22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы


18 августа 2022 года исполнилось 75 лет члену-корреспонденту Российской академии наук, доктору химических наук, профессору, научному руководителю Омского научного центра СО РАН, главному научному сотруднику ФИЦ «Институт катализа им. Г.К. Борескова СО РАН» Владимиру Александровичу Лихолобову.
В.А. Лихолобов – специалист в области создания катализаторов и каталитических процессов нефтехимического и органического синтеза, целенаправленного синтеза функциональных углеродных материалов и каталитических композиций, автор и соавтор более 600 научных публикаций, в том числе 141 охранного документа (Web of Sci – 354, Scopus – 335).
Известен отечественной и мировой научной общественности как один из инициаторов проведения исследований в области применения принципов гомогенного катализа для создания новых эффективных гетерогенных катализаторов органического синтеза, а также разработки методов каталитического матричного синтеза и их использования для получения практически важных материалов и каталитических композиций. Заметный вклад В.А. Лихолобов внес в разработку каталитических систем, имеющих перспективу значительного улучшения технико-экономических показателей продукции предприятий нефтехимического и органического синтеза. При активном участии проф. Лихолобова были разработаны научные основы и реализованы в опытно-промышленном масштабе технологии производства различных модификаций нового углеродного материала Сибунит и катализаторов на его основе для процессов органического синтеза; решены научно-технологические вопросы получения наноглобулярного углерода и его поверхностного и объемного модифицирования с целью создания новых функциональных материалов для решения вопросов экологии, защиты здоровья человека, обороноспособности государства.
Владимир Александрович имеет большой опыт научно-организационной работы. Член Президиума Сибирского отделения РАН (с 2000 г.). Председатель Президиума Омского научного центра СО РАН (2000-2015 гг.). Научный руководитель Омского научного центра СО РАН (с 2015 г.). Организатор и директор Института проблем переработки углеводородов СО РАН в Омске (2004-2015 гг.). Эксперт Российской академии наук. Член Бюро Научного совета ОХНМ РАН по катализу. Член Объединенного ученого совета СО РАН по химическим наукам, член редколлегий 5 научных журналов, член двух диссертационных советов при Омском государственном техническом университете.
В настоящее время В.А. Лихолобов продолжает активную научную и научно-организационную деятельность, являясь главным научным сотрудником ФИЦ «Институт катализа им. Г.К. Борескова СО РАН» и научным руководителем Омского научного центра СО РАН.
За время нахождения на посту председателя Президиума ОНЦ СО РАН В.А. Лихолобов существенно укрепил позиции Центра среди других региональных научных центров. Под его руководством сформирован квалифицированный научный коллектив, развиты интеграционные связи с вузами и промышленными предприятиями региона. Все научные организации, деятельность которых координирует Центр, обеспечены собственными производственными площадями. Создана приборная база научного оборудования, сконцентрированного в составе Омского регионального центра коллективного пользования СО РАН, входящего в сеть федеральных центров коллективного пользования научным оборудованием.
Владимир Александрович внес большой вклад в подготовку научных кадров. В 2003 году с его участием были созданы и работают две профильные университетские кафедры – кафедра химической технологии в Омском государственном университете им. Ф.М. Достоевского и кафедра «Химическая технология и биотехнология» организованного при его активном участии Нефтехимического института в Омском государственном техническом университете. Среди его учеников 9 докторов и 21 кандидат наук.
Успешная деятельность В.А. Лихолобова была отмечена многочисленными наградами; среди них медаль ордена «За заслуги перед отечеством» II степени (1999 г.), орден Дружбы (2007 г.), Государственная награда Омской области – медаль «За высокие достижения» (2007 г.), Почетная грамота Академии наук СССР (1974 г.), Почетное звание «Заслуженный ветеран Сибирского отделения Академии наук СССР» (1991 г.), Золотой Почетный знак «Общественное признание», номинация «Наука и образование» (2007 г.), Памятный знак «За труд на благо народа» в честь 115-летия со дня основания г. Новосибирска (2008 г.), Юбилейная медаль «65 лет Кемеровской области» (2008 г.), Почетный знак СО РАН «Золотая сигма» (2015 г.), Почетная грамота Федерального агентства научных организаций (2016 г.), Юбилейная медаль «Омск. 300-летие» (2016 г.), Памятная серебряная медаль в ознаменование 60-летия Сибирского отделения РАН (2017 г.), Почетная грамота Российской академии наук (2020 г.).
Научный совет по катализу ОХНМ РАН и редакция Каталитического бюллетеня сердечно поздравляют Владимира Александровича с юбилеем, желают ему крепкого здоровья и новых успехов!
12-17 сентября 2021 г.
в режиме онлайн
http://conf.nsc.ru/CR-24/en/


12-17 сентября 2021 года состоялась XXIV Международная конференция по химическим реакторам ХимРеактор-24, запланированная ранее на прохождение в 2020 году в кампусе Политехнического университета Милана в Италии. Эпидемиологическая ситуация в мире не позволила осуществить реальное взаимодействие участников, поэтому мероприятие прошло в режиме онлайн.
XXIV Международная конференция по химическим реакторам ХимРеактор-24 традиционно проходила под эгидой Европейской Федерации по химической технологии (EFCE). Организаторами конференции выступили Институт катализа им. Г.К. Борескова СО РАН и Политехнический университет Милана, Италия. Онлайн-трансляция осуществлялась из Института катализа Новосибирского научного центра.
