22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы


C 20 по 25 сентября 2021 года в Казани прошел IV Российский конгресс по катализу «РОСКАТАЛИЗ» («Роскатализ-2021»). Основным организатором Конгресса выступил ФИЦ «Институт катализа

Общая фотография участников Конгресса
Катализ – одна из самых наукоемких и перспективных областей науки, развивающаяся на стыке химии, физики, биологии и математики. Решение многих научных, технологических, экологических проблем – от многотоннажного производства органических веществ до управления жизненно важными биохимическими процессами в живой клетке – непосредственно связано с катализом. В мире более 90% всех реализованных в промышленности химических технологий используют катализаторы и каталитические процессы.
Разработка катализаторов и каталитических процессов, создание сопутствующих функциональных материалов и способов их конструирования входят в список основных приоритетов Стратегии научно-технологического развития (СНТР) Российской Федерации, которые позволят решить следующие приоритетные задачи, указанные в п. 20 СНТР РФ: осуществить переход к экологически чистой и ресурсосберегающей энергетике; повысить эффективность добычи и глубокой переработки углеводородного сырья; способствовать формированию новых источников энергии, способов ее транспортировки и хранения; осуществить смену сырьевой базы современной энергетики и химической промышленности и переход на активное использование возобновляемого растительного сырья и солнечной энергии; создать востребованные материалы для микроэлектроники, медицины, ракетной и космической отрасли.
Российский конгресс по катализу «Роскатализ» был задуман и трижды (в 2011, 2014, 2017 гг.) успешно реализован как дискуссионная площадка, место контакта между представителями науки и промышленности, продемонстрировал возросший интерес промышленности к отечественным катализаторам и каталитическим технологиям.
В рамках четвертого по счету конгресса «Роскатализ» были проведены продуктивные дискуссии, касающиеся перспектив решения задач общегосударственной значимости: импортозамещение катализаторов и стратегически важной химической продукции, подготовка высококвалифицированных кадров, создание и совершенствование технологической инфраструктуры для сравнительных испытаний важнейших промышленных катализаторов с целью разработки отечественных каталитических технологий.
Выбор Республики Татарстан в качестве места для проведения Конгресса был обусловлен высокой концентрацией в регионе нефтеперерабатывающих и химических предприятий, заинтересованных во внедрении самых современных промышленных технологий, наличием мощной сети научных и образовательных организаций, системы поддержки инноваций (Казанский федеральный университет, Казанский национальный исследовательский технологический университет, Иннополис), а также вниманием, которое Правительство Республики Татарстан уделяет развитию сферы науки и инноваций в регионе.
Научная программа Конгресса включала в себя 6 пленарных (45 мин.) и 9 ключевых (30 мин.) лекций, 4 приглашенных устных доклада, 127 устных (15 мин.) и более 100 стендовых докладов по следующим тематикам:
СЕКЦИЯ I. Фундаментальные основы катализа
СЕКЦИЯ II. Перспективные катализаторы и каталитические процессы
СЕКЦИЯ III. Катализаторы и каталитические процессы для энергетики будущего
СЕКЦИЯ IV Промышленные катализаторы и каталитические процессы
В рамках научной программы Конгресса также были проведены следующие важные сателлитные мероприятия:
 Молодёжная школа по катализу «Физико-химические методы исследования – ключ к пониманию принципов каталитического действия»
Молодёжная школа по катализу «Физико-химические методы исследования – ключ к пониманию принципов каталитического действия»
На Молодёжной школе было представлено 4 пленарные лекции (30 мин.) и 25 устных докладов (15 мин.).
 Круглый стол «Исследовательская карьера: от молодого ученого до лидера проекта»
Круглый стол «Исследовательская карьера: от молодого ученого до лидера проекта»
 Круглый стол «Катализ в добыче и переработке тяжелой и нетрадиционной нефти»
Круглый стол «Катализ в добыче и переработке тяжелой и нетрадиционной нефти»
 Сателлитный круглый стол «Химические технологии в борьбе с «углеродным» следом»
Сателлитный круглый стол «Химические технологии в борьбе с «углеродным» следом»
Всего на Конгресс приехали 378 очных участников из Беларуси (Минск), Нидерландов (Делфт), Узбекистана (Ташкент, Чирчик), Украины (Донецк) и России (Апатиты, Зеленоград, Новочеркасск, Санкт-Петербург, Уфа, Владивосток, Иваново, Мирный, Омск, Черноголовка, Гатчина, Иркутск, Москва, Пермь, Тверь, Йошкар-Ола, Нижний Новгород, Ростов-на-Дону, Тольятти, Казань, Новосибирск, Рязань, Томск, Екатеринбург, Красноярск, Новоуральск, Самара, Тюмень). Еще 118 участников опубликовали свои тезисы в сборнике трудов Конгресса в заочном режиме. Общее число очных и заочных участников составило 496 человек.
