22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы

XI Международный форум технологического развития «Технопром-2024»
27-30 августа 2024 г.
Новосибирск, Россия
XI Международный форум технологического развития «Технопром-2024» проходил в Новосибирске с 27 по 30 августа. Программа форума включала пленарные и панельные сессии, круглые столы, выставку инновационных технологий и множество других мероприятий. «Технопром» – эффективная площадка для обсуждения актуальных вопросов научно-технологического развития регионов страны. В Форуме по традиции участвуют представители научных организаций, ведущих технологических корпораций и компаний, органов государственной власти, а также иностранные делегации.
На «Технопроме-2024» обсудили, как России достичь технологической независимости в области катализаторов
На Форуме рассматривались проблемы России в области катализаторов. На мероприятии также был подписан меморандум о создании Ассоциации производителей катализаторов, призванной объединить научно-технический потенциал российских создателей этой продукции, чтобы обеспечить технологическую независимость и экономическую стабильность химического и топливно-химического комплекса.
По словам директора ФИЦ «Институт катализа им. Г. К. Борескова СО РАН» академика Валерия Ивановича Бухтиярова, одна из главных проблем создания отечественных катализаторов – это утрата масштабирующего звена.
В СССР существовал госзаказ и действовала линейка: академические научные институты и университеты занимались научными разработками новых технологий, множество отраслевых институтов переводили эти разработки с научного языка на промышленный, а также масштабировали их и осуществляли пилотные испытания, чтобы снять риски с производства. Однако после перестройки началась приватизация отдельных НИИ, в ходе которой большинство профильных институтов были утрачены. К тому же на рынок пришли за-рубежные поставщики, и они нередко выставляли специфические условия: продавали лучшие катализаторы по хорошей цене, но запрещали предприятиям использовать собственные. После санкций поставки иностранной продукции прекратились, а участок масштабирования технологий создания катализаторов так и остался невосполненным. Возникает вопрос, кто должен заниматься его воссозданием: наука, производство или, может быть, потребители катализаторов?
«Мы тесно работаем с компанией СИБУР и в рамках нашей дискуссии пришли к тому, что катализаторы от науки до внедрения должны пройти четыре стадии: концептуальные исследования, лабораторное пилотирование, масштабирование технологий и, наконец, промышленное испытание опытной продукции», – рассказал Валерий Бухтияров. Если за первую стадию отвечают научные лаборатории институтов и университетов, а за четвертую – предприятия, то с промежуточными возникают вопросы. «Наше общение с СИБУР показывает, что концептуальные исследования и лабораторное пилотирование – это, скорее, задача академических институтов, в третьем же направлении готовы быть активными некоторые предприятия. В отдельных институтах и университетах также есть инжиниринговые центры, способные осуществлять масштабирование».
По мнению Валерия Ивановича, эта проблема нуждается в помощи государства. «Если обеспечить госзаказ на производство высокотехнологичной продукции, страхование рисков для предприятий, кредитные деньги под низкий процент, это помогло бы сдвинуть дело с мертвой точки. Также мы считаем, что необходимо создавать центры масштабирования в академических институтах и университетах, для чего тоже нужно госзадание и проектное финансирование на разработку высокотехнологической продукции. В таком случае риски для бизнеса могут быть в большой степени сняты».
В настоящий момент ИК СО РАН совместно с Министерством промышленности и торговли РФ разрабатывает дорожную карту необходимых России катализаторов. Кроме того, чтобы объединить усилия в этом направлении, ученые, производители катализаторов и производители химической продукции объединяются в Ассоциацию производителей катализаторов.

На «Технопроме-2024» был подписан меморандум о со-здании Ассоциации производителей катализаторов с целью объединить научно-производственный потенциал отечественных разработчиков и производителей катализаторов и выработать совместные согласованные действия, которые будут направлены на обеспечение технологической независимости и экономической стабильности химического и топливно-экологического комплекса РФ. Документ подписали представители ИК СО РАН, Салаватского катализаторного завода, Научно-производственной компании «Синтез», Ред-кинского катализаторного завода, ЗАО «Нижегородские сорбенты», Института нефтехимического синтеза им. А. В. Топчиева РАН, химического факультета Московского государственного университета им. М. В. Ломоносова.
«Задачи этой ассоциации более-менее понятны. Это анализ и определение общеотраслевых проблем, выработка путей их решения, взаимодействие с различными министерствами и другими государственными и производственными структурами, кроме того, консолидирование по вопросам совершенствования механизмов налогообложения и финансовая поддержка отечественных производителей и разработчиков катализаторов. Также необходимо подготовить согласованые аналитические материалы по производству критически необходимых катализаторов в России и участвовать в экспертных группах профильных министерств», – отметил Валерий Бухтияров.