Традиционно программа конференции ХимРеактор фокусируется на фундаментальных аспектах и практическом применении каталитических процессов и химических реакторов, а также на разработке новых высокоэффективных каталитических технологий. Председателями конференции ХимРеактор-24 выступили профессор Александр Носков (Институт катализа им. Г.К. Борескова СО РАН, Новосибирск, Россия) и профессор Жанпьеро Гроппи (Политехнический университет Милана, Италия). Международный Научный комитет возглавил академик, профессор Валентин Пармон (ИК СО РАН, Новосибирск, Россия), Объединенный Программный комитет – д.т.н. Андрей Загоруйко (ИК СО РАН, Новосибирск, Россия), заместителем председателя Программного комитета выступил профессор Маттэо Маэстри (Политехнический университет Милана, Италия).


На официальном открытии конференции выступили председатель Научного комитета конференции, Президент Сибирского отделения Российской академии наук, лауреат премии «Глобальная энергия», научный руководитель Института катализа им. Г.К. Борескова СО РАН, академик РАН, профессор Валентин Николаевич Пармон; профессор Политехнического университета Милана Энрико Тронкони; со-председатель конференции, профессор Политехнического университета Милана Жанпьеро Гроппи; председатель Программного комитета конференции, ведущий научный сотрудник ИК СО РАН, д.т.н. Андрей Николаевич Загоруйко; заместитель председателя Программного комитета, профессор Политехнического университета Милана Маттэо Маэстри.
В своей приветственной речи В.Н. Пармон отразил историю становления конференции ХимРеактор, напомнив, что она уходит корнями в далекие 60-е годы прошлого века и, начавшись с небольших рабочих встреч всероссийского уровня, с годами мероприятие получило высокий статус, стало брендовым и широко признанным в мире. География конференций очень широка – Хельсинки, Берлин, Афины-Крит, Мальта, Вена, Люксембург, Дельфт, Лондон, Гент. Расширилась и география участников – на некоторых конференциях они представляли около 50 стран мира, от Американского континента до Дальнего Востока. Тематика форума, обсуждаемая и формируемая членами Международного научного совета, постоянно расширяется и модифицируется по мере появления новых прорывных направлений, благодаря чему она обязательно включает новейшие фундаментальные и практические достижения в области каталитических процессов и химических реакторов. Направления развития перспективных реакторов и технологий учитывали необходимость перехода на альтернативные источники энергии. Экологические тенденции также оказали сильное влияние на формирование тем. В своей приветственной речи д.т.н. А.Н. Загоруйко проиллюстрировал историю становления конференции ХимРеактор статистическими данными, рассказал об учреждении почетной лекции, посвященной памяти основателя конференции, профессора М.Г. Слинько. Дополняя историческое прошлое конференции ХимРеактор, профессор Енрико Тронкони предложил вспомнить, что Политехнический университет Милана участвовал в становлении форума в лице известного профессора Пио Форзатти, который был одним из немногих иностранных участников во времена его проведения в СССР.
Важным ноу-хау конференции ХимРеактор является система перекрестной квалифицированной оценки заявок на доклады, в которой участвуют эксперты из России и принимающей страны. Такая система обеспечивает высокую объективность отбора устных докладов, что гарантирует высокий научный уровень основной программы конференции. Она успешно применяется уже более 10 лет на конференциях Химреактор и некоторых иных научных мероприятиях Института катализа и даже была заимствована рядом европейских университетов для проведения своих крупных международных конференций и симпозиумов.
По итогам отбора в конференции ХимРеактор-24 приняли участие более 250 специалистов из 38 стран мира. Научная программа включала 6 пленарных лекций, 6 ключевых лекций, 113 устных докладов, 14 флэш-презентаций и около 80 стендовых докладов.
Научная программа конференции ХимРеактор-24, представленная на заседаниях четырех секций, фокусировалась на следующих научных направлениях:
По сложившейся традиции, пленарную сессию открыл доклад, посвященный основателю конференции, профессору М.Г. Слинько. Почетная лекция памяти профессора М.Г. Слинько была учреждена Программным комитетом конференции ХимРеактор в 2008 году. С тех пор она звучит на каждой конференции. Право чтения лекции по направлениям научной деятельности М.Г. Слинько предоставляется известным в мире ученым, работающим в области химической технологии, а право выбора лекторов всегда остается за членами Международного научного комитета конференции. На конференции ХимРеактор-24 эта честь выпала выдающемуся ученому, профессору Ги Марану (Университет Гента, Бельгия).

Основная идея лекции профессора Марана, отраженная в ее названии, заключалась в том, что ключевым моментом оптимизации и интенсификации химических процессов и разработки инновационных технологий является знание термодинамических и кинетических закономерностей протекания химических реакций и теории инжиниринга химических реакторов. В лекции рассматривается возможное использование этих знаний в промышленной практике для решения задач, связанных с глобальными климатическими изменениями, которые могут потребовать большего, чем постепенная оптимизация существующих процессов. Это открывает новые возможности для внедрения методов, основанных на интенсификации процессов и альтернативных энергоносителях, а также альтернативном сырье. Профессор сфокусировал свои доводы на процессе производства этилена, одном из многотоннажных процессов, на который приходится 10% выбросов CO2 во всей отрасли химической промышленности. Улавливание и использование выбросов CO2, производимых химическими и другими крупными промышленными источниками, такими как металлургическая и цементная промышленность, становится очень важным в контексте изменения климата. По мнению профессора Марана, параллельно с обсуждением прикладного аспекта химической инженерии, необходимо уделить внимание теоретическим аспектам, например, химической кинетике, которая не только демонстрирует количественные данные, но и дает представление о механизмах протекающих химических реакций. Огромный прогресс, достигнутый за последние десятилетия в области вычислительных методов в химии и инженерии, позволяет осуществить плавный масштабный переход на всех уровнях от выработки концепции до промышленной реализации. Однако экспериментальная проверка таких результатов вычислений всё-таки остается обязательной на ближайшие десятилетия. В лекции был представлен обзор существующих методов интенсификации каталитических процессов, а также их взаимных связей.
