22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы


18 апреля 2023 года отметил свое семидесятипятилетие Валентин Николаевич Пармон – академик РАН, вице-президент Российской академии наук, Председатель Сибирского отделения Российской академии наук, научный руководитель Института катализа им. Г.К. Борескова СО РАН, выдающийся ученый и организатор науки.
Валентин Николаевич, выпускник Московского физико-технического института, в 1977 г. был приглашен в Институт катализа Сибирского отделения АН СССР. В 1985 г. возглавил лабораторию каталитических методов преобразования солнечной энергии, с 1995 по 2015 гг. являлся директором Института катализа им. Г.К. Борескова СО РАН, сейчас – научный руководитель Института катализа СО РАН.
Основными направлениями научной деятельности В.Н. Пармона являются разработка и исследование катализаторов и каталитических процессов, в том числе для преобразования и аккумулирования различных видов энергии, катализ и фотокатализ в природе и в использовании возобновляемых и нетрадиционных энергоресурсов, а также выяснение роли абиогенных каталитических и фотокаталитических процессов в формировании состава атмосферы Земли и зарождении биосферы.
В.Н. Пармоном разработаны научные основы фотокаталитических методов преобразования солнечной энергии в химическую. Создано новое научное направление – радиационно-термический катализ. Под руководством В.Н. Пармона сконструированы и испытаны не имеющие мировых аналогов солнечные каталитические реакторы.
В.Н. Пармоном разработан принципиально новый подход к прямому преобразованию ионизирующего излучения в энергию химических топлив. В результате предложен и испытан в лабораторных условиях энергозапасающий и энергопреобразующий процесс “ИКАР”, который может быть эффективно использован для решения проблем ядерной и термоядерной энергетики будущего. Впервые созданы и испытаны не имеющие аналогов катализаторы на основе оксидов урана, комбинирующие функции ядерного топлива и катализатора для запасания химической энергии.
Под руководством В.Н. Пармона разработаны принципиально новые центробежные реакторы для термоударной обработки порошков твердых субстратов и отработана технология получения с помощью этих реакторов порошков активной окиси алюминия. Данная технология используется для промышленного производства новейшего поколения мелкосферических катализаторов дегидрирования легких алканов в олефины, уже нашедших промышленное применение для дегидрирования изобутана в изобутилен в кипящем слое катализатора на предприятии «Тобольск-Нефтехим» нефтехимической компании ОАО «СИБУР-холдинг».
Под руководством В.Н. Пармона разработаны и промышленно внедрены катализаторы нового поколения для производства моторных топлив. Ведутся работы по новым перспективным направлениям энергетики и транспорта (получение высококачественных топлив из возобновляемого растительного сырья, создание компактных генераторов водорода и др.).
В.Н. Пармон – вице-президент Российской академии наук, Председатель Сибирского отделения Российской академии наук, председатель Объединенного ученого совета по химическим наукам РАН, председатель Научного совета по катализу ОХНМ РАН.
В.Н. Пармон – автор и соавтор более 1000 научных работ, в том числе 8 монографий, 33 обзоров, 9 учебников для вузов, имеет более 150 авторских свидетельств и патентов на изобретения.
Валентин Николаевич активно участвует в подготовке научных кадров. Более 40 лет он возглавлял кафедру физической химии на факультете естественных наук Новосибирского государственного университета. В.Н. Пармон является почетным профессором Санкт-Петербургского государственного технологического института (Технологического университета), Новосибирского государственного университета и Томского государственного университета, в Томском государственном университете является председателем Совета Центра исследований в области материалов и технологий. Среди учеников Валентина Николаевича 13 докторов и 24 кандидата наук.
В.Н. Пармон – главный редактор-организатор журнала «Катализ в промышленности» и Каталитического бюллетеня, а также редактор журнала “Химия в России” (РХО), член редколлегий журналов “Успехи химии”, “Кинетика и катализ”, “Журнала физической химии” (РАН), ряда международных журналов, член Президиума Российского химического Общества имени Д.И. Менделеева, Сопрезидент российского каталитического общества.
Заслуги В.Н. Пармона отмечены высокими государственными наградами, среди которых Государственная премия РФ, премия им. В.А. Коптюга СО РАН и НАН Беларуси, премия за инновации в катализе EFCATS, международная премия «Глобальная энергия». Награждён орденом «За заслуги перед Отечеством» IV степени, орденом Почёта, медалью Франциска Скорины Республики Беларусь.

Институт катализа им. Г.К. Борескова СО РАН – один из крупнейших в России научно-исследовательских центров, деятельность которого связана с важнейшими промышленными сферами – нефтепереработкой, химией, нефтехимией, энергетикой, экологией, отмечает 65 лет со дня своего основания.
В основе деятельности ИК СО РАН – сочетание фундаментальный научных исследований с решением актуальных проблем отечественной промышленности.
Институт катализа был создан как Институт катализа Сибирского отделения Академии наук СССР Постановлением ЦК КПСС и Совета министров СССР № 795 от 23 июля 1958 г.
Основные направления деятельности Института, сформулированные его основателем и первым директором академиком Георгием Константиновичем Боресковым, включали разработку теории катализа, научных основ приготовления катализаторов, разработку и усовершенствование промышленных каталитических процессов, развитие методов математического моделирования каталитических реакторов.
С 1958 по 1984 г. директором Института был Георгий Константинович Боресков, с 1984 по 1995 г. – Кирилл Ильич Замараев.