На «Технопроме-2024» в рамках пленарного заседания обсуждали национальный проект «Новые материалы и химия», научно-технологическое, кадровое, информационное обеспечение
| Модератор: Шадрин Артем Евгеньевич – Генеральный директор, Автономная некоммерческая организация «Национальное агентство развития квалификаций» |
| Спикеры: |
| Артемьев Алексей Юрьевич – заместитель директора Департамента химической промышленности, Министерство промышленности и торговли Российской Федерации: Цели, задачи и принципы формирования национального проекта «Новые материалы и химия» |
| Секиринский Денис Сергеевич – заместитель министра, Министерство науки и высшего образования Российской Федерации: О развитии научно-производственной инфраструктуры для выпуска продукции малотоннажной химии научными и образовательными организациями в целях реализации приоритетов НТР |
| Алдошин Сергей Михайлович – Вице-президент, РАН: Участие научных советов РАН в выполнении Национального проекта «Новые материалы и химия» |
| Шадрин Артем Евгеньевич – Генеральный директор, Автономная некоммерческая организация.«Национальное агентство развития квалификаций»: Инструменты развития кадрового потенциала промышленности в рамках национального проекта «Новые материалы и химия» |
| Ретивов Василий Михайлович – заместитель директора по химическим исследованиям и технологиям, Национальный исследовательский центр «Курчатовский институт»: Наука – основа технологического лидерства в области создания материалов нового поколения |
| Кобзев Игорь Иванович – губернатор Иркутской области, правительство Иркутской области: Создание Федерального центра химии в Усолье-Сибирском – стратегический проект научно-технологического развития Иркутской области до 2036 года |
| Ситников Руслан Леонидович – первый заместитель председателя правительства Иркутской области: Реализация проекта по созданию в городе Усолье-Сибирское Федерального центра химии – якорного проекта государственной региональной программы научно-технологического развития до 2030 года |
| Иванец Дмитрий Васильевич – заместитель директора по технологическому развитию, Государственная корпорация по атомной энергии «Росатом» Развитие в Госкорпорации Росатом направления химии и новых материалов: наука, технологии, кадры |
| Огородова Людмила Михайловна – заместитель губернатора Томской области по научно-образовательному комплексу и цифровой трансформации, Администрация Томской области: Задачи региона по подготовке кадров для химических производств |
| Сутягинский Михаил Александрович – Председатель совета директоров, АО «Группа компаний ТИТАН»: Формирование кадрового резерва и наращивание технологического потенциала в рамках реализации деятельности промышленных предприятий и инжиниринговых центров. Промышленное предприятие – региональный центр компетенций |
Докладчики отметили важность и значимость национального проекта «Новые материалы и химия» для экономического развития России. В настоящее время происходит активное формирование принципов, целей и задач проекта.
В национальный проект «Новые материалы и химия» входят пять федеральных проектов:
На «Технопроме-2024» обсудили различные аспекты развития и применения ЦКП СКИФ
На XI Международном форуме технологического развития «Технопром-2024» в ходе мероприятий, посвященных ЦКП «Сибирский кольцевой источник фотонов», шла речь о технология синхротронного излучения применительно к нефтегазовой отрасли. Создатели специализированных станций на ЦКП СКИФ и представители крупных российских добывающих компаний рассказали, какие проблемы нефтегазовой отрасли будут решать с помощью синхротрона. Кроме того, на полях «Технопрома-2024» было подписано соглашение об организации консорциума ЦКП СКИФ и ведущих вузов.
Заместитель директора по научной работе ЦКП СКИФ доктор физико-математических наук Ян Витаутасович Зубавичус рассказал о текущем статусе реализации проекта. По его словам, сейчас завершается строительство зданий и идет активная стадия изготовления технического оборудования. «К концу 2024 года основные строительные работы будут выполнены, скорее всего, получится запустить инжекционный комплекс. К концу 2025 года мы готовы полностью сдать объект и ввести его в полноценную научную эксплуатацию. Оборудование комплекса сейчас готово на 70-80%. Идет создание шести станций полной очереди, всего на синхротроне может быть до 30 станций, у нас есть программа их развития, но всё будет зависеть от механизма финансирования со стороны Министерства науки и высшего образования РФ».
Для обучения кадров, которые будут работать на синхротроне, создан межвузовский консорциум по взаимодействию с ЦПК СКИФ. Он будет решать задачи по подготовке научных сотрудников, специалистов по эксплуатации комплексных инженерных систем, проектировщиков.
«Эксплуатация такой установки требует от 300 до 350 высококвалифицированных инженеров, которые смогут обеспечить работу сетей и оборудования. Кроме того, потребуется большое количество научных сотрудников, отвечающих за саму станцию. Поэтому необходимо самим обучать эти кадры. Это обеспечит поток молодых специалистов в отрасль», – отметил директор ФИЦ «Института катализа им. Г. К. Борескова СО РАН» академик Валерий Иванович Бухтияров.
На предложение об участии в консорциуме уже откликнулись организации со всей страны: вузы, учреждения среднего специального образования, школы. «СКИФ может стать удобным образовательным инструментом для того, чтобы вовлекать в исследования студентов младших курсов и, возможно, школьников 10-11-х классов», – прокомментировал Ян Зубавичус.