 Продолжил пленарную сессию доктор Роберто Зеннаро, представитель одной из крупнейших мировых нефтегазовых компаний Eni, Италия, имеющий 30-летний опыт работы в области химической, нефтеперерабатывающей, нефтегазовой и энергетической промышленности. Его лекция была посвящена прорывным направлениям, связанным с технологиями энергетического перехода, а именно с производством водорода, а также улавливанием, хранением и утилизацией СО2
Продолжил пленарную сессию доктор Роберто Зеннаро, представитель одной из крупнейших мировых нефтегазовых компаний Eni, Италия, имеющий 30-летний опыт работы в области химической, нефтеперерабатывающей, нефтегазовой и энергетической промышленности. Его лекция была посвящена прорывным направлениям, связанным с технологиями энергетического перехода, а именно с производством водорода, а также улавливанием, хранением и утилизацией СО2
Доктор Зеннаро проинформировал аудиторию о том, что Европейская комиссия в рамках «Зеленого курса» представила новые чрезвычайные цели по декарбонизации Европейского Союза с целью достижения нулевых выбросов к 2050 году и их промежуточного сокращения на 55% в 2030 году по сравнению с 1990 годом. Этот вопрос в ближайшие тридцать лет представляет собой уникальную проблему для мирового энергетического сектора, на который сегодня приходится около трех четвертей выбросов парниковых газов в Европе, затрагивая все отрасли промышленности. С этой целью в рамках европейской программы энергетического перехода и декарбонизации необходимо мобилизовать широкий спектр технических решений для обеспечения экономически безопасного энергоснабжения.
Доктор Зеннаро отметил, что хотя возобновляемые источники энергии, электрификация и энергоэффективность являются очевидными и признанными факторами декарбонизации, неясно, достаточны ли они для достижения амбициозной цели ЕС. Он констатировал, что возобновляемый и низкоуглеродистый водород – это источник полезной и чистой энергии, который в основном рассматривается в цепочке поставок энергии в качестве сырья для синтетического топлива и промышленных процессов (высокотемпературное отопление). Возобновляемый водород (зеленый водород) производится из биомассы или посредством электролиза, в то время как низкоуглеродистый водород (синий водород) – из ископаемого топлива с использованием технологий с низким уровнем выбросов. Голубой водород — это гибкий процесс, который уже давно и широко применяется для крупномасштабного производства водорода, и он будет использоваться до появления производств зеленого водорода, пока энергетический сектор не будет полностью декарбонизирован за счет возобновляемых источников энергии, т.е. не раньше 2040 г., возможно 2050 г.
Компания Eni считает, что технологии производства водорода дополняют друг друга, а не конкурируют друг с другом, при этом существует потребность в наличии различных технологий производства водорода, а также в разработке технологий экологически чистого производства водорода из отходов.
Далее доктор Зеннаро рассказал о способах производства водорода компанией Eni, обязательствах компании по снижению выбросов углекислого газа в этой отрасли, компаниях-компаньонах, присоединившихся к решению этой проблемы, а также о вопросах транспортировки водорода, улавливания и хранения CO2. Он отметил, что производится оценка технической и экономической осуществимости производства возобновляемого водорода и решения проблем декарбонизации.
 Большой интерес у аудитории вызвала лекция профессора Фрика Каптейна (Университет Дельфта, Нидерланды) об
интенсификации процессов с помощью структурированных катализаторов и реакторов.
Большой интерес у аудитории вызвала лекция профессора Фрика Каптейна (Университет Дельфта, Нидерланды) об
интенсификации процессов с помощью структурированных катализаторов и реакторов.
В своей лекции профессор Каптейн в первую очередь отметил, что интерес к структурированным катализаторам неуклонно растет в связи с тем, что уже давно доказаны потенциальные преимущества этого типа катализаторов. Создание принципиально новых гетерогенных каталитических процессов и работа над увеличением эффективности, интенсификации уже существующих – это задача, решение которой актуально во многих отраслях. В последние десятилетия, после успешного внедрения в систему контроля выбросов монолитных (блочных) катализаторов, благодаря их низкому перепаду давления, устойчивости к истиранию и возможностью управлять селективностью реакций, структурированные катализаторы широко исследовались и нашли практическое применение в различных каталитических процессах. Это открыло новые подходы к оптимизации реакторов с неподвижным или кипящим слоем катализатора, где структурирование позволяет найти эффективный компромисс между противонаправленными факторами процесса.
Стремление к интенсификации процессов привело к появлению новых направлений совершенствования катализаторов классических реакций. Использование металлических пен в качестве носителя катализатора оказалось успешным при получении чрезвычайно эффективных катализаторов для процесса Фишера-Тропша. Новые технологии, такие как аддитивное производство/3D-печать, открывают новые возможности для разработки принципиально новых каталитических систем.
 Профессор Фаусто Галуччи из Университета Эйндховена, Нидерланды, посвятил свою лекцию достижениям в области разработок мембранных реакторов и реакционно-сепарацинных процессов.
Профессор Фаусто Галуччи из Университета Эйндховена, Нидерланды, посвятил свою лекцию достижениям в области разработок мембранных реакторов и реакционно-сепарацинных процессов.