В 1991 году Институту катализа присвоено имя академика Г.К. Борескова. В 1995 году Институт возглавил Валентин Николаевич Пармон, перешедший в 2015 году на должность научного руководителя ИК СО РАН (с 2017 г. – председатель Сибирского отделения РАН и вице-президент Российской академии наук). С 2015 г. директором ИК СО РАН является Валерий Иванович Бухтияров

В 2019 году Институт катализа был преобразован в Федеральный исследовательский центр (ФИЦ) «Институт катализа СО РАН». Сейчас в состав ФИЦ входят Институт катализа СО РАН (в качестве головной организации) и три филиала: Центр новых химических технологий ИК СО РАН (г. Омск), Волгоградский филиал ИК СО РАН и Центр коллективного пользования «Сибирский кольцевой источник фотонов» (ЦКП «СКИФ»). ФИЦ «Институт катализа СО РАН» стал самым крупным в стране специализированным научным центром в области химического катализа и каталитических процессов.
Миссия Института:
Роль Института катализа в развитии химического комплекса СССР и России была подробно рассмотрена на расширенном совместном заседании Ученого совета ИК СО РАН и Президиума Сибирского отделения РАН.
Прозвучали доклады руководителей Института:
1. «Катализ в Сибирском отделении РАН: вехи истории».
Научный руководитель ИК СО РАН, академик РАН
Пармон Валентин Николаевич
2. «Разработка промышленных катализаторов для нефте(газо)химии: история, настоящее, будущее».
Заместитель директора, член-корреспондент РАН
Носков Александр Степанович
3. «Каталитические технологии для водородной энергетики».
Заместитель директора, д.х.н., профессор РАН
Мартьянов Олег Николаевич.
4. «Получение и свойства сверхвысокомолекулярного полиэтилена,
перерабатываемого твердофазным способом».
Заместитель директора, д.х.н., профессор РАН
Адонин Николай Юрьевич
5. «Источник синхротронного излучения ЦКП «СКИФ» – инструмент проведения
передовых исследований и развития новых технологий в Российской Федерации».
Директор ИК СО РАН, академик РАН
Бухтияров Валерий Иванович
В рамках мероприятий, посвященных юбилею Института катализа СО РАН, с 3 по 7 июля 2023 г. в Новосибирске был проведен 8-й Азиатский симпозиум по современным материалам (8th Asian Symposium on Advanced Materials, ASAM-8). Кроме российских ученых, в работе международного симпозиума приняли участие около 50 представителей Кореи, Японии, Тайваня, Китая и Австралии. Основные темы научной программы связаны с областями применения передовых материалов, их синтезом и структурой, биоматериалами и бионанокомпозитами.
16-18 мая 2023 г.
Омск, Россия
http://conf.nsc.ru/sigma-7/ru
С 16 по 18 мая 2023 г. в Омске прошла VII Всероссийская научная молодёжная школа-конференция «Химия под знаком СИГМА: исследования, инновации, технологии». Главным организатором мероприятия выступил Федеральный исследовательский центр «Институт катализа им. Г.К. Борескова Сибирского отделения Российской академии наук в лице своего Омского филиала – Центра новых химических технологий (ЦНХТ ИК СО РАН). Ключевыми партнерами конференции были Федеральное государственное автономное образовательное учреждение высшего образования «Омский государственный технический университет» (ОмГТУ), а также Центр компетенций Национальной технологической инициативы «Водород как основа низкоуглеродной экономики».
Школа-конференция проводилась при поддержке Министерства науки и высшего образования Российской Федерации, Министерства промышленности и научно-технического развития Омской области и Российского химического общества имени Д.И. Менделеева. В 2023 году это мероприятие впервые было проведено на территории Омского государственного технического университета (ОмГТУ), включая пространство «Точка кипения» ОмГТУ.
Школа-конференция «Химия под знаком СИГМА» каждый раз вызывает большой интерес как со стороны молодых исследователей, так и со стороны ведущих учёных-химиков. В этом году для участников Школы-конференции параллельно проводились четыре научные сессии по направлениям «Методы исследования функциональных материалов», «Новые функциональные материалы», «Экологический катализ, фотокатализ и катализ для альтернативной энергетики» и «Каталитические процессы переработки углеводородного сырья».

В первый день Школы-конференции состоялась панельная дискуссия на тему «Современные проблемы исследования функциональных материалов». Модератором мероприятия выступил д.х.н., профессор РАН Олег Николаевич Мартьянов (ИК СО РАН, Новосибирск), а в качестве спикеров в дискуссии приняли участие д.х.н. Василий Васильевич Каичев (ИК СО РАН, Новосибирск), к.х.н. Андрей Валерьевич Бухтияров (ИК СО РАН, Новосибирск), к.х.н. Вячеслав Леонидович Юрпалов (ЦНХТ ИК СО РАН, Омск) и к.т.н. Евгений Анатольевич Рогачев (ОмГТУ, Омск). Участники обсудили эффективность применения методов ЯМР и ЭПР, а также использования синхротронного излучения для исследования катализаторов. Особое внимание было уделено функционированию Центров коллективного пользования, действующих на базе научно-исследовательских и образовательных организаций. Большую заинтересованность у участников панельной дискуссии вызвал доклад А.В. Бухтиярова об исследовательских возможностях нового комплекса синхротронного излучения «СКИФ», создание которого полным ходом идёт в Новосибирске.

