22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы

C 3 по 7 июля 2023 г. в Новосибирске прошел 8-й Азиатский симпозиум по современным материалам ASAM-8. Самый первый Азиатский симпозиум по современным материалам (Asian Symposium on Advanced Materials, ASAM) был организован во Владивостоке в 2007 году. После этого симпозиум проводили в Китае, Японии, Тайване, Корее, Вьетнаме и, наконец, в восьмой раз он состоялся в Новосибирске – право принимать его в России отстаивали три года. Институт катализа СО РАН как организатор посвятил симпозиум ASAM-8 широкому спектру фундаментальных и прикладных тем — от получения и применения новых материалов в энергетике и экологических приложениях до биотехнологий и медицины.
Для удобства всех участников ASAM-8 был проведен в гибридном формате. Всего в симпозиуме приняло участие свыше 320 ученых, из них более 180 присутствовали на симпозиуме лично, и порядка 140 человек поучаствовали в работе симпозиума удаленно. Среди участников симпозиума ASAM-8 были представители ведущих университетов и научных организаций Алжира, Австралии, Китая, Японии, Кореи, Тайваня и Казахстана. Участники из российских научных организаций продемонстрировали высокий интерес к тематикам мероприятия. Симпозиум посетили ученые со всей страны: от Владивостока (Институт химии ДВО РАН) до Калининграда (БФУ им. И. Канта) и от Апатитов (ИХТРЭМС КНЦ РАН) до Ростова-на-Дону (Южный федеральный университет).
Темы и люди ASAM-8
Пленарные доклады представляли ученые с мировым именем. Первой была представлена лекция профессора Чанг-Сик Ха из Пусанского национального университета (Пусан, Корея) “Полиимиды и/или их гибридные пленки для нестандартных применений” (Polyimides and/or their hybrid films for tailor-made applications).
Полиимиды – это довольно известные высокоэффективные полимеры с уникальными свойствами, включающими их высокую термическую стабильность и хорошую механическую прочность, что делает их пригодными для применения во многих высокотехнологичных областях, таких как автомобильная, электронная и аэрокосмическая промышленность. Однако ароматические полиимидные пленки демонстрируют плохое оптическое пропускание желтого или темно-коричневого цвета, что обусловлено образованием комплексов с переносом заряда между диамином донора электронов и диангидридом акцептора электронов в основной цепочке.
Плохая оптическая прозрачность является одним из серьезных недостатков таких материалов для их применения в микроэлектронике и оптоэлектронике. Таким образом, разработка бесцветных и прозрачных полиимидов стала одной из передовых тем в индустрии микроэлектронных и электрооптических устройств. В последнее время в связи с быстрым ростом индустрии беспроводной мобильной связи также возросла значимость разработки полиимидов с низкой диэлектрической постоянной и низким поглощением влаги. Принимая во внимание такие важные проблемы в индустрии полиимидов, исследование профессора Ха посвящено нестандартному применению полиимидов и/или их гибридных пленок в органических светоизлучающих устройствах (OLED), гибких подложках, гибкой чувствительной коже и т.д.
Вторым пленарную лекцию представил профессор из Университета Ньюкасла (Ньюкасл, Австралия) Аджаян Вину. Тема его лекции – “Нанопористые материалы для энергетики и окружающей среды” (Nanoporous materials for energy and environment).
Профессор Вину в настоящее время работает директором Глобального инновационного центра передовых наноматериалов в Университете Ньюкасла. За 20 лет исследований профессор Вину внес огромный вклад в область нанопористых материалов и их применения в сенсорике, хранении энергии, топливных элементах, адсорбции и разделении, а также катализе.
Значимым событием для всех участников симпозиума стала лекция чл.-корр. РАН, д.х.н. Евгения Викторовича Антипова (МГУ имени Ломоносова, Москва, Россия) “Новые электродные материалы для металл-ионных аккумуляторов” (Novel electrode materials for metal-ion batteries).