Профессор Галуччи начал лекцию с констатации факта, что интенсификация процессов может дать импульс развитию химической промышленности с точки зрения повышения энергоэффективности, создания чистых, безопасных технологий и снижения выбросов. Интенсификация процессов может рассматриваться как очередная революция в химической промышленности, которая может привести к потенциальной экономии энергии, эквивалентной 1 млн тонн условного топлива в год.
Однако интенсификация – это очень широкая область, и во многих случаях это просто новое и более приятное название методов, которые уже применялись в химической промышленности. Но все-таки реальная оптимизация – это не только устранение узких мест уже работающих на промышленном уровне технологий, но и стратегии, которые могут открыть новые возможности для осуществления процессов, недоступные в обычных системах.
Профессор Галуччи резюмировал наиболее интересные концепции, представленные разными авторами. Он разделил стратегии на четыре категории, в которых интенсификация может быть достигнута либо в одной, либо в нескольких из этих областей:
Профессор Фаусто Галуччи считает, что интересная стратегия достигается в областях синергии факторов процесса. Как правило, интегрированными функциями являются реакция и разделение или управление реакцией и теплом. Интеграция функций позволит снизить капитальные и эксплуатационные расходы по сравнению с традиционными системами, в которых эти функции разделены
По мнению профессора Галуччи, перспективными концепциями являются концепция мембранного реактора, в которой мембранное разделение интегрировано с реакцией, и совмещённые реакционно-сепарационные процессы, в которых разделение веществ достигается с помощью средств, отличных от мембран (например, сорбентов).
 Лекция профессора Аннеми Богертс (Университет Антверпена, Бельгия) была посвящена инжинирингу плазменных реакций. Профессор Богертс констатировала, что плазменные технологии приобретают все больший интерес для осуществления различных процессов конверсии газов, таких как преобразование CO2 и CO4 в ценные продукты нефтехимии и получение азотсодержащих соединений, то есть вносят свой вклад в развитие зеленой химии.
Лекция профессора Аннеми Богертс (Университет Антверпена, Бельгия) была посвящена инжинирингу плазменных реакций. Профессор Богертс констатировала, что плазменные технологии приобретают все больший интерес для осуществления различных процессов конверсии газов, таких как преобразование CO2 и CO4 в ценные продукты нефтехимии и получение азотсодержащих соединений, то есть вносят свой вклад в развитие зеленой химии.
Профессор Богертс объяснила, что плазма – это частично или полностью ионизованный газ, создаваемый под действием электричества и образованный из нейтральных атомов (или молекул) и заряженных частиц (ионов и электронов). Важнейшей особенностью плазмы является ее квазинейтральность. Это означает, что объемные плотности положительных и отрицательных заряженных частиц, из которых она образована, оказываются почти одинаковыми. Перевод веществ в состояние плазмы увеличивает их реакционную способность. Термодинамически или кинетически лимитированные реакции могут протекать при умеренных температуре и давлении газа, поскольку активация газа осуществляется электронами. Обычно плазменные реакторы работают при атмосферном давлении, а газ вводится при комнатной температуре. Плазменная технология требует низких капитальных затрат. Кроме этого, плазменные реакторы можно быстро вводить в действие и останавливать; поскольку такие реакторы работают с электричеством, они весьма перспективны для аккумуляции энергии в системах выравнивания суточных колебаний производства и потребления электроэнергии в энергосетях.
Профессор Богертс отметила, что для более глубокого осмысления процессов преобразования, энергоэффективности и формирования продукта требуется хорошее понимание основных механизмов. Этого можно добиться с помощью компьютерного моделирования, подкрепленного экспериментами, что включает моделирование химии плазмы, а также моделирование конструкций реакторов различных типов, обычно используемых для конверсии газа. Аннеми Богертс дала краткое описание различных типов плазменных реакторов, используемых для конверсии газа и решения задач «зеленой химии», представила обзор современного состояния плазменной конверсии CO2 и CH4, связывания N2 с помощью различных типов плазменных реакторов, а также краткое обсуждение возможностей и основных возникающих при этом проблем.
Плазма – очень реактивная среда, но не селективная. Для повышения селективности используются катализаторы. Профессор Аннеми Богертс продемонстрировала аудитории результаты различных способов моделирования, позволяющих лучше понять основные механизмы плазменного катализа.
Посредством метода отслеживания частиц можно выявлять траектории движения молекул через реактор, что позволяет определять, с какой плотностью и температурным профилем газа они имеют дело при прохождении через реактор. Эта информаvия затем используется в качестве исходной при кинетическом моделировании процесса.
Заключительную пленарную лекцию «Преобразование устойчивой энергии: электрические реакторы» представил профессор Иб Чоркендорф (Датский технический университет, Копенгаген, Дания).
 Профессор Чоркендорф начал свою лекцию с размышления о том, что наше будущее многие рисуют без ископаемого топлива – энергия будет поступать в виде электричества, в основном от ветряных и фотоэлектрических устройств. Он обозначил, что Дания – мировой лидер в области ветроэнергетики. Солнечная энергетика в этой не самой солнечной стране развивается высокими темпами – солнечные электростанции за недавнее время произвели рекордное количество электроэнергии. Но нужно иметь в виду, что энергоресурсы будут непостоянны вследствие зависимости от климатических изменений; хотя они могут быть доступны на какое-то время, потребуется быстрая корректировка подходов к доступности энергии. Положительным факторам является то, что электрификация, прежде всего, приведет к значительной экономии энергии, поскольку электричество является гораздо более эффективным источником энергии из-за его высокой эксергии. Тем не менее, как отметил профессор Чоркендорф, не все можно электрифицировать, поэтому потребность в производстве топлива, например, для дальнемагистрального транспорта и, в частности, для авиации, все-таки останется. И по-прежнему будет существовать спрос на химикаты.