Ректор Новосибирского государственного технического университета профессор, доктор технических наук Анатолий Андреевич Батаев сообщил, что уже многие государственные университеты Новосибирска ввели дисциплину «Синхротронное излучение». При этом открываются как новые образовательные программы, так и адаптируются уже существующие.
«Станция “Диагностика в высокоэнергетическом рентгеновском диапазоне” будет особенно интересна для участников консорциума. Уже в следующем году можно будет приступить к реализации их идей и наработок. Тематика исследований будет связана с созданием цифрового керна и использованием синхротронного излучения для повышения нефтеотдачи месторождений», – прокомментировал Ян Зубавичус.
Заместитель директора Института нефтегазовой геологии и геофизики им. А.А. Трофимука СО РАН и ведущий научный сотрудник Научно-образовательного центра «Газпромнефть-НГУ», кандидат физико-математических наук Антон Альбертович Дучков рассказал о совместных исследованиях НГУ, Научно-образовательного центра «Газпромнефть-НГУ» и Института гидродинамики им. М.А. Лаврентьева СО РАН. Ученый отметил преимущества СКИФ по сравнению с рентгеновскими источниками.
«Чем хорош СКИФ – у него на порядки более яркий пучок, а значит, на порядки более быстрое сканирование, также он позволяет рассмотреть гораздо меньший размер образца. Это дает возможность фиксировать очень быстрые процессы и в реальном времени наблюдать за разрушением образца. Шаг между кадрами составляет 20 миллисекунд, и он отображается у оператора на экране во время сканирования. Мы сможем следить за протеканием жидкости в поле и даже за контрольными каплями. Кроме того, СКИФ позволяет осуществлять многомасштабное сканирование, то есть вы можете сделать скан большого образца, а затем тут же, не вынимая мишень, сменить фокус и увеличить разрешение», – сказал Антон Дучков.
Также ученый отметил, что высокие энергии пучка позволяют использовать так называемые фазоконтрастные методики обработки данных. Так, во время обычного сканирования кусочка угля не видно всех неровностей его структуры, однако они проявляются, если сделать фазовый контраст. Это важно для 3D-моделирования и создания цифровых двойников.
Руководитель Научно-технического центра «Газпром нефти» Владислав Вадимович Крутько рассказал о применении синхротронного излучения для исследования свойств залежей нефти и газа.
Сейчас компания работает над созданием цифрового керна, необходимого, чтобы проводить разработку пластов эффективнее и быстрее. На первом этапе этого процесса необходимо сканировать образец, что сейчас делается с помощью обычного рентгеновского электронного микроскопа высокого разрешения порядка 1,5 микрон.
«Этого разрешения недостаточно для того, чтобы визуализировать все особенности горных пород. Кроме того, время сканирования каждого образца исчисляется часами, что не позволяет нам в полной мере визуализировать динамические процессы. Работа со станцией СКИФ даст возможность увеличить разрешение нашей модели до 100 нанометров, а время сканирования будет исчисляться уже минутами и секундами», – сказал Владислав Крутько.
Начальник управления перспективных исследований Томского политехнического университета, где разрабатывается станция СКИФ «Микрофокус», кандидат физико-математических наук Алексей Сергеевич Гоголев прочитал доклад о расширении границ цифрового анализа на керне и рассказал, как в этом может помочь синхротронное излучение.
«Синхротрон позволяет напрямую наблюдать разные фазы (отдельно нефть в породе, отдельно воду, отдельно газ) в достаточно высоком разрешении, а также отдельные динамические процессы, например образование гидратов. Всё это хорошо визуализируется даже без применения фазоконтрастных методик. На нашей станции будут использоваться разные детекторы, в том числе спектральные, которые позволят на ходу определять минеральный состав образца. Характерные размеры, до которых можно спуститься, – 60 нанометров, скорости сканирования – до 20 миллисекунд и ниже, величина образцов может быть меньше миллиметра, синхротрон позволит их сканировать с высоким разрешением и контрастной чувствительностью», – прокомментировал Алексей Гоголев.
Главный менеджер управления разработки ПО для моделирования ООО «РН-БашНИПИнефть» кандидат физико-математических наук Юлия Айратовна Питюк также отметила возможности, которые предоставляет ЦКП СКИФ для создания цифрового керна. «Мы надеемся, что он позволит преодолеть ограничения существующих компьютерных томографов и сложность изучения динамических процессов, а также поможет с калибровкой цифровых моделей».
По материалам газеты «Наука в Сибири»
24 сентября 2024 г.
Павлец Ангелина Сергеевна
«Влияние метода синтеза и условий активации на состав, структуру и электрохимическое поведение PtCu/C катализаторов для катода топливного элемента
с протонообменной мембраной»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.6 - Электрохимия
Место защиты: ФГБУН Федеральный исследовательский центр проблем химической физики и медицинской химии Российской академии наук. Московская обл., г. Черноголовка
25 сентября 2024 г.