чл.-корр. РАН, д.х.н. Евгений Антипов
Девяносто процентов производимой сегодня энергии получают из ископаемого топлива. Быстрое потребление этого источников энергии оказывает крайне негативное влияние на наше будущее из-за экологического ущерба и изменения климата. В этом смысле развитие экологически чистых возобновляемых источников энергии, способных заменить ископаемое топливо, и одновременно эффективных крупных накопителей энергии не требует обоснования. Литий-ионные аккумуляторы изначально были разработаны для портативных электронных устройств, но в настоящее время предусмотрены новые ниши их применения в электромобилях и стационарных сетевых накопителях энергии.
Однако литий-ионные аккумуляторы не могут быть полностью адаптированы для этих применений. Географическая нехватка и чрезмерный спрос на литий активизируют развитие альтернативных технологий, основанных на широко доступных материалах, а именно натрий- и калий-ионных батарей.
Материалы на основе оксидов Na/K и полианионов переходных металлов рассматриваются в качестве катодов для этих батарей с целью повышения удельной энергии, долговечности и скорости работы.
В лекции был представлен обзор исследований новых фосфатов и фторид-фосфатов как перспективных электродных материалов для Na/K-ионных аккумуляторов с особым акцентом на взаимосвязи состава, условий синтеза, кристаллической структуры и электрохимических свойств материалов, предназначенных для практического применения.
Завершающей стала пленарная лекция профессора Дунъюань Чжао из Фуданьского университета (Шанхай, Китай), который входит в топ-15 самых цитируемых специалистов в области новых материалов. Название лекции – “Межфазно-ориентированная сборка функциональных мезопористых материалов с иерархическими порами из мономицелл” (Interfacial oriented assembly of hierarchical oriented functional mesoporous materials from monomicelles).
Функциональные мезопористые материалы представляют собой класс пористых твердых тел с размером пор от 2 до 50 нм. Они не только обладают уникальными свойствами, такими как высокая удельная поверхность, большой размер и объем пор, контролируемая однородность пор, но также имеют превосходные оптические, электрические и магнитные свойства неорганических функциональных наночастиц. Таким образом, многоуровневые функциональные мезопористые материалы имеют широкие перспективы применения во многих областях, таких как катализ, адсорбция, сепарация и биомедицина.
В лекции были рассмотрены последние достижения в области иерархических функциональных мезопористых материалов, синтезируемых путем ориентированной межфазной сборки мономицелл поверхностно-активных веществ.
Основываясь на идее регулирования межфазной сборки, был разработан ряд новых методов синтеза многоуровневых структурных и функциональных мезопористых материалов, таких как: самосборка замкнутой микроэмульсии, двухфазный синтез типа «жидкость-жидкость», испарительная ориентированно-агрегационная сборка, метод анизотропного роста, межфазное упорядочение, стратегия динамической миграции на границе раздела и т.д. Эти новые подходы позволяют синтезировать мезопористые наноматериалы с одноуровневой и многоуровневой архитектурой. К основным достоинствам таких материалов можно отнести однородность их морфологии, контролируемую мезоскопическую пористую структуру, высокую удельную площадь поверхности, большой объем пор и наличие открытых пор. Именно благодаря своей уникальной структуре эти материалы имеют очень хорошие перспективы применения в биомедицине и других областях.
“Помимо лекций очень высокого уровня можно отметить наши доклады, которые в большинстве своем посвящены фундаментальному и прикладному катализу. Зарубежные коллеги представляли не совсем обычные исследования в сфере биоматериалов и их приложений. Например, биоразлагаемые полимеры, биосенсоры, гели, которые сохраняют свою форму при механическом воздействии”, – рассказал заместитель председателя организационного комитета симпозиума к.х.н. Максим Казаков.

Сотрудники Университета ИТМО Павел Кривошапкин и Шантал Трэйси рассказывают о своих разработках
Российские ученые из ИК СО РАН, ИЦиГ СО РАН и Университета ИТМО поделились интересными разработками в области экологии, медицины и биотехнологий для пищевой промышленности. Младший научный сотрудник ИК СО РАН Алина Брагина рассказала про гетерогенные катализаторы Фентона для очистки воды от органических загрязнителей, таких как антибиотики, фенол, лигнин. Разработка позволяет удалять загрязнения в очень низких концентрациях, с чем не справляются другие системы.