Профессор Чоркендорф начал свою лекцию с размышления о том, что наше будущее многие рисуют без ископаемого топлива – энергия будет поступать в виде электричества, в основном от ветряных и фотоэлектрических устройств. Он обозначил, что Дания – мировой лидер в области ветроэнергетики. Солнечная энергетика в этой не самой солнечной стране развивается высокими темпами – солнечные электростанции за недавнее время произвели рекордное количество электроэнергии. Но нужно иметь в виду, что энергоресурсы будут непостоянны вследствие зависимости от климатических изменений; хотя они могут быть доступны на какое-то время, потребуется быстрая корректировка подходов к доступности энергии. Положительным факторам является то, что электрификация, прежде всего, приведет к значительной экономии энергии, поскольку электричество является гораздо более эффективным источником энергии из-за его высокой эксергии. Тем не менее, как отметил профессор Чоркендорф, не все можно электрифицировать, поэтому потребность в производстве топлива, например, для дальнемагистрального транспорта и, в частности, для авиации, все-таки останется. И по-прежнему будет существовать спрос на химикаты.
Профессор Чоркендорф заострил внимание аудитории на двух подходах к вопросу электрификации химической промышленности – это использование электрокатализа и замена ископаемых ресурсов, например, за счет использования эндотермических реакций с электрической компенсацией требуемых энергозатрат. В электрокаталитических процессах, на его взгляд, следует избегать высоких температурных градиентов, которые потенциально могут осложнять запуск реакторов и вызывать отложение углерода на катализаторах. На примере одной из наиболее сложных эндотермических реакций, а именно парового риформинга, Иб Чоркендорф продемонстрировал и проиллюстрировал возможности различных методов управления температурными режимами, их преимущества, особенности и перспективы.
Ключевые лекции на конференции ХимРеактор-24 представили профессор Владимир Сергеевич Арутюнов, Институт химической физики им. Н.Н. Семенова РАН (Москва, Россия); профессор Паул Дауэнхауэр, Университет Миннесоты (Миннеаполис, Миннесота, США); профессор Ханнсйорг Фройнд, Технический университет Дортмунда (Дортмунд, Германия); профессор Луис Гандиа, Государственный университет Наварры (Памплона, Испания); профессор Сулио Линич, Университет Мичигана (Анн-Арбор, Мичиган, США); профессор Руфат Шовкетович Абиев, Санкт-Петербургский государственный технологический институт (Технический университет).
Активность, с которой прошли сессии устных докладов, подтверждает интерес всей аудитории к тематике и высокий уровень представленных презентаций.
Не меньший интерес вызвала и стендовая сессия. 14 докладов были представлены в формате пятиминутных флэш-презентаций, позволявших отразить ключевую направленность доклада и продемонстрировать несколько слайдов. Остальные стендовые доклады были заранее размещены на сайте конференции в формате PDF-презентаций. С ними участники могли ознакомиться еще до конференции. К открытию конференции был создан онлайн-форум в Google Group со ссылками на PDF-презентации, на котором была возможность обсудить доклады с автором в интерактивном режиме. Окончательное обсуждение состоялось на двухчасовом заседании стендовой сессии в рамках научной программы конференции.
Проведение мероприятия в формате онлайн стало первым опытом в широком спектре организуемых Институтом катализа конференций ХимРеактор, традиционно проходящих в очном режиме. Приятно осознавать, что организаторам удалось создать атмосферу, которая позволила большому количеству участников, находящихся в разных частях света (от американского континента до Дальнего Востока) стать частью великолепного форума, окунуться в волну новой информации, дебатов, споров, получить удовольствие от общения друг с другом, и даже насладиться запоминающимся танцевально-певческим а-ля фуршетом с бокалом вина, музыкой, песнями и танцами.

Труды конференции ХимРеактор-24 традиционно будут опубликованы в виртуальном специальном выпуске Chemical Engineering Journal (Elsevier Science), который на сегодня является одним из ведущих мировых изданий в сфере химической технологии (импакт-фактор 13.273).
На закрытии конференции обсуждались вопросы модернизации тематических направлений конференции ХимРеактор, перспектив ее дальнейшего развития, выбора места проведения следующих мероприятий. Было получено несколько предложений по месту проведения следующей, XXV Международной конференции по химическим реакторам ХимРеактор-25, в том числе от представителей делегации Канады о проведении форума на американском континенте – в Канаде или Мексике. Аудитория с удовольствием поддержала выступление руководителя департамента Института микроинженерии и микросистем им. Фраунгофера (Майнц, Германия), профессора Гюнтера Колба с предложением провести конференцию ХимРеактор-25 в Германии.
Материал подготовили:
А.Н. Загоруйко, А.С. Носков, Т.В. Замулина
Институт катализа им. Г.К. Борескова СО РАН
Nickel catalyst enables versatile amine synthesis
Method creates hundreds of different amines from handy nitriles

Amines are ubiquitous in the pharmaceutical and chemical industries, and a nickel-based catalyst has now opened up a promising route to make these molecules from widely-available nitrile compounds (Science 2022, DOI: 10.1126/science.abn7565).
Primary amines, in which a nitrogen atom is bonded to just one carbon, are often made by reducing nitrile groups using hydrogen. It’s an efficient and cost-effective approach, widely used in the production of nylon, surfactants, and plastics. In contrast, the various methods used to make secondary or tertiary amines, where nitrogen carries two or three carbon groups, tend to be more complicated and involve more waste.
