора» и теории Л. Полинга «о катализе металлами» (о чём М. Будар специально написал краткое сообщение [M. Boudart /Pauling's Theory of Metals in Catalysis/ J. Am. Chem. Soc., 72, 1040 (1950)]).
Так, в работе [Mikovksy, R.J., Boudart, M., & Taylor, H.S. (1954). Hydrogen-Deuterium Exchange on Copper, Silver, Gold and Alloy Surfaces. Journal of the American Chemical Society, 76(14), 3814-3819; doi:10.1021/ja01643a067] было установлено, что гранулы частиц коллоидного серебра гораздо более активны в реакции изотопного H/D обмена, по сравнению с серебряной фольгой, и это отличие было связано с более низким значением энергии активации: таким образом этот результат повышения активности не мог быть объяснён более высокой поверхностью серебра в гранулах коллоидного серебра, но легко объяснялся предположением, что поверхность коллоидной частицы содержит атомы серебра «другой природы». Было также отмечено, что в случае медных катализаторов …энергия активации изучаемой реакции зависит от… «цвета металлической меди» (т.е., от цвета медного катализатора)… и что общий опыт приготовления катализатора показывает, что эффективный медный катализатор не должен выглядеть как медь (т.е. у такого катализатора атом меди должен быть «другой природы») … и если появляется характерный цвет меди… катализатор становится «мёртвым». Таким образом, в дисперсных медных катализаторах… "возбужденный" или "активный" атом меди напоминает никель и, таким образом, способен адсорбировать водород с достаточной силой (вышеуказанная работа, стр. 3818). Для понимания физико-химической природы такого "активного", похожего на никель, атома меди М. Будар в последующие годы осуществил постановку специальных исследований, которые были проведены уже его аспирантами. Так в работе [Benson, J. E., Walters, A. B., & Boudart, M. (1968). Activation of hydrogen at 79K by supported copper. The Journal of Physical Chemistry, 72(13), 4587–4589. doi:10.1021/j100859a037] было обнаружено, что катализатор, содержащий 5 моль.% меди на оксиде магния, активирует молекулярный водород уже при температуре 79 K, о чем свидетельствует его способность катализировать обмен H2-D2 при этой температуре со скоростью, отнесённой на атом поверхности металла, сравнимой с таковой, измеренной на металлическом никеле. Для объяснения появления у композиции Cu/MgO такой аномальной активности атомов меди («ведущих себя как атомы Ni») была выдвинута модель, согласно которой в композиции Cu/MgO у расположенных на поверхности MgO частиц меди появляются атомы, соседствующие с ионами кислорода носителя, из-за чего у таких атомов может измениться электронное состояние в сторону электронного состояния атома Ni (P.S.: можно отметить, что катион Cu+ является электронным аналогом атома Ni0: на последнем электронном слое находится такое же количество электронов – десять, как и у атома Ni (но в несколько отличающихся конфигурациях: 3d10 у Cu+ и 3d84s2 у Ni0), образуя с электронно-аналогичными молекулами-лигандами СО и CN– структурно-идентичные тетраэдрические комплексы Ni(CO)4 и Cu(CN)43–, соответственно). В последующем эти работы были развиты М. Бударом в направлении изучения каталитических свойств Ni-Cu-сплавов [например: E. Iglesia and M. Boudart, Decomposition of Formic Acid on Copper, Nickel, and Copper-Nickel Alloys. I. Preparation and Characterization of Catalysts, J. Catal., 81, 204-213 (1983); E. Iglesia and M. Boudart, Decomposition of Formic Acid on Copper, Nickel, and Copper-Nickel Alloys. II. Catalytic and Temperature-Programmed Desorption of Formic Acid on Cu/SiO2, Cu/Al2O3, and Cu Powder, J. Catal., 81, 214-223 (1983); E. Iglesia and M. Boudart, Decomposition of Formic Acid on Copper, Nickel, and Copper-Nickel Alloys. III. Catalytic Decomposition on Nickel and Copper-Nickel Alloys, J. Catal., 81, 224-238 (1983); E. Iglesia and M. Boudart, Decomposition of Formic Acid on Copper, Nickel, and Copper-Nickel Alloys. IV. Temperature-Programmed Decomposition of Bulk Nickel Formate and of Formic Acid Preadsorbed on Nickel Powder, J. Catal., 88, 325-332 (1984)] и, по аналогии, ряда других сплавов, в том числе включая железо, палладий, платину и золото [например: R. Chambers, M. Boudart/ Selectivity of gold for hydrogenation and dehydrogenation of cyclohexene/ Journal of Catalysis, 5(3), 517–528 (1966); F. L. Williams, M. Boudart/ Surface Composition of Nickel-Gold Alloys/ J. Catal., 30, 438 (1973); E. L. Kugler, M. Boudart/ Ligand and Ensemble Effects in the Adsorption of Carbon Monoxide on Supported Palladium-Gold Alloys/ J. Catal., 59, 201-210 (1979)] для понимания роли электронных и геометрических факторов в функционировании каталитически активных центров. Интересно, что в это же время такие же идейно-аналогичные исследования проводились в Англии Ч. Кемболлом (Charles Kemball), будущим министром Королевского общества Эдинбурга и президентом Королевского Института химии, стажировавшегося в рамках программы Commonwealth Fund Scholarship в период 1946–1947 г.г. в Принстоне у Х.С. Тэйлора и продолжившего в Англии (уже в составе своего коллектива) начатую у него тематику работ по исследованию Н/D обмена с участием углеводородов на металлических катализаторах [Wyn Roberts, M. (2000). Charles Kemball, C.B.E. 27 March 1923 – 4 September 1998. Biographical Memoirs of Fellows of the Royal Society, 46, 285–298. doi:10.1098/rsbm.1999.0085]. Нужно отметить, что возникшее таким образом «конкурентное» научное соревнование Х. Тэйлора и Ч. Кэмболла в области катализа металлами и их сплавами было взаимно полезным, привело к появлению совместных публикаций (например: Kemball, C., & Taylor, H. S. (1948). The Catalytic Decomposition of Ethane and Ethane—Hydrogen Mixtures. Journal of the American Chemical Society, 70(1), 345–351. doi:10.1021/ja01181a108) и, что более важно, к формированию уже между их учениками (М. Бударом – от Х. Тэйлора и Дж. Андерсоном (Jonh Robert Anderson – в будущем, всемирно известный исследователь в области процессов взаимодействия «газ-твёрдая поверхность» и фундаментальных основ гетерогенного катализа на металлах) – от Ч. Кэмболла) проектов совместных исследований (Exchange Projects) в этой области. Наиболее ярким результатом плодотворности научного сотрудничества М. Будара и Дж.Андерсона явилось их со-редактирование 11-ти выпусков издания «Catalysis: Science and Technology», Springer, Berlin, вышедших в период 1981-1996 г.г. [www.science.org.au/fellowship/fellows/biographical-memoirs/john-robert-anderson-1928-2007]. Отметим, что Дж.Р. Андерсон хорошо известен российским учёным благодаря его монографии «Structure of metallic Catalysts», переведённой на русский язык под редакцией Г.К. Борескова [Дж. Андерсон: «Структура металлических катализаторов», Изд-во «Мир», Москва, 1978 г.].