Предполагается, что по результатам конференции три лучших доклада будут опубликованы в журнале С – Journal of Carbon Research издательства MDPI. Однако жюри выделило еще одну работу — исследование младшего научного сотрудника ИК СО РАН Софьи Афонниковой, посвященное композитным материалам для модификации трубного гранулированного полиэтилена. В число победителей также вошел младший научный сотрудник Института Алексей Заворин с исследованием изменения структуры композитных нанотрубок при термообработке.
Лучшим стендовым докладом по итогам голосования участников Симпозиума стала совместная работа ИК СО РАН и ИНХ СО РАН “Микродисперсные никелевые катализаторы для синтеза углеродных нановолокон”, представленная Татьяной Максимовой.
Для участников симпозиума была подготовлена обширная социальная программа, включающая в себя экскурсии в научные институты и музеи новосибирского Академгородка, а также ставший традиционным вечер песни, на котором участники имели возможность пообщаться в неформальной обстановке и оценить музыкальные таланты друг друга.

Евгений Бакшеев (ООО «Экоальянс»), к.х.н. Илья Мишаков (ИК СО РАН),
к.х.н. Максим Мельгунов (ИК СО РАН) на вечере песни рассказывают о своих разработках
По словам Максима Казакова, и российские, и зарубежные участники высоко оценили уровень проведения ASAM-8. Следующий симпозиум, ASAM-9, должен состояться в 2025 году в Корее.
Материал подготовили:
А.А. Ведягин, С.С. Логунова, А.С. Аникина
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
фото: Е.К. Казакова
Photocatalytic reaction creates cyclopropanes from unactivated alkenes
The oxygen-mediated redox pathway makes carbon triangles without relying on hazardous reagents
Cyclopropanes are a common motif in medicinal chemistry. Three-carbon rings show up in drug structures for conditions including asthma, psoriasis, and COVID-19.
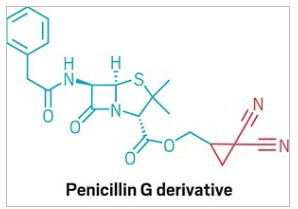
This new reaction makes it easier and safer to add cyclopropanes to complex molecules.
But despite its utility, cyclopropane is not easy to make compared with larger rings. There are a few established chemical methods for coaxing these highly strained molecular triangles into existence, but they require extra prefunctionalization steps or hazardous reagents such as diazo compounds.
Now, a team from Pennsylvania State University led by Ramesh Giri has developed a photocatalytic method for making cyclopropanes by combining alkenes and activated methylene derivatives such as 1,3-dicarbonyl compounds (Science 2023, DOI: 10.1126/science.adg3209). The reaction uses bench-stable reagents and isn’t air-sensitive—in fact, oxygen participates in it as an intermediate oxidant.
Rather than using a carbene or metal carbenoid as the most popular established methods do, this reaction was designed to go through a series of single-electron transfer steps initiated by blue light. “Carbenes are basically two radicals on one carbon. So instead of generating them in one step, we thought we would generate [radicals] stepwise,” Giri says. In addition to the starting materials, photocatalyst, and oxygen, the reaction also requires a catalytic amount of iodine to regenerate the catalyst.
The researchers found that the reaction works with a variety of mono- or disubstituted alkenes—though not dienes or styrene. It also works with multiple combinations of electron-withdrawing groups on methylene. Those groups also provide handles for further functionalization on the cyclopropane ring, which opens up synthetic possibilities that other methods can’t access.
Giri says he and his team are working on expanding the scope of alkenes that can be used and on scaling up the reaction. They also want to explore how to use this catalytic process for other alkene functionalization reactions. Giri also says he’s looking into incorporating the reaction into an undergraduate lab course. “This is how convenient it is. You couldn’t do that with any of the other reactions that you use to make cyclopropanes.”
Stereoenrichment with a single catalyst
Multitasking titanium catalyst erases and rewrites alcohols’ stereochemical information using two different mechanisms
Sometimes conformity is a good thing. Particularly when it comes to stereochemistry. While stereocenters arise in mirror-image enantiomer pairs when left to their own devices, the enantiomers can have very different biological activity. So synthetic and medicinal chemists would prefer to make only one of them at a time.