So chemists have been searching for ways to use nitriles to make secondary and tertiary amines using a process called hydrogenative coupling. This involves reacting a nitrile with hydrogen to produce a primary imine intermediate, and then coupling the imine to a primary or secondary amine. This coupling displaces the imine’s nitrogen in the form of ammonia, and forges a new C–N bond to produce a secondary or tertiary amine.
The problem is that route generally produces a mess of different products. One reason is that the imine intermediate can itself react with hydrogen to make an unwanted amine, which then muscles in on the coupling reactions.
A team led by Rajenahally Jagadeesh and Matthias Beller at the Leibniz Institute for Catalysis has now developed a catalyst that can steer the hydrogenative coupling reaction along the right course, ensuring high selectivity for the desired product. The key is to make sure that the reaction between the imine intermediate and hydrogen is much slower than the intended coupling reaction, which minimizes unwanted side reactions.
After testing a range of different catalysts and conditions, the researchers settled on a champion catalyst that contain nickel bound to a triphosphine ligand. The reaction worked well at 100–120 °C and 4000 kPa of hydrogen, and the team used this process to make more than 230 different amines. “We can make complex agrochemicals and pharma-ceuticals, and it also has a very wide applicability for the fine- and bulk-chemical industries,” says Jagadeesh.
The catalogue of compounds made with the reaction featured many chiral molecules, including the drug tecalcet, used to treat conditions related to the parathyroid glands. The method can also couple nitriles with ammonia, an approach the researchers used to make primary amines labeled with a nitrogen-15 isotope that could be used in metabolic studies. The team prepared a handful of products at the 10 g scale, and Jagadeesh says the reaction should be amenable to further scale-up.
“This research is excellent. I was overwhelmed to see how many amines could be prepared,” says Yasunari Monguchi at Daiichi University of Pharmacy. One particular advantage, Monguchi says, is that it uses a very simple catalyst system that relies on a commercially available ligand.
The reaction worked well with precursors containing a range of different functional groups, although it stumbled when faced with aldehydes, alkynes, and a few other groups. This versatility means the procedure could be used to modify nitrile-containing compounds at the end of a long synthesis—a strategy known as late-stage functionalization—to make many analogs of drug candidates for high-throughput screening, for example.
The team is now investigating the mechanism of the reaction and hopes to reduce the temperature and pressure of the reaction using a cobalt-based catalyst that is showing early promise.
Single catalyst molecules tracked in solution
Fluorescence microscopy traces Grubbs catalysts’ winding paths during polymerization
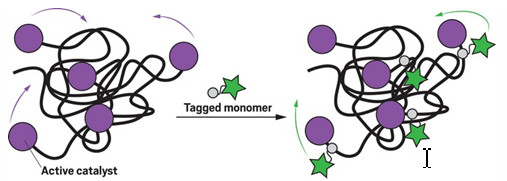
Fluorescent tags reveal the motion of active ruthenium catalysts stuck to the ends of polymer chains
The catalysts that buzz around inside a reaction are rather like a swarm of midges—you know they’re there, but each one is so small and fast that they seem impossibly difficult to track in flight. Until now.
For the first time, researchers have measured the motion of individual catalyst molecules during a reaction (J. Am. Chem. Soc. 2022, DOI: 10.1021/jacs.2c03566). Such insights could assist in the design of better catalysts, says Suzanne Blum of the University of California, Irvine, who led the work.
The research relied on superresolution fluorescence microscopy, which is already used to scrutinize the movements of single enzymes. But the technique has yet to tackle the challenges presented by imaging single molecules of chemical catalysts, Blum says. The team set their sights on a reaction that uses a ruthenium catalyst to add monomers to a growing polymer. Crucially, each catalyst molecule remains tethered to the end of a polymer chain, slowing the catalyst’s movement enough for reliable imaging.
During the experiment, the researchers interrupted the reaction once some polymer had formed and washed away any unreacted molecules. Then they added an extremely dilute solution of monomers tagged with fluorophores that briefly glow green when hit by blue light. These tagged monomers were incorporated into the polymer chains, right next to the ruthenium catalyst at the tip.
Giving the reaction a flash of blue light once every second provided regular updates on the positions of each polymer-bound fluorophore, and its adjacent catalyst. Meanwhile, unattached fluorophores still floating in solution moved around too quickly to register on the camera, so they did not muddle the images.
The microscope could pinpoint polymer-bound fluorophores to within 32 nm, enabling the team to calculate that about one-quarter of ruthenium catalysts were effectively stationary. A similar proportion moved around vigorously, traveling an average of 145 nm each second, while the remainder roamed more modestly.
This variation in movement may be due to the catalysts being more or less entangled in the spaghettilike mass of polymer chains, which could also affect the catalysts’ access to monomers or solvent molecules. “The most important finding from the experiment is that during polymeriza-tion the catalysts exist in many different environments,” Blum says.
If that environment is too cramped or restricts the supply of monomers, the polymer strand will grow more slowly. That could in turn affect how long different polymer strands grow, a key factor in determining the polymer’s bulk properties.
“A polymer is a complex sample; it’s always a distribution of molecular weights and lengths, and now we start to understand where this is orignating from,” says Johan Hofkens of KU Leuven, who works on single-molecule and superresolution microscopy. “I think it’s an interesting step forward to get this insight.”
Blum hopes that the method could help chemists to design catalysts that have more uniform motions during polymerization—for example, by tweaking their ligands so that they repel nearby polymer strands and ensure the catalysts can access a similar amount of monomers. This could produce more consistent chain lengths that improve the polymer’s material properties.
Her team is now applying the technique to other kinds of reactions. Not all reactions will be compatible with a fluorophore tag, Hofkens cautions. “But as long as you’re sure that you don’t disturb the process that you’re looking at, it’s a perfect technique, and it will find wider applications,” he says.