 В рамках работы Школы-конференции было представлено 13 пленарных докладов от ведущих учёных Российской Федерации. Открыла работу пленарной сессии в первый день Школы-конференции (16 мая) д.х.н. Екатерина Сергеевна Локтева (МГУ им. М.В. Ломоносова, Москва). Её лекция «Темплатные методы приготовления оксидных катализаторов» была посвящена возможностям синтеза носителей и катализаторов с заданными текстурными характеристиками, в том числе с полимодальным распределением пор по размерам.
В рамках работы Школы-конференции было представлено 13 пленарных докладов от ведущих учёных Российской Федерации. Открыла работу пленарной сессии в первый день Школы-конференции (16 мая) д.х.н. Екатерина Сергеевна Локтева (МГУ им. М.В. Ломоносова, Москва). Её лекция «Темплатные методы приготовления оксидных катализаторов» была посвящена возможностям синтеза носителей и катализаторов с заданными текстурными характеристиками, в том числе с полимодальным распределением пор по размерам.
 Большой интерес вызвала пленарная лекция д.х.н., профессора РАН Олега Николаевича Мартьянова (ИК СО РАН, Новосибирск) «Развитие и применение передовых методов in situ для исследования стабильности и физико-химических процессов в нефтяных системах». В своей лекции Олег Николаевич дал анализ потенциала современных физических методов исследований фазовой стабильности и физико-химических процессов, происходящих в тяжелых нефтях в режиме in situ , в том числе в условиях химических превращений тяжелых компонентов. Он представил основные принципы применения методов in situ , обсудил возможности и ограничения каждого из подходов.
Большой интерес вызвала пленарная лекция д.х.н., профессора РАН Олега Николаевича Мартьянова (ИК СО РАН, Новосибирск) «Развитие и применение передовых методов in situ для исследования стабильности и физико-химических процессов в нефтяных системах». В своей лекции Олег Николаевич дал анализ потенциала современных физических методов исследований фазовой стабильности и физико-химических процессов, происходящих в тяжелых нефтях в режиме in situ , в том числе в условиях химических превращений тяжелых компонентов. Он представил основные принципы применения методов in situ , обсудил возможности и ограничения каждого из подходов.
 Дальнейшее рассмотрение методов in situ последовало в пленарной лекции д.х.н. Василия Васильевича Каичева (ИК СО РАН, Новосибирск) «In situ и Operando исследования катализаторов и каталитических реакций». В ней подробно были рассмотрены особенности проведения ex situ, in situ и operando исследований катализаторов и каталитических реакций методами рентгеновской дифракции, РФЭС, XANES, EXAFS и ИК спектроскопии, обоснован выбор того или иного метода для изучения конкретных каталитических систем. Отдельная часть лекции была посвящена результатам operando исследований автоколебаний в реакции окисления пропана и метана на палладиевых и никелевых катализаторах методами рентгеновской дифракции, РФЭС и масс-спектрометрии.
Дальнейшее рассмотрение методов in situ последовало в пленарной лекции д.х.н. Василия Васильевича Каичева (ИК СО РАН, Новосибирск) «In situ и Operando исследования катализаторов и каталитических реакций». В ней подробно были рассмотрены особенности проведения ex situ, in situ и operando исследований катализаторов и каталитических реакций методами рентгеновской дифракции, РФЭС, XANES, EXAFS и ИК спектроскопии, обоснован выбор того или иного метода для изучения конкретных каталитических систем. Отдельная часть лекции была посвящена результатам operando исследований автоколебаний в реакции окисления пропана и метана на палладиевых и никелевых катализаторах методами рентгеновской дифракции, РФЭС и масс-спектрометрии.
 Пленарная лекция д.х.н., профессора РАН Оксаны Павловны Таран (ИХХТ СО РАН, ФИЦ КНЦ СО РАН, Красноярск) «Ароматические соединения из растений. Лигнин или целлюлоза?» затронула различные аспекты переработки растительного сырья в ароматические соединения. В ней подробно были рассмотрены процессы переработки лигнина (включая новую концепцию “ligninfirst”) и фурановых соединений, получаемых при кислотном катализе из полисахаридов.
Пленарная лекция д.х.н., профессора РАН Оксаны Павловны Таран (ИХХТ СО РАН, ФИЦ КНЦ СО РАН, Красноярск) «Ароматические соединения из растений. Лигнин или целлюлоза?» затронула различные аспекты переработки растительного сырья в ароматические соединения. В ней подробно были рассмотрены процессы переработки лигнина (включая новую концепцию “ligninfirst”) и фурановых соединений, получаемых при кислотном катализе из полисахаридов.
 Второй день конференции был открыт пленарной лекцией д.х.н. Игоря Викторовича Трушкова (НМИЦ ДГОИ им. Д. Рогачева, ИОХ им. Н.Д. Зелинского РАН, Москва) «Протонные ионные жидкости: растворители, катализаторы, реагенты». В ней было рассказано о новой концепции применения протонных ионных жидкостей в качестве реагентов тройного назначения (растворителей, кислотных катализаторов и реагентов). Эффективность такого подхода была продемонстрирована на модельных процессах раскрытия донорно-акцепторных циклопропанов и эпоксидов, а также успешной реализацией новых домино-процессов, в которых протонная ионная жидкость выступает в качестве растворителя, обеспечивает активацию субстрата в результате общего кислотного катализа и предоставляет анион для участия в реакции в качестве нуклеофила.