Живулин Дмитрий Евгеньевич
«Структура и физико-химические свойства допированных азотом графитоподобных материалов»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.4 - Физическая химия
Место защиты: ФГАОУ ВО «Южно-Уральский государственный университет (национальный исследовательский университет)», г. Челябинск
26 сентября 2024 г.
Корчак Петр Андреевич
«Равновесие жидкость-жидкость и распределение
биокомпонента в водно-солевых системах на основе ионных жидкостей разного строения»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.4 - Физическая химия
Место защиты: ФГБУН Институт химии растворов
им. Г.А. Крестова Российской академии наук, г. Иваново
26 сентября 2024 г.
Костина Анна Сергеевна
«Превращения метанола на модифицированных
силикагелевых адсорбентах в водо-метанольном отходе очистки природного газа»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.5.15 – Экология
Место защиты: ФГБОУ ВО «Кубанский государственный университет», г. Краснодар
26 сентября 2024 г.
Набиуллина Светлана Николаевна
«Комплекс аналитических методов для определения ультраследов элементов платиновой группы и золота
в геологических объектах и моделирование форм переноса этих элементов в природных средах»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.2 – Аналитическая химия
Место защиты: ФГБУН Институт геохимии и аналитической химии им. В.И. Вернадского Российской академии наук,
г. Москва
26 сентября 2024 г.
Червонная Татьяна Артемовна
«Эколого-аналитический контроль загрязнения водных экосистем и почв полиароматическими углеводородами
и полихлорбифенилами»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.5.15 – Экология
Место защиты: ФГБОУ ВО «Кубанский государственный университет», г. Краснодар
8 октября 2024 г.
Скрипников Андрей Михайлович
«Фракционирование биомассы древесины березы на ценные химические продукты с использованием экстракционных и каталитических процессов»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.4 - Физическая химия
Место защиты: ФГБНУ Федеральный исследовательский центр «Красноярский научный центр Сибирского отделения Российской академии наук», г. Красноярск
15 октября 2024 г.
Гринёв Вячеслав Сергеевич
«Молекулярное конструирование и функционализация новых N,O,S-полигетероциклических структур для создания перспективных биологически активных веществ»
Защита диссертации на соискание ученой степени доктора химических наук по специальности 1.4.3 - Органическая химия
Место защиты: ФГАОУ ВО «Национальный исследовательский Нижегородский государственный университет им. Н.И. Лобачевского», г. Нижний Новгород
16 октября 2024 г.
Оттенбахер Роман Викторович
«Каталитические системы на основе комплексов марганца для селективного жидкофазного окисления органических молекул»
Защита диссертации на соискание ученой степени доктора химических наук по специальности 1.4.14 – Кинетика и катализ
Место защиты: ФГБУН «Федеральный исследовательский центр «Институт катализа им. Г.К. Борескова Сибирского отделения Российской академии наук», г. Новосибирск
17 октября 2024 г.
Бардакова Ксения Николаевна
«Влияние структуры и физико-механических свойств трехмерных биодеградируемых полимерных материалов
на их биосовместимость и клеточную адгезию»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.7 - Высокомолекулярные соединения
Место защиты: ФГБУН Федеральный исследовательский центр химической физики им. Н.Н. Семенова Российской академии наук, г. Москва
17 октября 2024 г.
Гуляева Екатерина Сергеевна
«Кооперация металл-лиганд и металл-металл в катализируемых комплексами марганца реакциях (де)гидрирования»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.8 - Химия элементоорганических соединений, 1.4.4 - Физическая химия
Место защиты: ФГБУН Институт элементоорганических соединений им. А.Н. Несмеянова Российской академии наук, г. Москва
17 октября 2024 г.
Клюсик Оксана Николаевна
«Самораспространяющийся высокотемпературный синтез нанопорошков оксида скандия для оптической керамики»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.1 - Неорганическая химия
Место защиты: ФГАОУ ВО «Национальный исследовательский Нижегородский государственный университет им. Н.И. Лобачевского», г. Нижний Новгород
23 октября 2024 г.
Вегнер Маргарита Владимировна
«Октаэдрические иодидные кластерные комплексы молибдена с H2O и OH-лигандами: синтез, изучение оптических свойств и получение фотокаталитических систем на их основе»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.1 - Неорганическая химия
Место защиты: ФГБУН Институт неорганической химии
им. А.В. Николаева Сибирского отделения Российской академии наук, г. Новосибирск
24 октября 2024 г.
Павлов Владимир Сергеевич
«Дезактивация молекулярно-ситовых катализаторов конверсии метанола в углеводороды»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.12 – Нефтехимия
Место защиты: ФГБУН Ордена Трудового Красного Знамени Институт нефтехимического синтеза им. А.В. Топчиева
Российской академии наук, г. Москва
24 октября 2024 г.
Снатенкова Юлия Михайловна
«Конверсия оксигенатов в жидкие углеводороды на микро- и наноразмерных цинксодержащих цеолитах MFI»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.12 – Нефтехимия
Место защиты: ФГБУН Ордена Трудового Красного Знамени Институт нефтехимического синтеза им. А.В. Топчиева Российской академии наук, г. Москва
24 октября 2024 г.