A greener path toward vanillin from paper pulp
A new electrocatalytic process makes vanilla’s main flavor compound from kraft lignin
Vanillin is one of the world’s most popular aroma chemicals and fra-grances. However, 85% of it is synthesized from petrochemical precursors, and the food chemical industry is eager to find more sustainable sources. Researchers have now developed an electrochemical method to obtain vanillin from lignin, a tough biopolymer that is a by-product of the paper industry (ACS Sustainable Chem. Eng. 2020, DOI: 10.1021/acssuschemeng.0c00162).
A new electrochemical process could maximize the production of vanillin from lignin, a renewable by-product of the paper pulping process.
Lignin, left over after cellulose fibers are removed from wood to make paper, contains a mix of aromatic compounds that chemists have found ways to transform into a range of useful products. In the new study, Siegfried R. Waldvogel of Johannes Gutenberg University Mainz and colleagues created vanillin via a simple reaction. They first dissolved lignin and caustic soda in water and heated the solution. Next, they applied an electric current to the high pH solution using inexpensive nickel electrodes, breaking down the lignin and oxidizing it to produce vanillin in yields of up to 4.7%. “This may not seem impressive, but it is a remarkable selectivity,” Waldvogel says. No toxic or noxious side products are produced. Scandinavian company Borregaard already produces vanillin commercially from lignin via a copper-catalyzed oxidation. However, the process requires high temperature and pressure, and costly purification steps to remove the catalyst, Waldvogel says. Also, it uses a more specialized lignin raw material, whereas the new approach uses lignin from the kraft process, which yields 90% of the world’s paper pulp. About 150 million t of kraft lignin is generated per year, Waldvogel says, making it the most widely available carbon-based material after crude oil.
“This process is greener than currently available alternatives,” says Pablo Ortiz, a process chemist specializing in sustainable development at VITO, the Flemish Institute for Technological Research. Moreover, he values the use of kraft lignin as starting material. “Potentially, this method has the possibility to give higher yields of vanillin than Borregaard’s,” which is 0.3% by weight overall.
Waldvogel’s team has a new grant from the European Commission to build a pilot plant for continuous vanillin production at larger scale. The researchers would eventually like to use crude kraft lignin directly from the pulping process—a basic raw material known as black liquor—to reduce the need for caustic soda and water.
Chemical & Engineering News
Конференция Центра компетенций НТИ
«Водород как основа низкоуглеродной экономики»
27 ноября – 2 декабря 2022 г.
Шерегеш, Кемеровская область, Россия
http://conf.nsc.ru/h2nti/ru/
Конференция Центра компетенций НТИ «Водород как основа низкоуглеродной экономики» пройдет с 28 ноября по 1 декабря 2022 года в Шерегеше. День отъезда из Новосибирска 27 ноября, возвращение в Новосибирск 2 декабря 2022 г.
Организационный комитет
(ФИЦ Институт катализа им. Г.К. Борескова СО РАН, Новосибирск)
Председатель: Академик РАН Валерий Иванович Бухтияров
Заместители председателя:
д.х.н. профессор РАН Олег Николаевич Мартьянов
д.х.н. Павел Валерьевич Снытников
Организационный комитет :
д.х.н., профессор РАН Денис Владимирович Козлов
д.х.н., профессор РАН Екатерина Александровна Козлова
д.х.н. Вадим Анатольевич Яковлев
к.х.н. Дмитрий Игоревич Потемкин
Марина Валерьевна Верниковская
Анна Михайловна Ершова
Александра Романовна Иммен
Анастасия Станиславовна Аникина
Секретариат:
Марина Сергеевна Суворова
Светлана Сергеевна Логунова
Научная программа конференции включает доклады (20 минут) и устные сообщения (10 /15 минут) по направлениям:
Водородная заправка
Биоводород для генерации электроэнергии
Водород для Е-химии и Е-топлива
Крупнотоннажный водород для низкоуглеродной экономики
В рамках конференции пройдет круглый стол «Научная инфра-структура и образование».
Рабочий язык – русский.