Researchers from the Shanghai Institute of Organic Chemistry, led by Zhiwei Zuo, have now added a new reaction to the stereoselective toolbox. They have devised a clever method for turning mixtures of alcohol isomers into an enantiomerically pure product using a single catalyst (Science 2023, DOI: 10.1126/science.adj0040).
Once a mixture of stereoisomers is created, it’s very difficult to amend that without separating the isomers from each other and throwing out the unwanted one. This is because physics isn’t on chemists’ side in this matter. Mixtures have higher entropy, which the universe prefers. And something called the principle of microscopic reversibility says that any mechanistic step that flips an unwanted enantiomer into its twin can also flip it back. Fixing a mixture requires at least two distinct steps: one to erase stereochemical information from the unwanted isomer, and another to rewrite it to match the desired form.
Chemists developing deracemization reactions have previously accomplished this by using different catalysts for each step. “We saw a more straightforward way to do that,” Zuo says—with a single titanium catalyst that can play two different mechanistic roles, bound to a chiral phosphoric acid ligand.
Light provides the energy to kick an electron from the ligand to the metal center through a process called ligand-to-metal charge transfer. The reduced metal center can then facilitate a radical reaction to break a carbon-carbon bond adjacent to the alcohol, creating an achiral intermediate. The titanium changes roles to act like a typical transition metal catalyst to coordinate with the intermediate and stitch the molecule back together. Throughout the reaction, the ligand helps direct the stereochemistry, making it so that bonds are preferentially broken in the unwanted isomer and ensuring that the molecules are reassembled with the correct orientation.
Individually, each step is only moderately stereoselective but they amplify each other to give nearly complete conversion to a single isomer. “We can get a synergy effect,” says Zuo.
The reaction works on cyclic secondary and tertiary alcohols with ring sizes between 4 and 7 carbons as well as some acyclic amino alcohols. The ideal ligand is different for each type of ring, and the molecule has to be able to stabilize the radical intermediate for the transformation to work, Zuo says, but he and his team are far from finished working on this reaction. They are developing another paper that describes more mechanistic explorations and an expanded substrate scope, he says.
In an email, Alison Wendlandt of the Massachusetts Institute of Technology called the work “spectacular” and “literally amazing,” praising it for its elegance and simplicity. She said the method “significantly expands” the scope of molecules that can be deracemized, opening up new routes to making useful chiral building blocks.
Also in an email, Robert Knowles of Princeton University called the paper “an exciting addition to the literature on light-driven deracemization” and said it could pave the way for exciting future advances.
Zuo says he envisions improving the reaction to edit the stereochemistry of alcohols—or other key functional groups—on more complex molecules, as a form of molecular editing.
A new catalyst for breaking down nylon 6
Researchers turn commercial plastic into reusable monomer without solvent or extreme temperatures
Researchers at Northwestern University have taken a step toward making it easier to chemically recycle nylon 6. The polymer is used in products including carpets, textiles, packaging—and fishing nets, which are a major contributor to the Great Pacific Garbage Patch.
The team, headed up by catalysis chemist Tobin J. Marks, has designed a process that converts nylon 6 back into its starting material, caprolactam (Chem 2023, DOI: 10.1016/j.chempr.2023.10.022). This depolymerization process doesn’t require a solvent and takes less energy to run than other methods for chemically breaking down nylons.
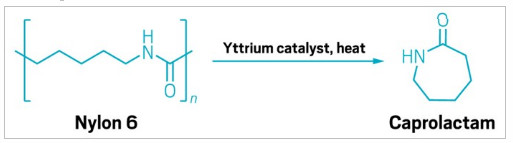
There are already industrial-scale processes for depolymerizing nylon trash and then using the caprolactam to make new nylon for textiles. Several brands, including Patagonia, use recycled nylon in some of their products. But the status quo chemical recycling process for nylon is time- and energy-intensive, making it quite expensive, says Yosi Kratish, who co-led the project. He says the team wanted to make nylon recycling easier, greener, and more cost-effective.
The polymers in plastic waste are simply huge molecules with bonds that can be manipulated like any other molecules, says Kratish. “It’s not a black box. We can zoom in on the weak spots and attack them.”