Второй день конференции был открыт пленарной лекцией д.х.н. Игоря Викторовича Трушкова (НМИЦ ДГОИ им. Д. Рогачева, ИОХ им. Н.Д. Зелинского РАН, Москва) «Протонные ионные жидкости: растворители, катализаторы, реагенты». В ней было рассказано о новой концепции применения протонных ионных жидкостей в качестве реагентов тройного назначения (растворителей, кислотных катализаторов и реагентов). Эффективность такого подхода была продемонстрирована на модельных процессах раскрытия донорно-акцепторных циклопропанов и эпоксидов, а также успешной реализацией новых домино-процессов, в которых протонная ионная жидкость выступает в качестве растворителя, обеспечивает активацию субстрата в результате общего кислотного катализа и предоставляет анион для участия в реакции в качестве нуклеофила.
 В своей пленарной лекции «Структурированные катализаторы и их применение в энергоустановках» д.х.н. Павел Валерьевич Снытников (ИК СО РАН, Новосибирск) рассказал о новых тенденциях по разработке энергоустановок на основе топливных элементов, поиску и исследованию катализаторов окислительной конверсии (паровой, автотермической конверсии или парциального окисления) углеводородных топлив, инженерных разработках конструкций топливного процессора, а также о разработке нового метода сорбционно-гидролитического осаждения для нанесения активного компонента на поверхность сформированного носителя.
В своей пленарной лекции «Структурированные катализаторы и их применение в энергоустановках» д.х.н. Павел Валерьевич Снытников (ИК СО РАН, Новосибирск) рассказал о новых тенденциях по разработке энергоустановок на основе топливных элементов, поиску и исследованию катализаторов окислительной конверсии (паровой, автотермической конверсии или парциального окисления) углеводородных топлив, инженерных разработках конструкций топливного процессора, а также о разработке нового метода сорбционно-гидролитического осаждения для нанесения активного компонента на поверхность сформированного носителя.
 Живой интерес вызвала лекция к.х.н. Владимира Львовича Кузнецова (ИК СО РАН, Новосибирск) «Керамические композиты, модифицированные углеродными нанотрубками». В ней автор отразил новые подходы к синтезу керамических композитов, модифицированных углеродными нанотрубками, с комплексом улучшенных свойств (механических, электрических, оптических), необходимых для использования в различных приложениях.
Живой интерес вызвала лекция к.х.н. Владимира Львовича Кузнецова (ИК СО РАН, Новосибирск) «Керамические композиты, модифицированные углеродными нанотрубками». В ней автор отразил новые подходы к синтезу керамических композитов, модифицированных углеродными нанотрубками, с комплексом улучшенных свойств (механических, электрических, оптических), необходимых для использования в различных приложениях.
 Свою пленарную лекцию «Эффекты адсорбционно-индуцированной сегрегации как инструмент для управления каталитическими свойствами биметаллических катализаторов» к.х.н. Андрей Валерьевич Бухтияров (ИК СО РАН, Новосибирск) посвятил обобщению результатов, полученных при исследовании биметаллических катализаторов для выяснения причин проявления в них синергетических эффектов. Особенно был отмечен факт установления корреляций между каталитическими свойствами и химическим состоянием/структурой активного компонента на поверхности биметаллических катализаторов. Андрей Валерьевич также рассказал об исследовании сегрегационных эффектов под воздействием газовых или реакционных сред и о разработке рекомендаций к «тонкой настройке» поверхности в «реальных» биметаллических катализаторах для достижения максимальной активности, стабильности, селективности в реакциях селективного гидрирования C≡C связи.
Свою пленарную лекцию «Эффекты адсорбционно-индуцированной сегрегации как инструмент для управления каталитическими свойствами биметаллических катализаторов» к.х.н. Андрей Валерьевич Бухтияров (ИК СО РАН, Новосибирск) посвятил обобщению результатов, полученных при исследовании биметаллических катализаторов для выяснения причин проявления в них синергетических эффектов. Особенно был отмечен факт установления корреляций между каталитическими свойствами и химическим состоянием/структурой активного компонента на поверхности биметаллических катализаторов. Андрей Валерьевич также рассказал об исследовании сегрегационных эффектов под воздействием газовых или реакционных сред и о разработке рекомендаций к «тонкой настройке» поверхности в «реальных» биметаллических катализаторах для достижения максимальной активности, стабильности, селективности в реакциях селективного гидрирования C≡C связи.
В заключительный день Школы-конференции вниманию участников были представлены пять пленарных лекций.
 В докладе к.х.н. Светланы Витальевны Черепановой (ИК СО РАН, Новосибирск) «Нанодоменные структуры и рентгеновская дифракция» были подробно обсуждены различные причины образования доменной структуры и подходы для ее исследования. Докладчик отобразил два основных подхода для определения параметров нанодоменной структуры методом рентгеновской дифракции, основанных на создании модели нанодоменной структуры, на базе которой рассчитывается порошковая рентгенограмма. В первом подходе создается вероятностная модель (усредненная по всем частицам) с пластинчатыми нанодоменами. Во втором подходе домены могут быть различной формы, но требуется усреднение по ансамблю частиц.
В докладе к.х.н. Светланы Витальевны Черепановой (ИК СО РАН, Новосибирск) «Нанодоменные структуры и рентгеновская дифракция» были подробно обсуждены различные причины образования доменной структуры и подходы для ее исследования. Докладчик отобразил два основных подхода для определения параметров нанодоменной структуры методом рентгеновской дифракции, основанных на создании модели нанодоменной структуры, на базе которой рассчитывается порошковая рентгенограмма. В первом подходе создается вероятностная модель (усредненная по всем частицам) с пластинчатыми нанодоменами. Во втором подходе домены могут быть различной формы, но требуется усреднение по ансамблю частиц.