После ухода в 1958 году Х.С. Тэйлора на пенсию М. Будар оставался в Принстоне до 1961 года, а затем его научная карьера продолжилась сначала в Калифорнийском, а затем в Стэнфордском университетах. Открывшиеся для него огромные возможности подбора молодых кадров и налаживания кооперации с другими университетами и ведущими учёными в области его научных интересов позволили ему активно проводить свои исследования по различным направлениям. Эта активная «научная жизнь» очень хорошо описана в воспоминаниях эго многочисленных учеников и коллег: ряд из этих воспоминаний [Ricardo B. Levy, Jim Dumesic, James A. Cusumano, and Enrique Iglesia/ "Michel Boudart"/ Biographical Memoirs of the National Academy of Sciences (2017): www.nasonline.org/wp-content/uploads/2024/06/boudart-michel.pdf; Ricardo B. Levy/ The extraordinary life of Michel Boudart: A very personal perspective/ Journal of Catalysis, 404, (2021), 987; doi.org/10.1016/j.jcat.2021.01.008; Gerhard Ertl/ Catalysis is a kinetic phenomenon: The legacy of Michel Boudart/ Journal of Catalysis, 404, (2021), 985; doi.org/10.1016/j.jcat.2021.01.009], наряду с другими, ссылки на которые даны по ходу текста, были использованы для написания нижеследующей части этой заметки.
БУДАР (Boudart), Мишель (Michel)
(18 июня 1924 года – 2 мая 2012 года)
 Мишель Будар родился в г. Брюсселе (Бельгия). Отец Мишеля, Франсуаз Будар, был известным бельгийским химиком-промыш-ленником, одним из первых руководителей UCB (Union Chimique Belge, Brussels, Belgium), и поэтому неудивительно, что Мишель увлекся химией и после окончания средней школы в 1940 г. поступил на химический факультет Лёвенского университета (University of Louvain, UCLouvain). Так как во время Второй мировой войны Университет был закрыт, Мишель изучал предметы, и особенно усиленно химию, в частном порядке, в связи с чем, когда Университет вновь открылся в 1944 году, Мишель окончил его в рекордно короткие сроки, получив степень бакалавра в 1945 году и степень магистра в 1947 году. Хотя отец убеждал его завершить учебу и получить степень Ph.D. в Бельгии, Мишель принял решение продолжить обучение у Хью С. Тэйлора (Hugh S. Taylor) в Принстоне (США) в области катализа, (о ком он узнал из настольного учебника по катализу, написанного Х.С. Тэйлором и Е.К. Риделом в 1919 г. [Catalysis in theory and practice. By E. K. Rideal and H. S. Taylor. Pp. xv.+ 496. (London: Macmillan and Co., Ltds., 1919); https://onlinelibrary.wiley.com/doi/10.1002/jctb.5000382203].
Мишель Будар родился в г. Брюсселе (Бельгия). Отец Мишеля, Франсуаз Будар, был известным бельгийским химиком-промыш-ленником, одним из первых руководителей UCB (Union Chimique Belge, Brussels, Belgium), и поэтому неудивительно, что Мишель увлекся химией и после окончания средней школы в 1940 г. поступил на химический факультет Лёвенского университета (University of Louvain, UCLouvain). Так как во время Второй мировой войны Университет был закрыт, Мишель изучал предметы, и особенно усиленно химию, в частном порядке, в связи с чем, когда Университет вновь открылся в 1944 году, Мишель окончил его в рекордно короткие сроки, получив степень бакалавра в 1945 году и степень магистра в 1947 году. Хотя отец убеждал его завершить учебу и получить степень Ph.D. в Бельгии, Мишель принял решение продолжить обучение у Хью С. Тэйлора (Hugh S. Taylor) в Принстоне (США) в области катализа, (о ком он узнал из настольного учебника по катализу, написанного Х.С. Тэйлором и Е.К. Риделом в 1919 г. [Catalysis in theory and practice. By E. K. Rideal and H. S. Taylor. Pp. xv.+ 496. (London: Macmillan and Co., Ltds., 1919); https://onlinelibrary.wiley.com/doi/10.1002/jctb.5000382203].