На открытии Конгресса с приветственным словом выступил Председатель Сибирского отделения РАН, вице-президент РАН, научный руководитель Института катализа СО РАН, академик РАН Валентин Николаевич Пармон. Валентин Николаевич отметил, что несмотря на все сложности и ограничения, вызванные эпидемиологической ситуацией, IV Российский конгресс по катализу был проведен в очном формате и стал крупнейшим в России и очень значительным даже по мировым масштабам мероприятием, посвященным обсуждению фундаментальных и прикладных проблем катализа и внедрению каталитических технологий в индустрию. Четыре года, прошедшие со времени проведения предыдущего мероприятия, были насыщены многочисленными событиями, повлиявшими на повестку Конгресса. В течение многих десятилетий углеводородное сырье было основным энергетическим ресурсом и базовым сырьем для химической промышленности, хотя научным сообществом неоднократно поднимался вопрос о грядущей смене традиционной сырьевой базы. Так и произошло. В самые последние годы на уровне Организации Объединенных Наций, а также ведущими странами Запада, Европейским Союзом были приняты решения, направленные на ускоренный переход к низкоуглеродной экономике, основанной на использовании возобновляемых источников энергии (энергии солнца, ветра), водородной энергетики. Соответствующие решения приняло недавно и Правительство Российской Федерации. Каталитические технологии переработки углеводородного сырья в целях получения энергетических ресурсов, химических веществ, функциональных материалов различного назначения были в центре научной повестки первых трех конгрессов. Однако последние решения привели к изменениям в этой повестке, в результате чего больше внимания было уделено «зеленым» технологиям, технологиям, направленным на снижение «углеродного следа» при переработке углеродсодержащего сырья. Углеродсодержащее сырье как основа энергетики и химической промышленности играет и будет продолжать играть определяющую роль в экономике России. Обеспечить постепенную трансформацию и адаптацию этого сектора промышленности к новым реалиям невозможно без каталитических технологий. По этой причине конгресс «Роскатализ» является очень важным для определения будущих направлений развития экономики России. Академик Пармон отметил также символическую значимость организации Конгресса на территории Республики Татарстан – одного из наиболее активных в инновационном плане регионов Российской Федерации с высоким научным, образовательным и промышленным потенциалом, колыбели нескольких ведущих химических школ России. В завершение своей речи Валентин Николаевич выразил надежду на то, что принятые на Конгрессе решения, озвученные здесь идеи и предложения станут основой развития науки о катализе и каталитических технологиях в России на годы и десятилетия вперед, а также пожелал всем участникам плодотворного общения, обмена идеями и опытом на четвертом конгрессе «Роскатализ».
Директор Института катализа СО РАН, академик РАН Валерий Иванович Бухтияров отметил, что конгресс «Роскатализ-2021», который традиционно организует Институт катализа СО РАН, проводится в четвертый раз и продолжает традицию проведения крупных научных мероприятий, заложенную еще во времена СССР, когда единственной конференцией по катализу была конференция по механизмам каталитических реакций. На нее приезжали не только ученые, но и промышленники, и это был обмен мнениями о научных достижениях и потребностях советской промышленности. Затем конференция стала международной, и появилась потребность в площадке для обсуждения проблем и задач именно российской промышленности, в которой используются каталитические технологии. Так появился «Роскатализ».
Научный руководитель направления «химия» ИОФХ им. А.Е. Арбузова ФИЦ КазНЦ РАН, академик РАН Олег Герольдович Синяшин подчеркнул роль катализа для промышленности Республики Татарстан: «Перед каталитическим сообществом стоят большие вызовы, которые связаны не только с традиционными направлениями, такими как химия, нефтехимия, биотехнологии, но и с экологическими и климатическими проблемами. Эти проблемы требуют совместного решения. В Татарстане собраны крупные химические и нефтехимические комплексы, это место, где сосредоточен мощный научный и образовательный потенциал. И руководство республики уделяет технологиям и инновациям большое внимание. Связь науки, образования, бизнеса и власти помогает решать многие проблемы» – сказал академик Синяшин.
Проректор Казанского федерального университета Дмитрий Альбертович Таюрский также отметил, что проведение Конгресса в Казани символично. «Каталитические технологии – это та область, где путь между фундаментальными разработками, их технологическим решением и готовой продукцией – самый короткий. Татарстан с его экономикой – мощным нефтехимическим комплексом, сельским хозяйством – является интересной площадкой, чтобы обсудить не только фундаментальные научные проблемы, но и поговорить об их прямом применении», – сказал он.
Начальник департамента развития нефтепереработки и нефтехимии ПАО «Газпром нефть» Олег Сергеевич Ведерников рассказал о том, как компания развивает технологическое направление. «Мы уделяем большое внимание технологическому лидерству, импортозамещению и разработке собственных технологий в части нефтепереработки, и делаем это как для повышения собственной эффективности производства, так и в целом для нефтеперерабатывающей отрасли России. Мы строим производство катализаторов в Омске – уверен, что в следующем году первые тонны продукции будут выпущены. В производстве используются полностью российские технологии, разработанные совместно с нашими отечественными институтами. Это основные катализаторы нефтепереработки, которыми мы можем обеспечить российскую нефтяную промышленность. В этом году мы создали свой научно-исследовательский центр «Газпромнефть – Промышленные инновации», основная задача которого – взять научные жизнеспособные труды и внедрить их в производство. «Роскатализ» – это хорошая платформа для живого общения и поиска разработок, которые можно быстро внедрить», – пояснил Олег Сергеевич.