The catalyst system that the researchers designed is an improved version of one that they reported last year (Angew. Chem. Int. Ed. 2022, DOI: 10.1002/anie.202212543). It uses yttrium or lanthanum metal and a “sandwich” ligand that helps stabilize and protect the metal as it hops between polymer strands, breaking amide bonds.
The researchers did the reaction in air- and water-free conditions just above the polymer’s melting point, using only nylon and the catalyst, which eliminates the added energy and waste typically associated with using a solvent. “The greenest solvent of all is no solvent,” says Marks.
Even with a very small amount of catalyst in the reactor, it took only a few hours to completely break down 1 g of polymer. The researchers simulated a continuous process by periodically adding new material to keep the reaction going and got nearly complete conversion across six batches without adding any extra catalyst. The caprolactam they produced was high-quality enough to polymerize into new nylon that’s virtually indistinguishable in quality from the original material.
The researchers used their catalyst to break down a selection of chopped-up nylon products, including fishing net, yarn, carpet, and a t-shirt. They also found that it works to remove the nylon from mixed plastics, including medical gloves made of a nylon blend.
Liwei Ye, the postdoctoral scholar who designed the catalyst and did many of the hands-on experiments, explains that the catalyst works on mixed waste because it’s very selective for amide bonds. “If the catalyst meets a polyolefin, it doesn’t care, it will not bind to it. The moment it meets a polyamide, it sits on it, and it does its job,” he says.
Geoffrey Coates of Cornell University, who was not involved in the work, says, in an email, that the catalyst is impressively productive and shows “great potential” for chemically recycling nylon 6. “The obvious next challenge will be to see if the catalyst works in the real world” where there are many more substances that could poison the catalyst, he adds.
The team is now working on improving the catalyst and extending the polymerization to other nylons. Kratish is leading efforts to commercialize the depolymerization technology. “This is the dream . . . to develop something and then make it real,” he says.
Chemical & Engineering News
|
February
26-29, 2024 International Conference on Advanced Functional Materials and Devices (AFMD‐2024) Chennai, India |
https://afmdsrmist2024.in/ |
|
March
6-8, 2024 European Hydrogen Energy Conference (EHEC 2024) Bilbao, Spain |
https://ehec.info/ |
| 1 марта
2024 г.
Всероссийская молодежная научная школа-конференция "Актуальные проблемы органической химии" (АПОХ-2024) п. Шерегеш, Кемеровская обл., Россия |
http://web3.nioch.nsc.ru/ctoc2024/ |
|
1-6
апреля 2024 г.
Всероссийская научная конференция студентов-физиков и молодых ученых (ВНКСФ-28) г. Новосибирск, Россия |
http://asf.ural.ru/VNKSF/Zayavki/cond.html |
|
April
3,
2024 2024 Catalysis Science & Technology Symposium London, UK |
https://www.rsc.org/events/detail/77852/2024-catalysis-science-and-technology-symposium |
|
15-19
апреля 2024 г.
Научно-практическая конференция «Фторидные материалы и технологии» г. Москва, Россия |
http://fluorchem.ru/ |
|
May
6-8,
2024 Annual Meeting on Reaction Engineering and Electrochemical Processes 2024 Würzburg, Germany |
https://dechema.de/en/REACT2024.html |
|
May
20-21, 2024 European Congress of Applied Chemistry (ECAC24) Prague, Czech Republic |
https://www.rsc.org/events/detail/77027/european-congress-of-applied-chemistry-ecac24 |
|
May
27-31, 2024 POLY-CHAR Conference 2024 - Polymers for our future Madrid, Spain |
https://congresosalcala.fgua.es/poly-char2024/ |
|
28-31
мая 2024 г.