Черкасова Полина Владимировна
«Разработка новых доступных каталитических систем
для фиксации углекислого газа в циклические органические карбонаты»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.3 - Органическая химия
Место защиты: ФГБУН Институт элементоорганических соединений им. А.Н. Несмеянова Российской академии наук,
г. Москва
29 октября 2024 г.
Вернигор Инна Евгеньевна
«Нанокомпозитные электрокатализаторы на основе углеродных нанотрубок: установление взаимосвязи природы активных центров и механизма токообразующих реакций в источниках тока»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.6 - Электрохимия
Место защиты: ФГБУН Институт физической химии и электрохимии им. А.Н. Фрумкина Российской академии наук, г. Москва
30 октября 2024 г.
Колоколов Даниил Игоревич
«2Н ЯМР спектроскопия в исследовании молекулярной подвижности в микропористых материалах: цеолитах
и металл-органических каркасах»
Защита диссертации на соискание ученой степени доктора химических наук по специальности 1.4.4 – Физическая химия
Место защиты: ФГБУН «Федеральный исследовательский центр «Институт катализа им. Г.К. Борескова Сибирского отделения Российской академии наук», г. Новосибирск
13 ноября 2024 г.
Лащинская Зоя Николаевна
«Исследование механизмов превращения С2–С4 алкенов
на цеолитах, модифицированных Zn, Cu, Ag, методами ЯМР и ИК-спектроскопии»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.4 - Физическая химия
Место защиты: ФГБУН «Федеральный исследовательский центр «Институт катализа им. Г.К. Борескова Сибирского отделения
Российской академии наук», г. Новосибирск
21 ноября 2024 г.
Бакунова Алина Константиновна
«Трансаминаза D-аминокислот из Haliscomenobacter hydrossis: каталитические свойства и структура»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.5.4 – Биохимия
Место защиты: ФГУ «Федеральный исследовательский центр «Фундаментальные основы биотехнологии» Российской академии наук», г. Москва
22 ноября 2024 г.
Каминский Олег Игоревич
«Разработка и исследование висмутовых фотокаталитических покрытий для очистки вод от органических
загрязняющих веществ»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.5.15 – Экология
Место защиты: ФГАОУ ВО «Дальневосточный федеральный университет», г. Владивосток
22 ноября 2024 г.
Якимова Людмила Сергеевна
«Полифункциональные частицы на основе макроциклических соединений и диоксида кремния: от синтеза
макроциклических структур к созданию функциональных материалов»
Защита диссертации на соискание ученой степени доктора химических наук по специальности 1.4.3 - Органическая химия
Место защиты: ФГБОУ ВО «Казанский национальный
исследовательский технологический университет», г. Казань
27 ноября 2024 г.
Журенок Ангелина Владимировна
«Разработка фотокатализаторов на основе графитоподобного нитрида углерода для получения водорода из водных
растворов триэтаноламина под действием видимого света»
Защита диссертации на соискание ученой степени кандидата химических наук по специальности 1.4.14 – Кинетика и катализ
Место защиты: ФГБУН «Федеральный исследовательский центр «Институт катализа им. Г.К. Борескова Сибирского отделения
Российской академии наук», г. Новосибирск
4 декабря 2024 г.
Кучеренко Александр Сергеевич
«Рециклизуемые органокатализаторы на основе хиральных аминов: дизайн и применение для асимметрического синтеза биологически активных веществ»
Защита диссертации на соискание ученой степени доктора химических наук по специальности 1.4.3 - Органическая химия
Место защиты: ФГБУН Институт органической химии им. Н.Д. Зелинского Российской академии наук, г. Москва
Scorching heat turns MOF into discerning hydrogenation catalyst
By selectively turning alkynes into alkenes, the porous material could pave the way for more efficient purification of polymer feedstocks
A catalyst that helps convert alkynes into alkenes could offer a more efficient and greener way to achieve the tricky industrial transformation (ACS Catal. 2024, DOI: 10.1021/acscatal.4c00310).
The alkenes used to manufacture polymers often contain troublesome traces of alkynes. To purify these feedstocks, chemists use semihydrogenation to turn carbon-carbon triple bonds into double bonds. This process requires a catalyst that preferentially binds and hydrogenates alkynes but shuns alkenes, sparing double-bonded carbons from further hydrogenation into unwanted alkanes.
One option is a palladium-based Lindlar catalyst, which contains additives such as lead that dial down palladium’s activity. But this type of catalyst sometimes suffers from poor stability and can cause overhydrogenation. And using toxic lead on an industrial scale is far from ideal.
A team led by Pascual Oña Burgos at the Institute of Chemical Technology (ITQ) in Valencia, Spain, has now created a semihydrogenation catalyst based on a metal-organic framework (MOF), a type of porous material containing a scaffold of metal-based nodes holding together organic molecular struts.