На открытии Конгресса. Слева направо: Олег Герольдович Синяшин,
Валерий Иванович Бухтияров, Дмитрий Альбертович Таюрский

Валентин Николаевич Пармон

Олег Сергеевич Ведерников

На открытии конгресса «Роскатализ-2021»
В ходе Конгресса были представлены уникальные лекции ведущих российских и мировых ученых.
Пленарные лекции:
ПЛ-1
Докладчик: Кашин Алексей Михайлович
«Альтернативная энергетика»
АО «Группа компаний ИнЭнерджи», Москва, Россия

Алексей Михайлович Кашин
Лекция А.М. Кашина, акционера и члена совета директоров Группы компаний «ИнЭнерджи», была посвящена вопросам перехода к безуглеродной энергетике. Было отмечено, что в мире происходит вытеснение традиционных видов топлива возобновляемыми источниками энергии. Развитие технологий и запрос общества на экологичность энергетики меняют облик энергосистем и модальность энергопотребления. Движущими силами «Новой энергетики» становятся: государственная политика, направленная на декарбонизацию, энергобезопасность, доступность электрической энергии, развитие технологий альтернативной энергетики, децентрализация.
По инициативе «ИнЭнерджи» разработана и согласована концепция программы развития Новых и Мобильных Источников Энергии (NAMES). Программа содержит три направления: Генерация, Накопители энергии, Топливо, и ориентирована на использование российских технологий и развитие отечественной науки и производства.
В решении проблемы декарбонизации развитие водородных технологий является ключевым компонентом. Использование водородного транспорта позволит значительно снизить выбросы СО2, так как на долю транспорта приходится большая часть выбросов углекислого газа. На фоне быстрого развития электротранспорта по всему миру всё больший интерес вызывают компании, владеющие технологиями производства накопителей энергии и технологиями экологически чистого получения электроэнергии для использования на транспорте. При этом весомую роль играет государственная поддержка, оказываемая компаниям, развивающим такого рода технологии.
В лекции были продемонстрированы разработки АО «ГК ИнЭнерджи» в области энергетических установок с топливными элементами транспортного назначения на основе платформенного решения «АСТРА».
ПЛ-2
Докладчик: д.х.н., академик РАН Стороженко Павел Аркадьевич
«Катализ в химии элементоорганических соединений»
Государственный научный центр Акционерное общество «Государственный научно-исследовательский институт химии и технологии элементоорганических соединений», Москва, Россия