 Вопросы, рассмотренные в пленарной лекции к.х.н. Светланы Анатольевны Яшник (ИК СО РАН, Новосибирск) «Современные подходы к улучшению характеристик окислительных катализаторов для систем очистки отработанных газов дизельных двигателей», позволили отобразить место окислительного катализатора в современной системе очистки отработанных газов автомобилей с дизельным двигателем, способы улучшения и оптимизации окислительных катализаторов, предназначенных для нейтрализации монооксида углерода и углеводородов до уровня действующих экологических стандартов, а также пути снижения содержания металлов Pt-группы в указанных каталитических системах.
Вопросы, рассмотренные в пленарной лекции к.х.н. Светланы Анатольевны Яшник (ИК СО РАН, Новосибирск) «Современные подходы к улучшению характеристик окислительных катализаторов для систем очистки отработанных газов дизельных двигателей», позволили отобразить место окислительного катализатора в современной системе очистки отработанных газов автомобилей с дизельным двигателем, способы улучшения и оптимизации окислительных катализаторов, предназначенных для нейтрализации монооксида углерода и углеводородов до уровня действующих экологических стандартов, а также пути снижения содержания металлов Pt-группы в указанных каталитических системах.
 В пленарной лекции к.х.н. Антона Алексеевича Габриенко (ИК СО РАН, Новосибирск) «Спектроскопия ЯМР высокого разрешения в твёрдом теле для изучения превращений углеводородов на металл-модифицированных цеолитах» были продемонстрированы возможности спектроскопии ЯМР высокого разрешения в твердом теле для изучения свойств гетерогенных катализаторов и механизмов их каталитического действия. Были представлены наиболее свежие и интересные результаты, полученные методами ЯМР ВМУ при исследованиях механизмов активации и превращения легких углеводородов (C2-C4), а также метана, на цеолитах ZSM-5 и BEA, модифицированных Zn, Cu, In и Ag.
В пленарной лекции к.х.н. Антона Алексеевича Габриенко (ИК СО РАН, Новосибирск) «Спектроскопия ЯМР высокого разрешения в твёрдом теле для изучения превращений углеводородов на металл-модифицированных цеолитах» были продемонстрированы возможности спектроскопии ЯМР высокого разрешения в твердом теле для изучения свойств гетерогенных катализаторов и механизмов их каталитического действия. Были представлены наиболее свежие и интересные результаты, полученные методами ЯМР ВМУ при исследованиях механизмов активации и превращения легких углеводородов (C2-C4), а также метана, на цеолитах ZSM-5 и BEA, модифицированных Zn, Cu, In и Ag.
К.х.н. Виталий Алексеевич Горбунов (ОмГТУ, Омск) в своём докладе «Модели самосборки поверхностных металлорганических структур» обобщил подходы к прогнозированию фазового поведения поверхностных металлорганических слоев (ПМОС), состоящих из функциональных органических молекул, координированных атомами переходных металлов (медь, железо, кобальт и др.). В докладе была подробно рассмотрена самосборка ПМОС на основе молекул-линкеров с С2 и С3 симметрией расположения функциональных групп. В качестве итога своего доклада Виталий Алексеевич заключил, что с помощью квантово-химических методов было показано, что ПМОС на основе 1,3,5- трис(пиридил)бензола и меди на окисленной поверхности карбида титана может быть активным и стабильным катализатором для водородных топливных ячеек.
Честь завершить сессию пленарных докладов в рамках Школы-конференции выпала к.х.н. Вячеславу Леонидовичу Юрпалову (ЦНХТ ИК СО РАН, Омск). В своей лекции «Новые возможности ЭПР-спектроскопии в изучении кислотных свойств алюмооксидных катализаторов» Вячеслав Леонидович отразил основные подходы по применению метода спектроскопии электронного парамагнитного резонанса (ЭПР) к исследованию кислотных центров, расположенных на поверхности сложных оксидных систем с использованием молекул-зондов различной природы. При этом особое внимание было уделено методическим аспектам применения ЭПР-спектроскопии молекул-зондов.
 С устными докладами в работе Школы-конференции приняли участие 127 человек, из которых 113 – молодые ученые. География Школы-конференции включала такие города, как Омск, Новосибирск, Томск, Бийск, Красноярск, Казань, Махачкала, Новочеркасск, Тверь, Санкт-Петербург, Москва и Харбин (КНР). В каждой секции были выделены два лучших доклада. Таким образом, восемь молодых учёных-исследователей были отмечены ценными призами и грамотами. Дипломами от Программного комитета были награждены Зоя Николаевна Лащинская (ИК СО РАН, Новосибирск), Софья Дмитриевна Афонникова (ИК СО РАН, Новосибирск), Наталья Викторовна Мальцева (НГУ, Новосибирск) и Анастасия Ивановна Золотухина (ТГУ, Томск). Дипломы от Российского химического общества им. Д.И. Менделеева получили Анастасия Сергеевна Жирнова (ИК СО РАН, Новосибирск), Данил Михайлович Шивцов (ИК СО РАН, Новосибирск), Мария Владимировна Черных (ТГУ, Томск) и Иван Николаевич Зубков (ЮРГПУ, Новочеркасск).