Переехав в 1947 году в Принстон и став аспирантом Х.С. Тэйлора, М. Будар в 1950 году защитил степень Ph.D. и снова, несмотря на постоянное давление родителей вернуться в Бельгию, М. Будар решил построить жизнь в Соединенных Штатах и получил в Принстоне должность научного сотрудника в Центре Форрестол (Forrestal Center, научно-исследовательский центр им Дж. Форрестола Принстонского Университета (имеет отделения аэронавтики, космических исследований, технических наук, лабораторию физики плазмы и др.)), где в 1953 году был привлечён к выполнению научно-исследовательских работ физико-химического направления в рамках проекта SQUID (связанного с разработкой жидкостных ракетных двигателей по заказу Управления военно-морских исследований США [arc.aiaa.org/doi/abs/10.2514/8.7104?journalCode=jjp]). В 1954 году М. Будар получил должность ассистента, а в 1958 году – доцента и оставался в Принстоне до 1961 года. Вспоминая «принстонский» период своей жизни, М. Будар отмечал, что он «….не смог бы выбрать другую, чем в Принстоне, более стимулирующую и захватывающую среду для получения академического образования и формирования его научного кругозора и интеллектуальной жизни…». Особенно это было связано с тем, что в г. Принстоне располагался не только университет, но и Институт перспективных исследований (Institute for Advanced Study, IAS, основан в 1930 г.), который взял на работу многих учёных, бежавших из Европы от угрозы нацизма (именно тут работали после эмиграции в США такие выдающиеся учёные как Альберт Эйнштейн (теоретическая физика), Джон фон Нейман (математическая физика), Курт Гёдель (математическая логика) и др.), а также знаменитых учёных-химиков, «освободившихся» после завершения Манхэттенского проекта [www.amnh.org/exhibitions/einstein/peace-and-war/the-manhattan-project]. Так, ещё при работе в группе Х.С. Тэйлора М. Будар познакомился и в будущем начал сотрудничать с Джоном Фенном (Нобелевская премия по химии 2002 г.), Лайнусом Полингом (дважды лауреатом Нобелевской премии, одна из них, 1954 г., по химии) и Полом Эмметом (одним из создателей в будущем методологии БЭТ).
После Принстона с 1961 года карьера М. Будара продолжалась уже в должности профессора на химико-технологическом факультете Калифорнийского университета в Беркли и далее, с 1964 г. – на факультете химической инженерии Стэнфордского университета, где с 1975 по 1978 год он возглавлял кафедру химической технологии. Несмотря на эти перемещения, центральной темой его исследований были каталитические свойства металлов, особенно очень мелких металлических частиц. В связи с этим нужно отметить, что в рамках этой тематики М. Будар принимал участие в проектах программы сотрудничества США и СССР по катализу (The Framework of the US–USSR exchange program in chemical catalysis), и один из таких проектов выполнялся в сотрудничестве с Институтом катализа СО АН СССР, с лабораторией Ю.И. Ермакова, занимавшейся в то время разработкой методов получения «точных активных центров». Намеченная программа совместных исследований по этому направлению была успешно выполнена Б.Н. Кузнецовым, сотрудником Ю.И. Ермакова, в ходе его длительной научной стажировки в Cтэнфорд для работы в исследовательском коллективе М. Будара; результаты этого исследования опубликованы в достаточно высоко цитируемой статье [Kuznetsov B.N., Yermakov Y.I., Boudart M., Collman J.P./ The conversion of neopentane on supported catalysts Pt + W/SiO2 and Pt + Mo/SiO2 obtained through organometallic compounds of Pt, W and Mo/ Journal of Molecular Catalysis. 4: 49-57. DOI: 10.1016/0304-5102(78)85034-2]. Будучи профессором, М. Будар неизменно руководил на кафедре в Стэнфорде одной из самых крупных групп выпускников, в конечном итоге подготовив более 70 докторантов на получение степени Ph.D. и проконсультировав около 100 аспирантов и приглашенных ученых. О масштабе этой титанической преподавательской работы М.Будара можно судить по его «химическому дереву», вырезка из которого [academictree.org/chemistry/tree.php?pid=57282/ Chemistry Tree – Michel Boudart Family Tree] дана ниже.
 >
>
В 1974 году, после первого мирового нефтяного кризиса, М. Будар и двое его сотрудников (Джеймс А. Кузумано /James A. Cusumano/ и Рикардо Леви /Ricardo B. Levy/) основали компанию Catalytica Associates, Inc. в Санта-Кларе, штат Калифорния, которая занималась решением сложных каталитических задач для нефтехимических, химических и фармацевтических фирм, а также государственных учреждений, и одной из важнейших областей её деятельности было производство синтетического топлива. В последующие три десятилетия компания разрослась, создав несколько дочерних компаний (наиболее крупные из них Catalytica Energy Systems, Inc., Catalytica Pharmaceuticals, Inc.). Так, к 2000 году фармацевтическая дочерняя компания Catalytica Pharmaceuticals, Inc. стала крупнейшим поставщиком прекурсоров для фармацевтической промышленности в Северной Америке.
Нужно особо отметить, что Мишель Будар высоко ценил работы Г.К. Борескова в области катализа. Так, в вводном разделе своей статьи, в которой появляются термины TOF и TON [M. Boudart, A. Aldag, J.E. Benson, N.A. Dougharty, G. Girvin Yarkins/ On the specific activity of platinum catalysts/ J. Catal. 6, 92-99, 1966; doi.org/10.1016/0021-9517(66)90113-8], он написал: «…первые систематические исследования… (P.S.: проблемы нормирования каталитической активности)… были проведены Боресковым и его сотрудниками, которые смогли усовершенствовать методику измерения площади поверхности платиновых катализаторов посредством селективной хемосорбции водорода… и имея эту необходимую основу, им удалось показать, что величина удельной, т.е. отнесённой на единицу поверхности платины, активности платины в окислении диоксида серы и водорода изменялась всего менее чем на порядок величины для образцов, отличающихся по величине площади поверхности платины на четыре порядка (платина, нанесенная на силикагель, платиновые губка, проволока и сетка)...», и, далее: «…ввиду далеко идущего значения результатов Борескова с сотрудниками нами было решено расширить их работу, воспользовавшись экстремальным состоянием дисперсии платины на ή-оксиде алюминия, системе, которая не была изучена советскими исследователями, ... в реакции гидрирования (P.S.: гидрогенолиза) циклопропана в пропан … с привлечением большого разнообразия дополнительных исследований, включающих … методы электронной микроскопии, спектроскопии рассеяния света, уширения линий рентгеновской дифракции и др.». В заключении статьи было написано: «… мы обнаружили примечательное отсутствие чувствительности числа оборотов (P.S. число оборотов рассчитывалось на один находящийся на поверхности атом платины, исходя из экспериментально измеренного адсорбционными методами их количества 1,1×1015 см–2) к размеру кристалла, природе носителя или деталям приготовления… и это относительное постоянство удельной активности, наблюдаемое школой Борескова, а теперь и нами, не являлось ожидаемым.».