В своем выступлении П.А. Стороженко рассказал о том, что в химии элементоорганических соединений без применения катализаторов невозможно получение многих ценных востребованных продуктов. Одним из важнейших каталитических процессов в этой области является прямой синтез кремний- и германийорганических соединений из металлургического кремния и германия, соответственно. Вопросами прямого синтеза кремнийорганических соединений, начиная от изучения механизма процесса до создания крупнотоннажных производств, ГНИИХТЭОС занимается более 50 лет. В настоящее время в институте проводятся работы по расширению сырьевой базы получения кремнийорганических мономеров, включая альтернативные бесхлорные методы синтеза соединений, имеющих связь Si–C. На базе ГНИИХТЭОС также были изучены каталитический процесс прямого синтеза алкоксисиланов из металлургического кремния и низших спиртов – метилового и этилового в среде высококипящего растворителя, разработан способ получения SiH4 и MeSiH3 высокой чистоты диспропорционированием триэтоксисилана на гомогенных и гетерогенных катализаторах, были тщательно изучены процессы гидросилилирования этилена, пропилена (с привлечением квантово-химических расчетов), высших олефинов (С14-С18), непредельных соединений с аминными, перфторалкильными, перфторциклоалкильными, сложноэфирными, арильными, алкиларильными, эпоксидными, нитрильными и другими группами. Были синтезированы олигомерные жидкости, часть которых показала повышенную смазывающую способность, а некоторые могут быть использованы в качестве защитных покрытий для оргстекла.
ПЛ-3
Докладчик: к.х.н., профессор Пидько Евгений Александрович
«Расчетная химия каталитических систем: скоро ли восстание машин?»
Делфтский технологический университет, Делфт, Нидерланды
Университет ИТМО, Санкт-Петербург, Россия
Тюменский государственный университет, Тюмень, Россия

Евгений Александрович Пидько
В своем выступлении лектор отметил, что в современной химии и катализе расчетные методы заняли достойное место в инструментарии исследователей для характеризации и анализа механизмов реакций наравне с такими физико-химическими методами как ядерный магнитный резонанс, колебательная спектроскопия, фотометрия и рентгеновская дифракция. Сегодняшний прогресс в фундаментальных исследованиях каталитических систем в огромной степени основывается на результатах квантово-химических расчетов. Современные методы расчета функционала электронной плотности достигли уровня точности, достаточного для обеспечения поддержки кинетических и спектроскопических исследований в катализе, и даже для определения направления экспериментального поиска новых улучшенных катализаторов для химических процессов. Однако точность подобных расчетов, являющаяся краеугольным камнем теоретических исследований, упирается в несовершенство используемых моделей, развитие которых стало ведущей темой данного доклада.
Точность квантово-химических методов и атомистических моделей каталитических систем особенно важна для разработки передовых прогностических моделей в катализе, которые требуют знания структуры активных центров на молекулярном уровне, механизма их действия и эволюции в условиях каталитического процесса. Традиционные статические модели, основанные на приближении поверхности потенциальной энергии при 0 К в пределе низкой концентрации реагента, не способны описать динамическую природу каталитических систем. Дальнейшее увеличение предсказательной способности теоретических исследований требует расширения и пересмотра моделей реакционной способности каталитических систем с использованием «операндо» подходов, явно учитывающих условия реакции и структурную сложность образующихся каталитических ансамблей. В настоящем докладе вопросы точности моделирования каталитических систем были рассмотрены на примере гомогенных катализаторов селективного гидрирования, катион-модифицированных цеолитов и нанесенных оксидных систем.
ПЛ-4
Докладчик: академик РАН Белецкая Ирина Петровна
«Прогресс в металлокомплексном катализе на рубеже 20 и 21 веков»
Московский государственный университет имени М.В. Ломоносова, Химический факультет, Москва, Россия