С устными докладами в работе Школы-конференции приняли участие 127 человек, из которых 113 – молодые ученые. География Школы-конференции включала такие города, как Омск, Новосибирск, Томск, Бийск, Красноярск, Казань, Махачкала, Новочеркасск, Тверь, Санкт-Петербург, Москва и Харбин (КНР). В каждой секции были выделены два лучших доклада. Таким образом, восемь молодых учёных-исследователей были отмечены ценными призами и грамотами. Дипломами от Программного комитета были награждены Зоя Николаевна Лащинская (ИК СО РАН, Новосибирск), Софья Дмитриевна Афонникова (ИК СО РАН, Новосибирск), Наталья Викторовна Мальцева (НГУ, Новосибирск) и Анастасия Ивановна Золотухина (ТГУ, Томск). Дипломы от Российского химического общества им. Д.И. Менделеева получили Анастасия Сергеевна Жирнова (ИК СО РАН, Новосибирск), Данил Михайлович Шивцов (ИК СО РАН, Новосибирск), Мария Владимировна Черных (ТГУ, Томск) и Иван Николаевич Зубков (ЮРГПУ, Новочеркасск).
Уникальной особенностью для участников VII Всероссийской научной молодёжной школы-конференции «Химия под знаком СИГМА: исследования, инновации, технологии» стала возможность прослушать программу дополнительного профессионального образования – курс повышения квалификации «Водородная энергетика и химическая технология топлива».
Организаторы Школы-конференции выражают свою глубокую благодарность гостям за активное участие в работе и приглашают принять участие в следующей конференции «Химия под знаком СИГМА: исследования, инновации, технологии».

Организационный комитет школы-конференции
Материал подготовили:
Л.Н. Степанова, А.В. Лавренов
Центр новых химических технологий
Института катализа им. Г.К. Борескова СО РАН, г. Омск
An up-close view of catalysis in real time
Scientists observe how different temperatures alter interactions between metal nanoparticles and support material
One way to fight global warming is to capture carbon dioxide before it enters the atmosphere and convert it into something useful, such as methane, which can be a fuel or a chemical feedstock. That conversion, which bonds hydrogen atoms to carbon atoms, requires a catalyst, and now a group of researchers has uncovered how a certain class of catalysts can be treated to make them more efficient (Science 2023, DOI: 10.1126/science.adf6984).
Scientists have long known that placing a metal catalyst atop a different material could improve catalytic performance, a phenomenon called strong metal–support interaction (SMSI). The new study marks the first time researchers have actually seen in real time what’s going on in SMSI and how it can be adjusted for optimum performance.
The team set nickel nanoparticles atop titanium dioxide and used a combination of electron microscopy and vibrational spectroscopy to examine how individual atoms behaved when the catalyst was preheated and then exposed to CO2 and hydrogen. When they heated the catalyst to 400 °C, the TiO2 formed thin overlayers coating the nanoparticles. When they subsequently introduced the gases, the overlayers crawled off the nickel, leaving it completely exposed. With pretreatment to 600 °C, however, the titanium only slid off certain facets of the nanoparticle during catalysis, leaving the nickel only partially exposed. That left behind more interface sites between the titanium and the nickel where carbon reactions could take place, which made the catalysis more active and selective, working at a faster rate and creating fewer unwanted byproducts.
Bert Weckhuysen, a chemistry professor at Utrecht University in the Netherlands and one of the leaders of the work, says other temperatures probably lead to different amounts of interfaces between the materials, and he plans to study those to see if they give even better results. He also hopes to look at other SMSI systems, such as cobalt on titania.
Redesigning the nanoparticles to create different facets to which the overlayers cling might also provide a way to fine-tune the catalysis, says Sara Bals, a professor of physics at the University of Antwerp in Belgium and another leader of the study. “This is a completely new playground, and we look forward to exploring that new playground,” she says.
Wenyu Huang, a professor of chemistry at Iowa State University, calls the study “a substantial advancement” in the field. “This work will profoundly impact catalyst design for many important reactions that require harsh reaction conditions,” he says.
And Ding Ma, a professor of chemistry at Peking University in China not involved in the study, says the results are a “groundbreaking finding” in SMSI, which “has been the hot topic in catalysis.” He says the knowledge provided by the research “could be surely used to design new efficient/effective catalyst systems.”
Chemists make alkyl chlorides with less waste
Method swaps out oxidants and reductants for a visible-light catalyst
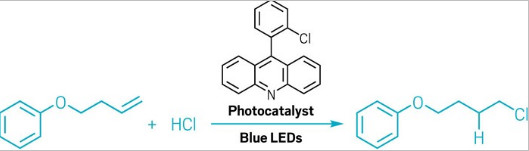
Chemists have figured out how to produce an industrially important reaction in a very economical way. Usually, adding a hydrochloric acid across a double bond involves two steps—an oxidation and a reduction—and requires a 1:1 ratio of both the oxidant and the reductant. Now Tobias Ritter and coworkers at the Max Planck Institute for Kohlenforschung and RWTH Aachen University devised a method that requires only adding a photocatalyst and visible light to the starting material and hydrochloric acid (Nat. Catal. 2023, DOI:10.1038/s41929-023-00914-7). This new finding represents reactivity that’s the opposite of a well-established mechanism in organic chemistry, and it’s a way to make commodity chemicals with less waste. In college organic chemistry class, students usually learn about the Markovnikov rule: when adding a mineral acid across a carbon-carbon double bond, the compound that comes from the stablest carbocation is the one that will form. Anti-Markovnikov reactions, which have the opposite selectivity, are well known to come out of radical reactions of mineral acids, but these require some sort of stoichiometric initiator. This reaction does not, Ritter says. The researchers used cheap available mineral acids, such as HCl, and added them across simple olefins to form an alkyl chloride, he says. This is not the first time that chemists have made an alkyl chloride from an olefin, Ritter says. “There are a lot of other robust, cheaper methods than what we have here,” he says. But this is the first time anyone has done this reaction with high atom economy, he says. The real target for industrial chemists is taking a cheap feedstock chemical and adding water across a double bond to make primary alcohols, a starting material for a massive amount of industrial chemicals, Ritter says. This new method represents a step toward making those reactions with less waste.