XIII Всероссийская научная конференция с международным участием и школа молодых ученых «Химия и технология растительных веществ» г. Сыктывкар, Россия |
https://chemi.komisc.ru/ru/page/menu.conf.CTPS_XIII/ |
|
June
2-7, 2024 International School of Photochemistry: from Photocatalysis to Photobiology (PHOTOCAT 24) Padova, Italy |
https://photocat24.com/ |
|
June
9-14, 2024 4th International summer school on catalyst preparation: fundamental concepts and industrial requirements (CatPrep2024) Vogüé, France |
https://catprep2024.sciencesconf.org/ |
|
June
16-19, 2024 International Symposium on Chemical Reaction Engineering Turku/Åbo, Finland |
https://www.iscre28.org/ |
|
June
17-21, 2024 12th European Conference on Solar Chemistry and Photocatalysis: Energy and Environmental Applications (SPEA12) Belfast, UK |
https://www.ulster.ac.uk/conference/spea12 |
|
17-21
июня
2024 г.
XII Международная конференция “Механизмы каталитических реакций” (MCR-XII) г. Владимир, Россия |
http://conf.nsc.ru/mcr-12/en |
|
June
24-28, 2024 85th Prague Meeting on Macromolecules - Polymers for Sustainable Future Prague, Czech Republic |
https://www.imc.cas.cz/sympo/85pmm/ |
|
June
30 – July 3, 2024 Industrial Biotechnology International Conference (IBIC 2024) Bologna, Italy |
https://www.aidic.it/ibic2024/ |
|
1-5
июля 2024 г.
XXIV Международная конференция по химической термодинамике в России г. Иваново, Россия |
http://rcct.isc-ras.ru/ |
|
2-6
июля 2024 г.
3-я международная конференция «Физика и химия горения и экстремальных процессов» (ComPhysChem'24) г. Самара, Россия |
http://comphyschem.ssau.ru/en/ |
|
July
10-12, 2024 International Symposium on Catalytic Chemistry of C1 Molecules Lille, France |
https://www.c1chem.org/ |
|
July
14-19, 2024 18th International Congress on Catalysis Lyon, France |
https://www.icc-lyon2024.fr/ |
|
August
25-29, 2024 27th International Congress of Chemical and Process Engineering (CHISA 2024) Prague, Czech Republic |
https://2024.chisa.cz/ |
|
September
2-6, 2024 XIII International Conference on Chemistry for Young Scientists “Mendeleev 2024” Saint Petersburg, Russia |
https://events.spbu.ru/events/mendeleev-2024?lang=Eng |
|
9-14
сентября 2024 г.
V Российский конгресс по катализу «РОСКАТАЛИЗ» г. Санкт-Петербург, Россия |
http://conf.nsc.ru/RusCat-5/ru |
|
September
10-12, 2024 Beilstein Organic Chemistry Symposium – Main-group Chemistry for Modern Catalysis and Synthesis Limburg an der Lahn, Germany |
https://www.beilstein-institut.de/en/symposia/main-group-chemistry/ |
|
September
17-20, 2024 4th International Conference on Fundamentals and Applications of Cerium Dioxide in Catalysis (CERIA 2024) Portorož-Portorose, Slovenia |
https://ceria2024.chem-soc.si/ |
|
3-5 октября 2024 г.
V Научно-технологический симпозиум «Гидропроцессы в катализе» (HydroCat-V) г. Сочи, Россия |
|
|
октябрь/ноябрь
2024 г.
III Международная конференция “Использование синхротронного излучения для исследования катализаторов и функциональных материалов” г. Новосибирск, Россия |
Памяти
Евгения Зиновьевича ГОЛОСМАНА
(1937 – 2023 гг.)

26 августа 2023 года ушел из жизни известный специалист в области промышленного катализа, главный научный сотрудник ООО НИАП-КАТАЛИЗАТОР, Заслуженный химик России, общественный деятель, доктор химических наук, профессор Евгений Зиновьевич Голосман.
Е.З. Голосман известен своими достижениями в научной деятельности, созданием научной школы, масштабным внедрением разработанных катализаторов с многомиллиардным экономическим эффектом в химической, металлургической, нефтехимической, пищевой, медицинской, атомной, оборонной и других отраслях промышленности, а также в сфере коммунального хозяйства, преподавательской деятельностью в НИ РХТУ им. Д.И. Менделеева и активной общественной работой.