Мишль Будар встречался с Г.К. Боресковым во время работы IV Международного конгресса по катализу (Москва, 23-29 июня 1968 г.), где они активно принимали участие в дискуссиях об электронных, энергетических и структурных факторах в катализе (Proceedings of the Fourth International Congress on Catalysis, Moscow, USSR, 23-29 June, 1968; Akademiai Kiado, Budapest, 1971: v. I, p. 492; v. II, p. 311, 318), а также VIII Международного конгресса по катализу (Берлин-Зап., 2-6 июля 1984 г.), на котором Г.К. Боресков выступил с докладом о необходимости учёта при исследованиях каталитических реакций влияния реакционной среды на катализатор (VIII International Congress on Catalysis, Berlin (West), 2-6 July 1984; Verlag Chemie, Proceedings: v. III, p.231-242), а Мишель Будар – как reviewer of discussion-remarks (Proceedings: v. VI, p. vi: 03-010).
М. Будар в 1975 году работал вместе с Г.К. Боресковым в составе Editorial board for “Catalysis Reviews: Science and Engineering”, Volume 11, Issue 1, DOI: 10.1080/01614947508079979, а в 1990 году – с К.И. Замараевым в подготовке выпуска Faraday Discussions, 1991, 92, The Chemistry and Physics of Small Metallic Particles; Thomas J.M [Ed.] касательно раздела (GENERAL DISCUSSION) p.79-107; doi.org/10.1039/FD9919200079, в котором основное внимание было уделено дискуссиям о корректности использования кластеров металлов как модельных систем по отношению к высокодисперсным металлическим частицам и о возможных причинах изменения «структурной чувствительности» каталитический реакции в ходе её протекания.
В 1997 году М. Будар приезжал в Институт катализа для участия в работе Мемориальной конференции, приуроченной к 90-летию со дня рождения Г.К. Борескова (2-nd International Conference “Catalysis on the eve of the XXI Century. Science and Engineering”; July 7-11, 1997, Novosibirsk), на которой он выступил с пленарным докладом “Kinetic coupling within and between catalytic cycles” и оставил памятную запись (Материалы из архивов музейного отдела Института катализа СО РАН).

Мишель Будар представляет свою пленарную лекцию
в Большом зале Дома учёных СО РАН
Памятная запись Мишеля Будара:
| Мишель Будар Стенфордский университет, США Июль 1997 Боресков создал Институт, заслуженно названный его именем. В качестве Основателя он выбрал для Института катализа центры активности, в том числе связанные с исследованиями поверхности, органометалликой, материаловедением, кинетикой и разработкой химических реакций. Благодаря такому многогранному подходу Институт приобрел международную известность и влияние в период работы под руководством Борескова. Боресков скончался в 1984 году, сразу после Международного Конгресса по катализу (ICC) в Берлине, на котором он представил сообщение о реконструкции каталитических поверхностей в результате взаимодействия между этими поверхностями и реакционной средой. Это была основополагающая идея, впервые намеченная Боресковым в шестидесятые годы. Сегодня эта идея активно разрабатывается методами науки о поверхности. Преемник Борескова, Кирилл Замараев значительно расширил известность Института благодаря харизматическому влиянию его личности в международном масштабе. Он провел реконструкцию Института, необходимую для выживания в условиях шоковой перестройки общества. Благодаря этой реконструкции Институт оказался способен организовать Вторую Боресковскую конференцию. Замараев скончался перед самым открытием Международного Конгресса по катализу в Балтиморе. Его пленарную лекцию представил В.Н. Пармон, преемник Замараева на посту директора Института. Название доклада было “Фотокатализ: состояние науки и перспективы”. Замараев был поистине мультидисциплинарным провидцем. И Борескова, и Замараева будут долго помнить в связи с их научной работой, управленческими достижениями, обаянием личности, уникальным сочетанием достоинств. |
На протяжении более 40 лет Мишель Будар являлся всемирно признанным лидером в области фундаментальных основ гетерогенного катализа благодаря его существенному вкладу в концептуальное, молекулярное и количественное понимание химии каталитических явлений. Хотя изначально поставленная задача по «поиску и описанию активных центров Тэйлора» во многом определила эволюцию современной науки о катализе, сегодняшние успехи в этой области в большей степени уже основаны на положениях, которые Мишель Будар развивал на протяжении всей своей научной карьеры: а) о показателе «частота оборотов активного центра»; б) о градации каталитических реакций по типу активного центра на «структурно-чувствительные и структурно-нечувствительные»; в) о необходимости применения специальных микро-кинетических методов для получения данных об активности именно «активного центра», а не частицы катализатора в целом; г) о продуктивности рассмотрения ультрадисперсных полиметаллических частиц не в термине «сплавные», а как полиметаллические кластеры, применяя для описания их термодинамических и химических свойств такой показатель как «d-характер связей металл-металл, формирующих структуру кластеров». Эти развиваемые им положения он защищал от «размывания» их сути (blur the concept) и некорректных применений, публикуя для этого специально написанные статьи. Так, в работе [Boudart M. /Turnover Rates in Heterogeneous Catalysis/ Chemical Reviews, (1995), 95: 661-666. DOI: 10.1021/cr00035a009] в разделе Reasons for the Review он отмечал, что …сложность измерения TOF заключается не только в определении скорости, но и в подсчете активных участков… что первой задачей является их идентификация…и что их идентификация и подсчет в идеале должны проводиться во время каталитической реакции… В другой статье [Madon R.J, Boudart M./ Experimental criterion for the absence of artifacts in the measurement of rates of heterogeneous catalytic reactions/ Ind. Eng. Chem. Fundam. (1982), 438-447. DOI: 10.1021/I100008A022] на примере нанесённых металлических катализаторов были представлены «правила» измерения их каталитической активности, позволяющие получить её значения в отсутствие артефактов (связанных, главным образом, с влиянием процессов массо- и теплопереноса), что важно для последующего корректного вычисления TOF. В последующей работе [Boudart M./ Classical catalytic kinetics: A placebo or the real thing?/ Industrial and Engineering Chemistry Fundamentals. (1986), 25: 656-658. DOI: 10.1021/I100024A029] был критически проанализирован вопрос о приемлемости и наличии смысла применения уравнений классической кинетики для вычисления констант скоростей реакций, протекающих на катализаторах с явно неоднородной поверхностью; выводом явилось заключение, что …структурно-нечувствительные реакции подчиняются классической каталитической кинетике, она не является placebo (P.S. от латинского: вещество без явных лечебных свойств, используемое для имитации лекарственного средства: иногда капсулу или таблетку с плацебо называют «пустышкой») …и таким образом, для таких реакций использование классической каталитической кинетики не только полезно, но и правильно.