Researchers had already found that when MOFs are pyrolyzed at several hundred degrees Celsius, they can form metal nanoparticles embedded in porous carbon, potentially making stable and active catalysts. Oña Burgos’s team has now shown that chemical pretreatment of a MOF before pyrolysis can produce an even more effective catalyst.
The researchers reacted a palladium-indium MOF with aniline and then pyrolyzed the material at 800 °C. That caused the MOF’s metals to form palladium-indium nanoparticles and its organic molecules to degrade into porous carbon peppered with nitrogen atoms. Compared with pyrolyzed MOFs that didn’t get the pretreatment, the strategy produced smaller nanoparticles—and hence a higher density of catalytic sites. Indium improves palladium’s preference for binding alkynes, and nitrogen helps to activate incoming hydrogen molecules.
The catalyst semihydrogenates phenylacetylene to styrene with 96% selectivity, and with 96% conversion at ambient temperature and pressure. In contrast, a commercial palladium-on-carbon catalyst offers only 50% selectivity, generating loads of the unwanted alkane product.
Having proved that their catalyst works well with liquid alkynes, “the next step is to move to the gas phase for industrial applications like the semihydrogenation of acetylene to ethylene,” Oña Burgos says.
Natural minerals catalyze phosphorus cycling
New research shows that natural iron oxides actively catalyze organic phosphorus into its inorganic form
Phosphorus is a key element for all life on Earth. For decades, researchers thought that, in nature, only enzymes could transform organic phosphorus—phosphates within biomolecules—into its bioavailable, inorganic form, free phosphate ions. Minerals in sediment and soil were thought to only adsorb phosphorus, not participate in the dephosphorylation reaction. But now new research shows that naturally occurring iron oxide minerals also act as catalysts (Nat. Commun. 2024, DOI: 10.1038/s41467-024-47931-z).
The work began years ago, when lead researcher Ludmilla Aristilde, an environmental chemist at Northwestern University, designed an initial experiment to follow the dephosphorylation products that developed when aden-osine triphosphate was mixed with pure ferrihydrite, an iron oxide mineral commonly found in soil. Using high-resolution mass spectrometry, the team was able to search for more than just phosphorus, the only target in past experiments. This expertise paid off; the team found organic products—adenosine diphosphate, adenosine monophosphate, and adenosine—but no inorganic phosphate. “If you were only following phosphate,” Aristilde says, “you would say there is no catalysis,” and might incorrectly assume iron oxides are noncatalytic.
A serendipitous encounter with Sharon Bone, a former lab mate and now beamline scientist at SLAC National Accelerator Laboratory, helped Aristilde search for the missing phosphate. Bone agreed to collaborate and initiated an urgent request for beam time so the researchers could analyze the surface of the ferrihydrite with advanced X-ray scattering techniques. Not only did they find phosphate adsorbed to the pure mineral, Aristilde says they also found even more organic, dephosphorylated products. Their findings indicated that the pure minerals were clearly acting as catalysts.
But other researchers remained unconvinced after Aristilde published the work in 2019 (J. Colloid Interface Sci. 2019, DOI: 10.1016/j.jcis.2019.03.086). Fellow scientists asked her, What if real-world samples are too complex to behave in the same way? So Aristilde searched for more collaborators. Two field scientists sent her samples: sediment from the bottom of a lake and soil from a forest floor. Both contained a fraction of iron oxide minerals.
With those real-world samples, Aristilde and her team repeated their initial experiments. After they analyzed the mass spectra and X-ray data, the role of minerals was clear. “Iron oxide in a soil matrix and sediment matrix is also acting as a catalyst,” Aristilde says. And not only are the minerals cata-lyzing dephosphorylation, she says, but the rate of mineral catalysis is comparable to that of an enzyme.
Finding abiotic pathways of producing bioavailable phosphorus in the natural environment has important implications for how researchers understand the phosphorus cycle, says Elizabeth Herndon, an environmental geochemist at Oak Ridge National Laboratory. “With some of the quantitative information that they provide here,” she says, “there’s the potential to incor-porate mineral-catalyzed pathways into biogeochemical models.”
Herndon thinks there are plenty more ecosystems to investigate. “This study opens the door to looking at this catalysis across more environments,” she says, “Maybe it’ll be important in some places and less in other places.”
Chemists make the popular fragrance compound ambrox via asymmetric catalysis
Synthetic route uses an imidodiphosphorimidate catalyst and a fluorinated alcohol solvent
Chemists have made (–)-ambrox—a compound that’s popular in perfumes—starting from (3E,7E)-homofarnesol. A team led by the Max Planck Institute for Kohlenforschung’s Benjamin List developed the route, which achieves asymmetric cyclization of a polyene using a scant amount of a chiral imidodiphosphorimidate catalyst in a fluorinated alcohol solvent (Nature 2024, DOI: 10.1038/s41586-024-07757-7).