Деятельность профессора Е.З. Голосмана тесно связана с Новомосковским филиалом государственного института азотной промышленности и продуктов органического синтеза (НИАП-КАТАЛИЗАТОР), куда он пришел в 1962 г. после окончания Московского химико-технологического института им. Д.И. Менделеева. Евгений Зиновьевич создал и возглавил сектор (1966 г.), лабораторию (1971 г.), а затем (1978 г.) хорошо оснащенный приборами и установками большой отдел физико-химических и аналитических исследований, стандартизации и качества в институте, который являлся одним из крупнейших центров страны по разработке, исследованию и наработке промышленных катализаторов. С 2011 года работал на должности главного научного сотрудника «НИАП-КАТАЛИЗАТОР».
Евгений Зиновьевич – действительный член Российской инженерной академии и Международной академии экологии, профессор кафедры химической технологии неорганических веществ Новомосковского института РХТУ им. Д.И. Менделеева.
Под руководством Е.З. Голосмана и при его непосредственном участии создано новое научное направление – химия приготовления оксидных и металлоксидных цементсодержащих катализаторов для широкого круга органических, неорганических и экологических процессов. В соответствии с разработанной малоотходной технологией организовано промышленное производство цементсодержащих катализаторов в катализаторном цехе «НИАП-КАТАЛИЗАТОР», катализаторном производстве Дорогобужского ЗАУ и др.
Е.З. Голосман принимал участие в испытаниях и внедрении в промышленность ряда разработанных катализаторов на химических, металлургических, машиностроительных, нефтехимических заводах. В первую очередь, для процессов метанирования, получения защитных атмосфер, синтеза бутиловых спиртов, анилина, низкотемпературной конверсии оксида углерода и др. на Новомосковской АК «Азот», «Салаватнефтеоргсинтез», Новолипецком, Магнитогорском, Белорецком, Мариупольском, меткомбинатах, Запсибе, Омском заводе «Химпром», Черкасском, Придонском, Северодонецком, Череповецком, Гродненском, Кирово-Чепецком, Березниковском, Щекинском, Одесском, Невинномысском, Тольяттинском, Куйбышевском, Дорогобужском азотных заводах, «Нижнекамскнефтехим», «Ангарскнефтехим», Кировоградском заводе «Пишмаш», заводе «Микропровод» (Подольск), Северском, Первоуральском трубных заводах, Ленинабадском комбинате редких металлов (г. Чорух-Дайрон, Таджикистан), Узбекском металлургическом заводе, адронном коллайдере в Швейцарии и др.
Под научным руководством профессора Е.З. Голосмана разработаны и внедрены в промышленность катализаторы разложения озона, созданы высокоэффективный катализатор метанирования, катализаторы очистки газов от закиси азота, катализаторы получения водородсодержащего газа для топливных элементов. Разработанные и синтезированные неплатиновые катализаторы внедрены и эксплуатируются на 170 заводах и организациях РФ, СНГ и дальнего зарубежья.
Профессор Е.З. Голосман внес существенный вклад в подготовку научных кадров: под его руководством выполнено более 200 научно-исследовательских дипломных работ студентов, защищено 12 кандидатских диссертаций. Автор более 600 публикаций, в том числе более 300 статей, четырех монографий, около 100 изобретений и патентов (как российских, так и иностранных), более 300 тезисов докладов научных конференций и др.
Евгений Зиновьевич активно занимался научно-организационной работой. Вице-президент Тульского областного правления Союза научных и инженерных общественных объединений, заместитель председателя Российского химического общества им. Менделеева Тульской области, член центрального правления РХО им. Менделеева. Член редколлегии журнала «Катализ в промышленности» и бюллетеня «Химия в России». Занесен в Книгу Почета НИАП, награжден Почетным Знаком Губернатора Тульской области «Общественное признание. В 2017 году профессор Е.З. Голосман стал лауреатом премии Правительства Российской Федерации в области науки и техники, в 2018 году награжден золотой медалью им. В.Г. Шухова и медалью «Лучший изобретатель Тульской области», в 2021 году получил звание Ветерана Химпрома и медаль имени академика В.А. Легасова, в 2022 году – орден «Инженерная слава» Российской инженерной академии.
Евгений Зиновьевич останется в памяти своих коллег и учеников как активный, творческий человек, посвятивший свою жизнь служению науке.