Другая серия работ Мишеля Будара была связана с вопросами применения фундаментальных знаний о физико-химических основах каталитических процессов для решения задач воплощения этих знаний в совершенствование и создание новых катализаторов. М. Будар и его ученики были первыми исследователями, которые экспериментально установили, что небольшие кластеры платины, нанесенные на цеолиты, находятся внутри цеолитовых суперклеток, и оценили их средний размер, который составил ~ 5 атомов на кластер, как сообщалось в статье [Dalla Betta, R.A. and M. Boudart. “Well-Dispersed Platinum on Y Zeolite: Preparation and Catalytic Activity”. Catalysis: Proceedings of the Fifth International Congress of Catalysis (Amsterdam: North-Holland) 1973, 1329–1341]. Было также показано, что эти кластеры платины сильно взаимодействуют с алюмо-кремний-оксидной структурой цеолита, что приводит к изменению их зарядового состояния, и к получению материала с новыми, важными для применения в практике каталитическими свойствами. Возможно, лучшим примером получения неожиданных результатов было открытие М. Бударом факта, что карбиды вольфрама проявляют свойства, подобные платине [R.B. Levy, M. Boudart/ Platinum-like behavior of tungsten carbide in surface catalysis. Science (1973), 181, 547-549; 10.1126/science.181.4099.547], которое привело к созданию широкого спектра нового класса каталитически активных материалов – карбидов, нитридов и карбонитридов металлов. Другие исследования в области биметаллических кластеров привели к созданию катализаторов процессов высоко-температурного гидрирования, исключающих нежелательный крекинг углеводородного сырья.
В серии работ, связанных с практическими вопросами разработки состава, способов получения и методов исследования гетерогенных катализаторов, можно выделить четыре обзора, написанных в период 1990-2000 г.г.: [Wrobleski J.T, Boudart M./ Preparation of solid catalysts: an appraisal/ Catalysis Today (1992), 15: 349-360. DOI: 10.1016/0920-5861(92)85002-4]; [Boudart M./ Heterogeneous molecular catalysis: Oxymoron or reality?/ Journal of Molecular Catalysis a: Chemical (1997), 120: 271-280. DOI: 10.1016/S1381-1169(96)00421-9]; [Boudart M./ Model catalysts: Reductionism for understanding/ Topics in Catalysis (2000), 13: 147-149. DOI: 10.1023/A:1009080821550]; [Boudart M./ From the century of the rate equation to the century of the rate constants: A revolution in catalytic kinetics and assisted catalyst design/ Catalysis Letters (2000), 65: 1-3. DOI: 10.1023/A:1019057002970] и выделяющихся наличием в их названиях слов-парафраз (т.е., слов, перевод смысла которых требует применения комбинации нескольких слов), подчёркивающих ключевое предназначение обзора: appraisal (от старофран. – apreisier – «соображения о ценности»); oxymoron (от греч. – «остроумная глупость»); reductionism (от лат. reductio – «разумное упрощение»); revolution (от лат. revolūtiō – «поворот, движение для начала нового движения»).
М. Будар являлся автором или соавтором более 280 журнальных статей и членом редколлегий по меньшей мере десятка журналов. Он был одним из главных редакторов серии из одиннадцати томов "Catalysis: Science and Technology". М. Будар был удостоен многочисленных наград, в том числе: премии Вильгельма (Wilhelm Award) в области разработки химических реакций от Американского института инженеров-химиков (1974), премии Кендалла (Kendall Award) (1977) и премии Мерфи (Murphee Award) (1985) от Американского химического общества, а также премии "Пионер химии" (Chemical Pioneer Award of the American Institute of Chemists) (1991); был избран членом Национальной академии наук и Национальной инженерной академии (the National Academy of Science and the National Academy of Engineering) (США), иностранным членом Королевской академии наук, литературы и изящных искусств Бельгии и ее Совета по прикладным наукам при Королевской Бельгийской академии (the Academia Royale des Sciences, des Lettres et des Beaux-Arts de Belgique and its Royal Belgian Academy Council for Applied Sciences). Будар получил почетные докторские степени от Льежского университета (the University of Liege), Университета Нотр-Дам (the University of Notre Damethe University of Ghent) и Национального политехнического института Лотарингии (the Institute National Polytechnique de Lorraine). Он также многократно был приглашенным профессором в университетах Лувена (Louvain), Рио-де-Жанейро, Токио и Парижа. В 1994 году он стал почетным профессором (professor emeritus) химической инженерии Стэнфордского университета.