В своей лекции академик РАН Ирина Петровна Белецкая на примере реакций кросс-сочетания и присоединения к кратным связям с образованием связей углерод-углерод и углерод-гетероатом (N, S, Se, P, O, Si, B) рассмотрела изменения, произошедшие в области металлокомплексного катализа: переход от катализа палладием к катализу медью и другими дешевыми и менее токсичными металлами, гетерогенизацию комплексов металлов на разных подложках, а также новые перспективные пути синтеза металлокомплексных катализаторов.
ПЛ-5
Докладчики: к.х.н. Ощепков Александр Геннадьевич
Институт катализа СО РАН, Новосибирск, Россия
д.х.н. Савинова Елена Романовна
Страсбургский Университет, Страсбург, Франция
«Электрокатализ в системах преобразования энергии»
В связи с эпидемиологическими ограничениями Е.Р. Савинова не смогла присутствовать на Конгрессе лично, поэтому лекцию по результатам совместной работы прочитал А.Г. Ощепков.
Совместная работа коллектива ученых из Института катализа СО РАН и Страсбургского университета посвящена поиску перехода от ископаемого топлива к низкоуглеродной экономике, основанной на использовании возобновляемых источников энергии, что является одним из основных глобальных вызовов, с которыми сталкивается человечество в настоящее время. Это, в свою очередь, повышает значимость электрохимических технологий, которые становятся важной составляющей современного технологического уклада. Одним из перспективных направлений представляется преобразование электрической энергии в химическую энергию экологически чистого топлива (например, водорода) с последующим ее преобразованием в электричество в топливных элементах.

Александр Геннадьевич Ощепков
Следует отметить, что разработка эффективных, долговечных и доступных материалов для электрохимических систем преобразования энергии невозможна без понимания механизма электрокаталитических процессов, протекающих на поверхности электрокатализатора, и факторов, определяющих их скорость. Существуют различные подходы для получения прямой или косвенной информации о структуре и составе активных центров электрокатализатора и промежуточных продуктов электрокаталитических реакций, а также их механизмов. Oни включают использование электрохимических методов, in situ и operando спектральных и микроскопических методов, ab initio расчетов и кинетического моделирования. В лекции были проиллюстрированы перечисленные подходы, рассмотрен ряд важных электрокаталитических процессов, протекающих в низкотемпературных топливных элементах и электролизерах, и показаны примеры установления корреляций между структурой и составом активного компонента электрокатализатора и его активностью и стабильностью.
ПЛ-6
Докладчик: д.х.н. Яковлев Вадим Анатольевич
«Каталитические подходы переработки растительной биомассы»
Институт катализа СО РАН, Новосибирск, Россия

Вадим Анатольевич Яковлев
Получение различных биотоплив и их интеграция в традиционную нефтепереработку – перспективное направление зеленой энергетики. Руководитель инжинирингового центра Института катализа СО РАН, д.х.н. В.А. Яковлев в ходе конгресса «Роскатализ» рассказал о технологиях переработки биомассы и способах улучшения качества таких топлив. Современный технологический уровень переработки биомассы значительно ниже, чем в нефтепереработке и традиционной химической промышленности, ориентированной на ископаемое сырье. Это в свою очередь приводит к более высокой себестоимости биопродуктов. Очевидно, что разработка технологий переработки возобновляемого сырья должна быть, по возможности, нацелена на мягкую интеграцию в существующую инфраструктуру химической и нефтеперерабатывающей промышленности.
Роль катализа в переработке биосырья представляется ключевой. В связи с этим разработка катализаторов селективного гидрирования, окисления, а также для процессов этерификации, гидролиза и дегидратации продуктов первичной переработки является актуальной и представлена в данной работе.
В работе сделан акцент на каталитическую переработку фурфурола – продукта гидролиза гемицеллюлоз, растительных липидов – триглицеридов жирных кислот, свободных жирных кислот, жидких продуктов быстрого пиролиза растительной лигноцеллюлозы, иловых осадков коммунальных очистных сооружений. В основе переработки биосырья лежат процессы селективного гидрирования, гидрогенолиза, гидроизомеризации и крекинга. Отдельно рассмотрены каталитические процессы окислительной карбонизации биомассы и глубокого окисления отходов жизнедеятельности человека на примере иловых осадков очистных сооружений. Показано, что применение каталитических технологий позволяет решить, с одной стороны, проблему «углеродного следа», а с другой стороны, экологические проблемы, связанные с отходами.
Ключевые лекции:
КЛ-1
Докладчик: к.х.н. Варфоломеев Михаил Алексеевич
Варфоломеев М.А., Юань Ч., Вахин А.В., Нургалиев Д.К.
«Применение катализаторов для повышения эффективности добычи тяжелой нефти: от лабораторных исследований до промысловых испытаний»
Казанский (Приволжский) федеральный университет, Казань, Россия
В первой ключевой лекции рассказывалось о том, что в настоящее время в условиях ухудшения структуры запасов углеводородного сырья и возрастающего потребления энергии остро стоит проблема освоения трудноизвлекаемых запасов углеводородов, к которым, в частности, относятся тяжелые нефти. Сложности при добыче таких нефтей вызваны высокой вязкостью, плотностью и значительным содержанием смолисто-асфальтеновых веществ. Высокая энергоемкость применяемых тепловых технологий нефтедобычи требует использования принципиально новых технологических подходов. Одним из них может стать использование катализаторов