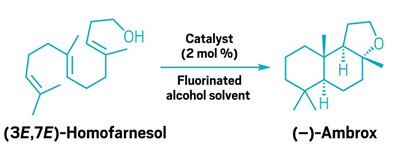
This type of asymmetric polyene cyclization has long eluded synthetic chemists, who have been looking for alternatives to gathering (–)-ambrox from ambergris, a waxy substance vomited by sperm whales. List calls the reaction “a provocation by nature to us chemists” because nature can guide the polyene to fold itself in a way that it easily makes the desired isomer, but synthetic chemists struggle to achieve the same selectivity. Previous attempts have used stoichiometric amounts of chiral acids and were not as successful as the new route.
David Sarlah, a synthetic chemist at Rice University who was not involved in the work, calls the synthesis a significant leap forward. “It exemplifies the best case of achieving catalytic and highly selective classical polyene cyclization that mimics nature,” he says in an email. “Although they reported a limited substrate scope, this work can pave the way for translating biomimetic polyene cyclizations toward asymmetric synthesis of many important molecules.”
In recent years, fragrance scientists have developed enzymes that can make (–)-ambrox, and List says his group’s route might be competitive with that biocatalytic synthesis. The team demonstrates that its synthesis works on a multigram scale. The synthesis uses a chiral imidodiphosphorimidate catalyst, which possesses an enzyme-like microenvironment. This catalyst coaxes (3E,7E)-homofarnesol into the perfect position to become (–)-ambrox upon protonation. List says using a fluorinated alcohol solvent was key to the reaction’s success because it helped boost the ionizability of the reactants. The use of fluorinated solvents raises environmental concerns, but Mathias Turberg, a graduate student in List’s lab, says it was easy to recover and recycle the solvent.
The chemists say the approach might also be used on the polyene cycliza-tion of squalene, which is an important step in sterol synthesis. “We’ve barely scratched the surface of this exciting type of transformation,” Turberg says. “It holds great promise for efficiently producing natural products” that are currently made only by enzymes.
Chemical & Engineering News
| 23-27 сентября 2024 г. XIII Международная конференция «Химия нефти и газа» г. Томск, Россия |
http://petroleum-chemistry.ru/ |
|
26-27
сентября 2024 г.
Школа-конференция Центра компетенций НТИ «Водород как основа низкоуглеродной экономики» г. Киров, Россия |
https://h2nti-2024.tilda.ws/ |
|
30
сентября – 3 октября 2024 г.
IX Международный симпозиум «Химия и химическое образование» г. Владивосток, Россия |
https://www.iscce.ru/ |
|
September
30 – October 3, 2024 VIII International School-Conference for Young Scientists “Catalysis: from Science to Industry” (CatConf2024) Tomsk, Russia |
chttp://catconf.tsu.ru |
|
3-6
октября 2024 г.
V Научно-технологический симпозиум «Гидропроцессы в катализе» г. Сочи, Роза-Хутор, Россия |
https://sts-5.tilda.ws/ |
|
7-12
октября 2024 г.
XXII Менделеевский съезд по общей и прикладной химии Федеральная территория «Сириус», Россия |
https://mendeleevcongress.ru |
|
October
10, 2024 Advanced Energy Materials 2024 London, UK |
https://www.aemlondon.com/ |
|
October
14-15, 2024 1st Maruoka Conference on the Frontier of Organic Synthesis and Catalysis Nagoya, Japan |
https://www.chembio.nagoya-u.ac.jp/labhp/organic3/maruoka_conference/index.html |
|
15-17
октября 2024 г.
XIII Международный российско-казахстанский симпозиум «Углехимия и экология Кузбасса» Посвящается памяти академика РАН З.Р. Исмагилова г. Кемерово, Россия |
http://www.iccms.sbras.ru/ccsymp-2024 |
|
October
18-22, 2024 10th IUPAC International Conference on Green Chemistry Beijing, China |
https://iupac.org/event/10th-iupac-international-conference-on-green-chemistry/ |
|
October
21-25, 2024 5th International Conference on Materials Science & Nanotechnology Athens, Greece |
https://materialsconference.yuktan.com/ |
|
24-25
октября 2024 г. V Всероссийская научно-практическая конференция с международным участием «Водород. Технологии. Будущее» г. Пермь, Россия |
https://htf.tpu.ru/ |
|
28-30
октября 2024 г.
III Школа молодых ученых по синхротронным методам исследования в материаловедении г. Новосибирск, Россия |
https://conf.nsu.ru/Radiation_technologies_2024 |
|
28
октября – 1 ноября 2024 г. Международная конференция «Динамические процессы в каталитических структурах» (ДПКС 2024) г. Тюмень, Россия |
https://dpcs24.org/ |
|
30
октября – 1 ноября 2024 г. 16-я Международная конференция «Углерод: фундаментальные проблемы науки, материаловедение, технология» г. Москва, Россия |
http://www.ruscarbon.org/2024_index.html |
|
1-4
ноября 2024 г.