Признавая заслуги этого выдающегося ученого, в 2006 году датская компания Haldor Topsøe выступила спонсором премии Мишеля Будара за достижения в области катализа (The Michel Boudart Award for the Advancement of Catalysis), которая присуждается совместным решением Европейской федерации каталитических обществ и Североамериканского каталитического общества (the European Federation of Catalysis Societies and the North American Catalysis Society) [efcats.org/Awards/Awards+ Directory/Michel+Boudart+Award.html%C2%A0].
Панфилова Алина Дмитриевна
«Высокодисперсный никель на пористом азотсодержащем углероде: синтез и каталитические свойства в реакции разложения газообразной муравьиной кислоты»
Защита
диссертации на соискание ученой степени
кандидата химических наук
по специальности
1.4.4 - Физическая химия
Место
защиты:
Институт неорганической химии
им.
А.В. Николаева Сибирского отделения
Российской академии наук , г. Новосибирск
9 октября 2025 г.
Козлов Даниил Андреевич
«Композитные материалы на основе оксида вольфрама и графитоподобного нитрида углерода для применения в фотокатализе»
Защита
диссертации на соискание ученой степени
кандидата химических наук
по специальности
1.4.15 - Химия твердого тела
Место
защиты:
Институт общей и неорганической химии
им. Н.С. Курнакова Российской академии
наук , г. Москва
9 октября 2025 г.
Уродкова Екатерина Константиновна
«Стабилизированные олигохитозанами нанодисперсии серебра для аналитических и биомедицинских приложений»
Защита
диссертации на соискание ученой степени
кандидата химических наук
по специальности
1.4.4 - Физическая химия
Место
защиты: Институт
физической химии и электрохимии
им.
А.Н. Фрумкина Российской академии наук ,
г. Москва
14 октября 2025 г.
Харитонов Владимир Борисович
«Инденильные комплексы родия: синтез и каталитическая активность»
Защита
диссертации на соискание ученой степени
кандидата
химических наук
по
специальности 1.4.8 - Химия
элементоорганических
соединений
Место
защиты: Институт
элементоорганических соединений
им.
А.Н. Несмеянова Российской академии
наук , г. Москва
14 октября 2025 г.
Бирюков Клим Олегович
«Влияние лигандов L- и X-типа на эффективность каталитических систем на основе 5d-металлов в реакциях восстановительного аминирования и циклоприсоединения СО2»
Защита
диссертации на соискание ученой степени
кандидата
химических наук
по
специальности 1.4.3 - Органическая химия
Место
защиты:
Институт
элементоорганических соединений
им.
А.Н. Несмеянова Российской академии
наук , г. Москва
22 октября 2025 г.
Захватова Наталья Владимировна
«Супрамолекулярные катализаторы радикального распада пероксидов на основе четвертичных аммониевых соединений»
Защита
диссертации на соискание ученой степени
кандидата
химических наук
по
специальности 1.4.4 - Физическая химия
Место
защиты: Федеральный
исследовательский центр
химической
физики
им. Н.Н. Семенова Российской
академии наук , г. Москва
28 октября 2025 г.
Бушков Николай Сергеевич
«Имидные комплексы титана и вольфрама, иммобилизованные на SiO2, как катализаторы оксо-имидного гетерометатезиса»
Защита
диссертации на соискание ученой степени
кандидата
химических наук
по
специальности 1.4.8 - Химия
элементоорганических
соединений
Место
защиты: Институт
элементоорганических соединений
им.
А.Н. Несмеянова Российской академии
наук , г. Москва
11 ноября 2025 г.
Курганский Владимир Иванович
«Селективная окислительная функционализация C=C и C–H групп в присутствии хиральных бис-амино-бис-пиридиновых комплексов Mn»
Защита
диссертации на соискание ученой степени
кандидата химических наук
по специальности
1.4.14. - Кинетика и катализ
Место
защиты: Институт органической химии
им. Н.Д. Зелинского Российской академии
наук , г. Москва
13 ноября 2025 г.
Ленгерт Екатерина Владимировна
«Альгинатные микроконтейнеры, модифицированные серебряными наночастицами: получение, свойства и применение»
Защита
диссертации на соискание ученой степени
кандидата
химических наук
по
специальности 1.4.4. - Физическая химия
Место защиты:
«Саратовский
национальный исследовательский
государственный университет имени
Н. Г. Чернышевского»,
г. Саратов
19 ноября 2025 г.
Гариева Гульназ Фаниловна
«Кристаллизация, физико-химические и адсорбционные свойства катионообменных форм цеолита LSX»
Защита
диссертации на соискание ученой степени
кандидата
химических наук
по
специальности 1.4.4. - Физическая химия
Место защиты: Уфимский федеральный исследовательский центр Российской академии наук , г. Уфа
19 ноября 2025 г.
Дмитрачков Алексей Михайлович
«Взаимодействие NO с оксидными носителями и нанесенными платиновыми катализаторами в ходе их приготовления и в условиях реакции нейтрализации оксидов азота»
Защита
диссертации на соискание ученой степени
кандидата
химических наук
по
специальности 1.4.14. - Кинетика и катализ
Место
защиты:
Федеральный исследовательский центр
«Институт катализа
им. Г.К. Борескова
Сибирского отделения Российской академии
наук , г. Новосибирск
4 декабря 2025 г.
Волков Михаил Александрович
«Новые аспекты координационной химии технеция в различных степенях окисления»
Защита
диссертации на соискание ученой степени
доктора химических наук
по специальности
1.4.13. – Радиохимия
Место
защиты: Институт
физической химии и электрохимии
им.
А.Н. Фрумкина Российской академии наук ,
г. Москва
More cross-coupling with less metal
Low-cost sodium salt may enable chemists to assemble molecules more efficiently and safely

This new cross-coupling method is compatible with the precursors
to Suzuki-Miyaura coupling and other popular molecule-building methods.