Iron-rich meteorites and volcanic ash catalyzed prebiotic molecules on early Earth
Hydrogenation catalysts may have rained down on the early Earth
Researchers have long debated how the molecular components needed for life came to exist in the bleak environment of the early Earth about 4 billion years ago. Researchers now present experimental evidence for a new possibility–that metals from iron-rich meteorites pelting the planet from above, as well as from volcanic particles spewing from within, catalyzed the fixation of carbon dioxide, the dominant gas in the atmosphere at the time. This helped build basic carbon-containing molecules such as hydrocarbons, aldehydes, and alcohols, which could then perform the reactions that generated more complex biotic molecules.
“We have closed a gap in the story of initiating life,” says Oliver Trapp, a chemist at Ludwig Maximilian University of Munich, who led the work (Sci. Rep. 2023, DOI: 10.1038/s41598-023-33741-8).
The idea hit Trapp like a meteorite—literally. One day about 6 years ago, he was examining a meteorite he had purchased that was part of the Campo del Cielo, a cluster of meteorites thought to have fallen to Earth more than 4,000 years ago in Argentina. Reading the certificate listing the rock’s components–92% iron, 7% nickel, 0,5% cobalt, and a sprinkle of iridium—he imagined a meteorite hurtling through the atmosphere. It would slow down and heat up, causing the outside of the rocks to form nanoparticles. “Of course, these nanoparticles are highly reactive,” Trapp says. “I immediately realized this was the perfect Fischer-Tropsch catalyst.”
To test their idea experimentally, he and his colleagues made nanoparticles from meteorites and from volcanic ash and minerals. They put these materials into chambers that simulated a variety of atmospheric and climate conditions of that time–specifically, different atmospheric pressures and temperatures, different ratios of CO2 and hydrogen, and wet versus dry climate conditions. Across climate scenarios, the iron and other metals in the meteorite and ash particles catalyzed the synthesis of prebiotic molecules.
“Under [all] these conditions you are getting really, really similar compositions of these oxygenated compounds, which are really nice building blocks that can continue to generate chemistry,” Trapp says. Based on the rate of reactions observed, as well as estimates from other studies of ancient meteorite activity, the researchers calculated that this mechanism could have churned out 600,000 metric tons of these molecules per year, for tens to hundreds of millions of years. The team is now conducting large-scale simulation models and experiments to explore what kinds of reactions this initial prebiotic mixture molecules might have undergone and what larger compounds it might have produced.
“It’s surprising that chemists haven’t until recently systematically looked at how inorganic volcanic and meteoritic particles react with CO2,”, says Joseph Moran, a chemist at the University of Strasbourg. “The early Earth was producing hydrogen and hydrogenation catalysts were literally raining down from the sky,” Moran says. “Perhaps it is no coincidence that very ancient organisms were running their metabolisms on hydrogenation.”
Chemical & Engineering News
| July 23-28, 2023 31st International Conference on Photochemistry Sapporo, Japan |
https://icp2023.jp/ |
| July 30 – August 4, 2023 Gordon Research Conference – Photochemistry Lewiston, United States |
https://www.grc.org/photochemistry-conference/2023/ |
| 14-18 августа 2023 г. 4-я Российская конференция «Графен: молекула и 2 D кристалл» Новосибирск, Россия |
http://grapheneconf.nsu.ru/ |
| August 20-25, 2023 49th IUPAC World Chemistry Congress 2023 (IUPAC|CHAINS 2023) Hague, the Netherlands |
https://iupac2023.org/ |
| August 27 – September 1, 2023 15th European Congress on Catalysis (EuropaCat 2023) Prague, Czech Republic |
https://www.europacat2023.cz/ |
| 4-8 сентября 2023 г. Международная конференция по химии «Байкальские чтения-2023» Иркутск, Россия |
https://irkinstchem.ru/conf/baikalreading23 |
| September 5-8, 2023 14th International Workshop on Polymer Reaction Engineering Potsdam, Germany |
https://dechema.de/en/PRE2023.html |
| 6-8 сентября 2023 г. VII Всероссийская научно-практическая конференция молодых ученых и специалистов «Материалы и технологии XXI века» Бийск, Алтайский край, Россия |
uda@ frpc.secna.ru +7 (3854) 30-18-15 8 963 574 8985 |
| September 10-13, 2023 14th European Adhesion Conference & 7th World Congress on Adhesion and Related Phenomena (EURADH/WCARP 2023) Garmisch-Partenkirchen, Germany |
https://dechema.de/en/euradh_wcarp_2023.html |
| 11-13 сентября 2023 г. 3-я Школа молодых ученых «Электрохимические устройства: процессы, материалы, технологии» Новосибирск, Россия |
http://www.solid.nsc.ru/school2023/ |
| September 11-15, 2023 8th International Conference on Semiconductor Photochemistry Strasbourg, France |
https://sp8.unistra.fr/ |
| September 14-16, 2023 16th Edition of Global Conference on Catalysis, Chemical Engineering & Technology (CAT 2023) Valencia, Spain |
https://magnusconferences.info/catalysis |