|
3-7
октября 2022 г. XXIII Международная Черняевская конференция по химии, аналитике и технологии платиновых металлов Новосибирск, Россия |
https://chernyaev2022.affinaz.ru/ |
|
6-7
октября 2022 г. Всероссийская школа-конференция “Фотокатализ – от фундаментальных исследований до практического применения” Новосибирск, Россия (в online-режиме, без оргвзноса) |
http://conf.nsc.ru/photocat-50/ru |
|
October
17-19, 2022 New Frontiers of Natural Sciences: 1st International Selçuk Meeting (NFNS 2022) Konya, Turkey |
http://nfns2022.selcuk.edu.tr/ |
|
October
25-28, 2022 7th Ertl Symposium on Catalysis in Electrochemistry Gwangju, South Korea |
http://ertlsymp2007.com/ |
|
31
октября – 2 ноября 2022 г. Всероссийская научно-техническая конференция «Проблемы науки. Химия, химическая технология и экология» Новомосковск, Россия (без оргвзноса) |
gez75@yandex.ru 301665 Тульская область, г. Новомосковск, ул. Дружбы, д. 8 Новомосковский институт (филиал) ФГБОУ ВО «Российский химико-технологический университет имени Д.И. Менделеева» |
|
October
31 – November 3, 2022 International Conference “Synchrotron Radiation Techniques for Catalysts and Functional Materials Novosibirsk, Russia |
http://conf.nsc.ru/SRTCFM-2022/en |
|
October
31 – November 4, 2022 Conference on Advances in Catalysis for Energy and Environment (CACEE-2022) & CO2India Network 1st Annual Meet Mumbai, India |
https://www.cacee.org/ |
|
2-4 ноября 2022 г. V Международный научно-технический форум по химическим технологиям и нефтегазопереработке «Нефтегазохимия - 2022» Минск, Беларусь |
https://petro.belstu.by/ |
|
November
7-8, 2022 4th European Congress on Graphene & 2D Materials (Graphene 2022) Paris, France |
https://crgconferences.com/graphene |
|
November
8, 2022 Zeolites meet RDM – Research Data Management for Porous Materials DECHEMA, Germany (online event) |
https://dechema.de/en/Zeo_WS_2022.html |
|
November
9-10, 2022 6th International Conference on Catalysis and Chemical Engineering (CatChem 2022) Dubai, UAE |
https://catchemconference.com |
|
November
10-11, 2022 3rd International Conference on Biofuels and Bioenergy Paris, France |
https://crgconferences.com/biofuels/ |
|
November
18-19, 2022 5th International Symposium on Hydrogen Energy and Energy Technologies (HEET 2022) Osaka, Japan |
http://www.heet-18.org/ |
|
November
21-24, 2022 VI International Conference “Fundamental Basesof Mechanochemical Technologies” (FBMT-2022) Novosibirsk, Russia |
http://www.solid.nsc.ru/en/fbmt2022/ |
|
23-25
ноября 2022 г. V Международная научно-техническая конференция “Минские научные чтения-2022: Научно-техническая и экономическая безопасность Союзного Государства” Минск, Беларусь |
akalinich@belstu.by |
|
28
ноября – 1 декабря 2022 г. Конференция Центра компетенций НТИ “Водород как снова низкоуглеродной экономики” Шерегеш, Кемеровская область, Россия |
http://conf.nsc.ru/h2nti/ru |
|
28-30
ноября 2022 г. Вторая школа молодых ученых “Электрохимические устройства: процессы, материалы, технологии” Новосибирск, Россия (без оргвзноса) |
http://www.solid.nsc.ru/school2022/ |
|
December
15-16, 2022 International Conference on Catalysis Engineering and Applications (ICCEA 2022) Cairo, Egypt |
https://waset.org/catalysis-engineering-and-applications-conference-in-december-2022-in-cairo |
|
January
9-11, 2023 10th Annual Global Congress of Catalysis 2023 (GCC-2023) Sapporo, Japan |
https://www.bitcongress.com/gcc2023-japan/ |
|
February
8-10, 2023 12th Annual World Congress of Nano Science & Technology (Nano S&T-2023) Sapporo, Japan |
https://www.bitcongress.com/nanobiox2023/ |
|
February
20-22, 2023 7th International Conference on Catalysis and Chemical Engineering (CCE-2023) Las Vegas, NV, USA |
https://catalysis.unitedscientificgroup.org/conference-info |
|
March
8-9, 2023 6th Hydrogen & Fuel Cells Energy Summit Lisbon, Portugal |
https://www.wplgroup.com/aci/event/hydrogen-fuel-cells-energy-summit/ |
|
March
20-21, 2023 International Conference on Catalysis & Chemical Engineering Rome, Italy |
https://scisynopsisconferences.com/catalysis/ |
|
March
20-21, 2023 International Conference on Graphene & 2D Materials Dubai, UAE |
https://scisynopsisconferences.com/graphene/ |
|
April
13-14, 2023 4th Global Summit on Catalysis and Chemical Engineering (Chemical Catalyst 2023) Rome, Italy |
https://catalysis.mindauthors.com/ |
|
May
15-17, 2023 Annual Meeting on Reaction Engineering DECHEMA-Haus, Frankfurt, Germany |
https://dechema.de/en/REACT2023.html |
|
June
14-15,
2023 33rd Edition of Chemistry World Conference (Chemistry-2023) Rome, Italy |
https://chemistryworldconference.com/ |
|
June
19-23, 2023 IV International Symposium “Applied Nanotechnology & Nanotoxicology” (ANT-2023) Kaliningrad, Russia |
http://conf.nsc.ru/ant-2022/en |
|
July
9-13, 2023 13th International Symposium on Scientific Bases for the Preparation of Heterogeneous Catalysts (PREPA 13) Louvain-la-Neuve, Belgium |
https://www.prepa13.org/ |
|
August
18-25, 2023 49th IUPAC World Chemistry Congress 2023 (IUPAC|CHAINS 2023) Hague, the Netherlands |
https://iupac2023.org/ |
|
August
27 – September 1, 2023 15th European Congress on Catalysis (EuropaCat 2023) Prague, Czech Republic |
https://www.europacat2023.cz/ |
|
September
17-21, 2023 14th European Congress of Chemical Engineering and 7th European Congress of Applied Biotechnology (ECCE/ECAB 2023) Berlin, Germany |
https://ecce-ecab2023.eu/ |
|
2023 VI German-Russian Seminar “Bridging the Gap between Model and Real Catalysis” Energy Conversion and Hydrogen Related Technologies |
http://conf.nsc.ru/rgs-2022/en/ |
|
2023 6th International Conference “Catalysis for Renewable Sources: Fuel, Energy, Chemicals” (CRS-6) |
http://conf.nsc.ru/CRS6/en |
|
2023 XII International Conference “Mechanisms of Catalytic Reactions” (MCR-XII) |
http://conf.nsc.ru/mcr2022/en |
|
2023 VII International Conference on Structured Catalysts and Reactors (ICOSCAR7) |
http://conf.nsc.ru/ICOSCAR7/en |