Cross-coupling has been one of the most popular types of reactions in organic chemistry for decades. The pharma and agrochemical industries use these methods on a large scale to forge bonds between aromatic rings. One downside of these classic reactions is that they require one of the coupling partners to be fitted with an organometallic group before the coupling step.
Methods that let chemists bypass that premetalation step in favor of directly stitching together two aryl halides have been gaining momentum. Such methods could shorten syntheses and cut waste. But they often still require a reductant such as zinc or manganese for the catalytic cycle to work, which can pose safety problems on scale-up.
“These are useful reactions at the discovery level, but they're a little challenging to deploy on scale” because metal reductants can plate onto reactors and cause a fire hazard, says Michael J. Krische of the University of Texas at Austin. “You don't want to do ton scale chemistry using elemental zinc or elemental manganese.” Krische and his team, along with collaborators at Genentech, the University of Minnesota Twin Cities, and the University of Pittsburgh, may have come up with a cost-effective solution to the problem.
They’ve unveiled a new coupling method that joins an aryl bromide with an aryl iodide using inexpensive sodium formate as a reductant, along with a commercially available palladium catalyst and an iodide source (Nat. Chem. 2025, DOI: 10.1038/s41557-024-01729-0).
Sodium formate is “so cheap it's even used for deicing runways at airports,” Krische says. His team previously deployed it to put a new spin on other classic reactions, such as the Grignard (J. Am. Chem Soc. 2019, DOI: 10.1021/jacs.8b13652) and Heck couplings (J. Am. Chem. Soc. 2023, DOI: 10.1021/jacs.3c09876) before setting their sights on cross-coupling.
The reaction can also handle coupling partners that don’t work as well with other cross-couplings, such as nitrogen-containing rings. And it works with anilines and aryl boronates, which can be used for subsequent Buchwald-Hartwig and Suzuki-Miyaura reactions, respectively.
Krische says the reaction works best on electron-rich aryl iodides and aryl bromides with an adjacent nitrogen or other heteroatom. The mechanism is also somewhat unusual: the catalyst is a negatively charged complex with two palladium atoms and four iodides. The palladiums cycle between the +1 and +2 oxidation states as they shuffle aryl groups and iodines between the metal centers until the aryl groups are next to each other and can bond together to make the cross-coupled product. “People had never really identified any unique reactivity associated with palladium(I). And this is unique,” Krische says.
Harvard University organic chemist Richard Y. Liu, who was not involved in the project, calls the study “very inspiring work” that “will certainly get a lot of attention from the community.” He adds that the ability to use this reaction in tandem with other coupling methods “really improves its potential for chemical discovery.”
Krische says he and his team will continue working to make the reaction as industry-friendly as possible. For example, they would love to figure out how to make it work with cheaper, greener nickel or cobalt catalysts.
Protein catalysts designed to do non-natural chemistry
Researchers couple AI and chemical know-how to create tools for cyclopropanation and silylation reactions
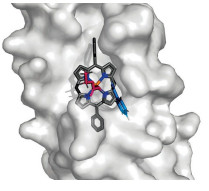 A protein catalyst’s synthetic porphyrin cofactor binds to styrene and a diazo compound, which will form a cyclopropane.
A protein catalyst’s synthetic porphyrin cofactor binds to styrene and a diazo compound, which will form a cyclopropane.
Enzymes are masters of making molecules. They accomplish exquisitely selective chemistry in water, and scientists have used the technique of directed evolution to modify existing enzymes to carry out chemical reactions not found in nature, like creating cyclopropanes and doing silicon-hydrogen bond insertions. Now, researchers have combined artificial intelligence (AI) and their own chemical intuition to create protein catalysts from scratch that can perform these same transformations (Science 2025, DOI: 10.1126/science.adt7268).
AI has become successful at designing proteins, and those who use it to predict and design protein structures have won Nobel Prizes. But chemical know-how is still important, says Yang Yang, a chemistry professor at the University of California, Santa Barbara, who led the project with the University of California, San Francisco’s William F. DeGrado and the University of Pittsburgh’s Peng Liu. “Protein design, even with the best AI-based methods, is not a solved problem. The most efficient way to generate effective designs for catalysis is perhaps to combine the AI-based method and also the in-house experience and knowledge of protein structure,” Yang says.
Compared with enzymes, protein catalysts are simpler, more stable at high temperatures, and can also be used in environmentally friendly organic solvents like ethanol, allowing chemists to load up on organic substrates. In this case, the researchers wanted to create protein catalysts that could do cyclopropanation reactions stereoselectively.
Their first attempts, which primarily relied on AI to design the protein catalysts, had decent stereoselectivity. To create catalysts that built cyclopropanes with an enantiomeric ratio of 99:1, they had to study the structure of their protein catalysts and make adjustments based on their chemical knowledge. The team similarly used directed evolution to improve upon AI-designed protein catalysts for Si–H insertion reactions.
“We think AI tools are definitely very powerful and very transformative. But we do need some additional input from either human expertise or additional chemistry simulation to further enhance the reliability of those AI predictions,” Liu says.
J. L. Ross Anderson, who studies de novo protein design at the University of Bristol and was not involved in the work, calls the project a “tour de force” in terms of combining strategies for creating protein catalysts. He says these new protein catalysts are able to perform particularly tough chemical transformations. “Not only are they challenging just by their very nature but also because of the stereospecificity that they're managing to tap into—it’s another layer of complexity in the design process,” Anderson says.
DeGrado says the new catalysts, which contain either synthetic porphyrin or heme cofactors, could be used instead of expensive metal catalysts to create drug candidates in a medicinal chemistry campaign or when making certain molecules on a large scale.
DeGrado wants other scientists to know that the tools the researchers used are easy to get up and running. “All of these things have gone from being difficult to very fairly simple,” he says. “To me, the impact isn't so much that this was such a difficult thing, but that it was a relatively simple thing that can translate very easily to new labs.”