Международная научно-техническая конференция «Катализ в промышленности и проблемы экологии» г. Ташкент, Узбекистан |
https://conference-katalizes.uz/ |
|
November
4-5, 2024 Research Data Management School of Catalysis Frankfurt am Main, Germany |
https://dechema.de/en/RDM_School2024.html |
|
November
4-8, 2024 5th International Conference on Emerging Advanced Nanomaterials (ICEAN 2024) Newcastle, Australia |
https://www.newcastle.edu.au/research/centre/gican/icean-2024 |
|
11-14
ноября 2024 г.
III Международная конференция “Использование синхротронного излучения для исследования катализаторов и функциональных материалов” г. Томск, Россия |
https://srtcfm-2024.tilda.ws/ |
|
11-15
ноября 2024 г.
Всероссийская конференция им. академика В.И. Овчаренко «Органические радикалы и органическая электрохимия: фундаментальные и прикладные аспекты» г. Новосибирск, Россия |
http://or-2024.tomo.nsc.ru/index.php?lang=ru |
|
November
14-15, 2024 7th International Symposium on Hydrogen Energy and Energy Technologies (HEET 2024) Osaka, Japan |
https://www.heet-18.org/ |
|
November
15-19, 2024 г.
IX International Conference of Young Scientists “Synthesis of Electrode Materials” Moscow, Russia |
https://crei.skoltech.ru/cest/conference-of-young-scientists-2024/ |
|
November
17-22, 2024 9th International Symposium on Practical Surface Analysis (PSA-24) Pusan, Korea |
https://surfaceanalysis.kr/PSA/PSA24/ |
|
21-22
ноября 2024 г.
VIII Всероссийская молодежная конференция «Проблемы и достижения химии кислород- и азотсодержащих биологически активных соединений» г. Уфа, Россия |
https://uust.ru/events/get/1540/ |
|
November
25-27, 2024 2nd International Conference on Renewable and Sustainable Energy (RENEWABLEENG-2024 – Hybrid Edition) Paris, France |
https://renewableengconfex.org/ |
|
November
25-29, 2024 Quantum Chemistry of Excited States (QCES-2024) Cluj-Napoca, Romania |
https://www.molcas.org/QC-ES/ |
|
November
28-29, 2024 Global Summit on Renewable and Sustainable Energy (GSERSE2024) Porto, Portugal |
https://unitedresearchforum.com/gserse2024/ |
|
December
2, 2024 GeCatS Infoday "Catalysis and Process Engineering as Enablers for Sustainable Energy Carriers and Chemicals" Frankfurt am Main, Germany |
https://dechema.de/en/GeCatS_Infoday2024.html |
|
December
9-11, 2024 International Conference on Nanoscience and Nanotechnology 2024 Paris, France |
https://www.sciconx.com/nanoscience |
|
December
13, 2024 5th European Forum on New Technologies: Artificial Intelligence in Chemical Engineering Paris, France |
https://efce.info/5th European Forum on New Technologies.html |
|
December
16-20, 2024 Conference on Advances in Chemistry for Energy and Environment (CACEE-2024) Mumbai, India |
https://www.cacee2024.org/ |
|
|
|
|
February
2-7, 2025 European Winter School on Physical Organic Chemistry (E-WISPOC) Bressanone, Italy |
https://www.ewispoc.com/ |
|
February
13-15, 2025 International Meet & Expo on Chemical Engineering and Catalysis Dubai, United Arab Emirates |
https://chemcatmeet.org/ |
|
February
24-26, 2025 9th International Conference on Catalysis and Chemical Engineering (ССE-2025) San Francisco, CA, USA |
https://catalysis.unitedscientificgroup.org/ |
|
April
8-10, 2025 7th International Conference on Advanced Oxidation Processes Frankfurt am Main, Germany |
https://iwa-aop.org/ |
|
21-26
апреля 2025 г.
V Российский конгресс по катализу «РОСКАТАЛИЗ» г. Санкт-Петербург, Россия |
https://ruscat-5.tilda.ws/ |
|
May
26-28, 2025 Annual Meeting on Reaction Engineering 2025 Wuerzburg, Germany |
https://dechema.de/en/Events/2025/ Annual+Meeting+on+Reaction+Engineering+2025.html |
|
July
13-18, 2025 IUPAC World Chemistry Congress 2025 Kuala Lumpur, Malaysia |
https://iupac.org/event/iupac-world-chemistry-congress-2025/ |
|
July
14-18, 2025
12th World Congress of Chemical Engineering and 21st Asian Pacific Confederation of Chemical Engineering 2025 (WCCE12 & APCChE 2025) Beijing, China |
https://www.wcce12.com/
|
|
August
24-28, 2025 Fast Ionic Transport Systems Prague, Czech Republic |
https://www.imc.cas.cz/sympo/86pmm/ |
|
September
24-26, 2025 Faraday Discussion – High-entropy alloy nanostructures: from theory to application London, United Kingdom |
https://www.rsc.org/events/detail/77926/high-entropy-alloy- nanostructures-from-theory-to-application-faraday-discussion |
|
October
12-15, 2025 3rd International Conference on Energy, Environment & Digital Transition (E2DT 2025) Palermo, Italy |
https://www.aidic.it/e2dt2025/ |