| September 17-21, 2023 14th European Congress of Chemical Engineering and 7th European Congress of Applied Biotechnology (ECCE/ECAB 2023) Berlin, Germany | https://ecce-ecab2023.eu/ |
| September 24-27, 2023 15th European Conference on Metal Organic Frameworks and Porous Polymers (EUROMOF 2023) Granada, Spain |
https://euromof2023.com/index.php |
| September 25-29, 2023 Workshop on Mechanisms of High Temperature Corrosion and Oxidation 2023 Marktheidenfeld, Germany |
https://dechema.de/en/WS_HTC2023.html |
| 2-6 октября 2023 г. X Международная конференция «Добыча, подготовка, транспорт нефти и газа» Томск, Россия |
http://ipc-petroleum.su/ |
| 2-6 октября 2023 г. VII Школа молодых ученых «Новые каталитические процессы глубокой переработки углеводородного сырья и биомассы» Красноярск, Россия |
http://bic-school-2023.tilda.ws/ |
| 5-6 октября 2023 г. IV Всероссисйкая научно-практическая конференция с международным участием «Водород. Технологии. Будущее» Новосибирск, Россия |
|
| 5-6 октября 2023 г . V Всероссийская научная конференция с международным участием «Переработка углеводородного сырья. Комплексные решения. Самара, Россия |
https://samgtu.ru/levinter |
| 8-13 октября 2023 г. XXV Всероссийская конференция по химическим реакторам ХимРеактор-25 Тюмень, Россия |
https://chemreactor.org/ |
| 23-27 октября 2023 г. Международная конференция «Использование синхротронного излучения для исследования катализаторов и функциональных материалов» Новосибирск, Россия |
http://conf.nsc.ru/SRTCFM-2023/en |
| 13-17 ноября 2023 г . Всероссийская конференция с международным участием «Современные проблемы науки о полимерах» Санкт-Петербург, Россия |
https://young.macro.ru/ |
| November 22-24, 2023 13th International Vanadium Symposium Lisbon, Portugal |
https://vanadium13.events.chemistry.pt/ |
| 26 ноября – 1 декабря 2023 г. Конференция Центра компетенций НТИ «Водород как основа низкоуглеродной экономики» |
https://catalysis.ru/block/index.php?ID=2&SECTION_ID=16 |
| July 10-12, 2024 International Symposium on Catalytic Chemistry of C1 Molecules Lille, France |
https://www.c1chem.org/ |
| July 14-19, 2024 18th ICC – International Congress on Catalysis Lyon, France |
https://www.icc-lyon2024.fr/ |
| September 17-20, 2024 4th International Conference on Fundamentals and Applications of Cerium Dioxide in Catalysis (CERIA 2024) Portoroz-Portorose, Slovenia |
https://ceria2024.chem-soc.si/ |

21 июня 2023 года ушел из жизни академик РАН Зинфер Ришатович Исмагилов – ученый с мировым именем в области гетерогенного катализа, углехимии и химии углеродных материалов, специалист в области экологического катализа, научный руководитель Федерального исследовательского центра угля и углехимии СО РАН, главный научный сотрудник ИК СО РАН, Заслуженный деятель науки РФ, Лауреат премии Правительства РФ в области науки и техники, Лауреат премии имени В.А. Коптюга и премии «Глобальная энергия».
Зинфер Ришатович Исмагилов окончил Новосибирский государственный университет по специальности «химия» в 1969 году, поступил в аспирантуру Института катализа СО АН СССР. С 1972 года трудился в ИК СО АН СССР, пройдя путь от младшего научного сотрудника до заведующего лабораторией и руководителя отделом. В 1973 году защитил кандидатскую, а 1988-м – докторскую диссертацию.
В 1988-1992 годах – заместитель генерального директора МНТК «Катализатор».
С 2010 года – директор Института углехимии и химического материаловедения СО РАН в городе Кемерово, с 2015 года – директор Института углехимии и химического материаловедения в составе ФИЦ угля и углехимии СО РАН, с 2020 года – научный руководитель ФИЦ УУХ СО РАН, научный руководитель Центра коллективного пользования ФИЦ УУХ СО РАН.
Успехи, достигнутые коллективом Института катализа им. Г.К. Борескова СО РАН в значительной мере определялись, в том числе, творческим мышлением и вкладом в научные исследования З.Р. Исмагилова. Именно под его руководством разработаны научные основы синтеза носителей и катализаторов, выполнены фундаментальные исследования механизма ряда каталитических реакций изотопными, кинетическими и физико-химическими методами. В химии углеродных наноматериалов выполнен большой цикл работ по синтезу и исследованию закономерностей формирования углеродных нановолокон, детально изучены их структурные, текстурные, адсорбционные и электрофизические свойства. Разработаны оригинальные методики модифицирования углеродных наноматериалов.
Исследования, выполненные под руководством З.Р. Исмагилова, имеют инновационное направление с реальным выходом на создание новых продуктов и технологий.
Зинфер Ришатович был удивительный человек – выдающийся ученый, крупный организатор науки и педагог, яркий пример жизненной и гражданской позиции, осознанного служения российской науке.
Он всегда работал с полной отдачей сил. Ответственный, принципиальный руководитель, требовательный к себе и подчиненным, до конца своих дней он оставался полным мудрости, идей, желания успеть поделиться своими знаниями, мыслями с учениками, коллегами, друзьями.
Его научное наследие еще долго будет служить нынешним и будущим поколениям ученых и инженеров.
Зинфер Ришатович Исмагилов остается живым в своих публикациях, в сердцах своих коллег, родных и друзей.