Chemical & Engineering News
|
September
28-30, 2025 NINETEENTH Conference, Nanotechnology Based Innovation for the Environment, Energy, and Health Salerno, Italy |
https://www.aidic.it/nineteenth25/index.php |
|
29
сентября – 3 октября 2025 г.
VII семинар памяти профессора Ю.И. Ермакова «Гомогенные и закрепленные металлокомплексы в качестве катализаторов для процессов полимеризации, нефтехимии и тонкого органического синтеза» г. Казань, Республика Татарстан, Россия |
https://ermakov-2025.tilda.ws/ |
|
29
сентября – 3 октября 2025 г.
XI Международная научно-практическая конференция«Добыча, подготовка, транспорт нефти и газа» г. Томск, Россия |
http://ipc-petroleum.su/ |
|
29
сентября – 3 октября 2025 г.
Конференция по использованию рассеяния нейтронов в исследовании конденсированных сред (РНИКС-2025) г. Томск, Россия |
https://rniks2025.imp.uran.ru/ |
|
6-10
октября 2025 г. Международная конференция “Функциональные материалы” (ICFM’2025) г. Форос, Крым, Россия |
https://lomonosov-msu.ru/rus/event/9501/ |
|
6-10
октября 2025 г. VII Российская конференция (с международным участием) «Актуальные проблемы нефтехимии» г. Пятигорск, Россия |
http://conference.forenewchemistry.ras.ru/ |
|
7-9
октября 2025 г. XIV Международный российско-казахстанский симпозиум «Углехимия и экология Кузбасса» г. Кемерово, Россия |
http://www.iccms.sbras.ru/ccsymp-2025 |
|
October
12-15, 2025 3rd International Conference on Energy, Environment & Digital Transition (E2DT 2025) Palermo, Italy |
https://www.aidic.it/e2dt2025/ |
|
14-16
октября 2025 г. Конференция «Российское научное приборостроение: состояние и проблемы» г. Черноголовка, Московская область, Россия |
https://научныйприбор.рф/ |
|
20-24
октября 2025 г.
II Сибирский химический симпозиум (СХС-2025) г. Томск, Россия |
https://www.irkinstchem.ru/announcements/ii-sibirckiy-khimicheskiy-simpozium-skhs-2025 |
|
21-25
октября 2025 г.
Курчатовский форум «Исследования с применением синхротронного излучения, нейтронов и электронов» (Курчатов ФСНЭ 2025) г. Москва, Россия |
https://rsini.ru/ |
|
25-26
октября 2025 г.
XXVIII Международная экологическая студенческая конференция (МЭСК-2025) г. Новосибирск, Россия |
http://eco.nsu.ru |
|
27-31
октября 2025 г.
XXVI Международная конференция по химическим реакторам (ХимРеактор-26) г. Минск, Республика Беларусь |
https://chemreactor.org/ |
|
November
3-5, 2025 4th International Online Conference on Materials (IOCM2025) Switzerland |
https://sciforum.net/event/IOCM2025 |
|
17-21
ноября 2025 г. Международная конференция «Конгресс пользователей ЦКП СКИФ: перспективные исследования с использованием синхротронного излучения» г. Новосибирск, Россия |
https://indico.inp.nsk.su/e/SKIF-congress |
|
24-28
ноября 2025 г. V Всероссийская конференция с международным участием «Исследования и разработки в области химии и технологии функциональных материалов» г. Апатиты, Россия |
http://chemi-ksc.ru/m-osnovnoe/konferentsii/643-konferentsii-2025 |
|
December
3-5, 2025 3-day International Conference on Materials Science (3d-ICOMAS) Verona, Italy |
https://3d-icomas.org/ |
|
December
15-20, 2025 Computational Photocatalysis: Photophysics & Photochemistry at Interfaces. Machine Learning Bridges Theory and Experiment (PACIFICHEM) Honolulu, United States |
http://www.pacifichem.org/ |
|
19-20
февраля 2026 г.
Лидеры России и стран СНГ: НЕФТЕПЕРЕРАБОТКА 2026 Конференция и выставка по нефтепереработке: проекты, технологии, оборудование, катализаторы г. Москва, Россия |
https://enleader.ru/events/refining2026 |
|
7-8
апреля 2026 г.
Лидеры России и стран СНГ: ГАЗ И ХИМИЯ 2026 Конференция и выставка по технологиям и оборудованию для газовой и химической промышленности г. Москва, Россия |
https://enleader.ru/events |
|
9-10
апреля 2026 г.
Лидеры России и стран СНГ: КАТАЛИЗАТОРЫ 2026 Конференция и выставка по катализаторам нефтепереработки и нефтегазохимии г. Москва, Россия |
https://enleader.ru/events |
|
May
4-6, 2026 12th International Symposium on Characterization of Porous Solids Dresden, Germany |
https://cops-xii.org/ |
|
May
10-14, 2026 8th International Congress on Operando Spectroscopy (Operando VIII) Asilomar, CA (USA) |
https://events.slac.stanford.edu/operando-viii/ |
|
May
26-28, 2026 Annual Meeting on Reaction Engineering 2026 Würzburg, Germany |
https://dechema.de/en/ReactEng2026.html |
|
June
28 – July 1, 2026 Balticum Organicum Syntheticum 2026 (BOS2026) Tallinn, Estonia |
https://boschem.eu/ |
|
7-8
сентября 2026 г.
Лидеры России и стран СНГ: РАЗНОТОННАЖНАЯ ХИМИЯ 2026 Конференция и выставка по нефтехимии: проекты, технологии, оборудование, катализаторы г. Москва, Россия |
https://enleader.ru/events |
|
September
21-23, 2026 Annual Global Summit on Polymers and Composite Materials Prague, Czech Republic |
https://vividglobalsummits.com/2026/composite-materials |
|
September
24-26, 2026 International Summit on Catalysis and Chemical Engineering Barcelona, Spain |
https://catalysisvision.org/ |