Михаил Алексеевич Варфоломеев
В работе были представлены результаты систематического исследования, начиная от разработки и характеризации катализаторов процессов окисления нефти и акватермолиза, их тестирования в свободном объеме, пористой среде и при пластовых условиях в режиме фильтрации на образцах керна до промысловых испытаний на месторождениях. Показано, что разработанные каталитические композиции смещают температурный интервал процессов окисления нефти в более низкие значения, снижают энергию активации, особенно стадии высоко-температурного окисления, стабилизируют фронт горения, что подтверждается данными состава газообразных продуктов окисления нефти и объемом добытой нефти. Также при паро-тепловом воздействии они приводят к снижению содержания тяжелых компонентов в нефти (смолы, асфальтены) и уменьшению ее вязкости, существенно повышают коэффициент вытеснения и дебит скважин. Дальнейшее развитие и практическое использование результатов данной работы позволит найти широкое применение катализаторов в процессах добычи тяжелой нефти
КЛ-2
Докладчик: д.х.н. Сидельников Владимир Николаевич
Сидельников В.Н., Патрушев Ю.В., Шашков М.В.
«Современные методы анализа многокомпонентных смесей методом газовой хроматографии»
Институт катализа СО РАН, Новосибирск, Россия

Владимир Николаевич Сидельников
Газовая хроматография – консервативный аналитический метод химии, но тем не менее он постоянно развивается. Главный научный сотрудник Института катализа СО РАН, д.х.н. В.Н. Сидельников рассказал в ходе конгресса «Роскатализ» о проблемах, которые возникают при использовании этого метода, и их решении за счет новых технологий разделения.
Одной из относительно новых технологий разделения является комплексная двумерная хроматография (ГХ×ГХ). Метод двумерной газовой хроматографии позволяет решать сложные задачи аналитического разделения с одновременным использованием двух колонок различной полярности – полярной и неполярной. Пару колонок подбирают таким образом, что вторая колонка позволяет разделить вещества, разделение которых на первой затруднено или невозможно.
Сравнительно недавно был открыт новый класс полярных и высокополярных неподвижных фаз для хроматографии – ионные жидкости (ИЖ). Преимущество ИЖ заключается в более высокой термостабильности по сравнению с любыми известными фазами аналогичной полярности. Колонки с ИЖ имеют уникальную селективность, что позволяет проводить разделения, которые ранее были невозможны. Использование ИЖ в двумерной хроматографии позволило проводить высокоселективные разделения полярных соединений при повышенной температуре.
Еще одним современным достижением в области хроматографии стала разработка капиллярных и полунасадочных колонок, изготовленных на основе технологий микромеханики. Внедрение этих колонок и появление аппаратуры на их основе приводит к миниатюризации и удешевлению хроматографического оборудования, направленного на решение специализированных аналитических задач, решаемых методом газовой хроматографии.
КЛ-3
Докладчик: академик РАН Анаников Валентин Павлович
«В чем разница между гомогенными и гетерогенными каталитическими системами в процессах органического синтеза?»
Институт органической химии им. Н. Д. Зелинского РАН, Москва, Россия
Традиционно для решения задач органического синтеза используются как гомогенные, так и гетерогенные каталитические системы. Как показали работы, проводимые в лаборатории академика В.П. Ананикова, динамический катализ комплексами и наночастицами переходных металлов, нанесенными катали-заторами, а также собственная каталитическая активность углеродных центров на поверхности составляют универсальный и гибкий инструментарий для решения задач тонкого органического синтеза. Открытие фундаментальной роли динамических явлений в катализе поменяло представление о функционировании катализаторов и поставило вопрос о взаимопревращении гомогенных и гетерогенных каталитических систем. Исследование динамических каталитических систем имеет ключевое значение для создания катализаторов нового поколения, обладающих свойствами динамической подстройки и пригодных для решения широкого круга практически важных задач.

Александр Валентинович Лавренов,
Валентин Павлович Анаников,
Евгений Александрович Пидько
В настоящем докладе были проанализированы современные исследования природы каталитических систем и обсуждены достоинства и недостатки каждого из типов катализаторов. Существенное значение имеют вопросы интеграции передовых методов контроля типа каталитических систем, визуализации процессов с участием наноразмерных систем. Важное значение имеет сравнение катализаторов на основе никеля и палладия и выбор оптимальных каталитически активных центров.
КЛ-4
Докладчик: д.т.н. Загоруйко Андрей Николаевич
«Микроволокнистые катализаторы: история и перспективы»
Институт катализа СО РАН, Новосибирск, Россия

Микроволокнистые катализаторы – это структуры, в которых в качестве носителя используются волокна различных материалов, в частности, стекловолокно. Такие структуры показывают высокую актив¬ность и термическую устойчивость в различных промышленных процессах по сравнению с другими типами. Ведущий научный сотрудник Института катализа СО РАН, д.т.н. А.Н. Загоруйко в рамках конгресса «Роскатализ» рассказал о том, какие микроволокнистые катализаторы разрабатывают в Центре, как их применяют и какие у них перспективы.
Использование многоуровневого геометрического структурирования каталитических систем открывает путь для создания новых высокоэффективных типов катализаторов с улучшенной активностью, высокой эффективностью массопереноса и низким перепадом давления. В течение последних 20 лет прорыв в этой области был достигнут за счет применения микроволокнистых, в частности, стекловолокнистых катализаторов (СВК). СВК демонстрируют высокую активность и стойкость к дезактивации в различных каталитических реакциях. В качестве активных компонентов в таких катализаторах могут использоваться различные благородные металлы (Pt, Pd и др.), а также оксиды переходных металлов (Cu, Fe, V, Ni и пр.).
Различные подходы к синтезу СВК позволяют стабилизировать высокодисперсные моно- или биметаллические частицы как на поверхности микроволокон, так и в их объеме, что влияет на их структуру и электронные свойства: так на поверхности стабилизируются частицы в виде металла, тогда как в объеме – металл-оксидные Pt0-PtOx кластеры, что может существенно влиять как на каталитическую активность, так и на термостабильность и устойчивость к дезактивации катализатора.
Важным инженерным достоинством СВК является возможность их структурирования в виде картриджей с однородной структурой, интенсивным тепло- и массообменом, низким гидравлическим сопротивлением. Наиболее перспективными областями применения таких катализаторов являются быстрые реакции в газовой и жидкой фазах, а также реакции, в которых селективность чувствительна к диффузионным ограничениям.
В докладе были представлены существующие и перспективные применения каталитических процессов на основе СВК.
КЛ-5
Докладчик: д.х.н. Водянкина Ольга Владимировна
«Катализаторы на основе оксидов марганца/церия для окислительных превращений органических соединений и СО»
Томский государственный университет, Томск, Россия

В лекции было рассказано о том, что каталитические системы, используемые в окислительных процессах, постоянно совершенствуются в связи с растущим загрязнением атмосферы и необходимостью контролировать качество воздуха в больших городах и промышленных комплексах. Наряду с использованием нанесенных катализаторов, содержащих благородные металлы подгруппы Pt, разработаны и широко используются сложные оксидные катализаторы, состав которых может варьироваться в достаточно широких пределах в зависимости от решаемых задач. Оксиды церия и марганца привлекают внимание исследователей как активный компонент или модификатор для создания активных катализаторов на основе сложных и/или нанесенных оксидных материалов для процессов окисления летучих органических соединений и монооксида углерода.
В докладе анализируются современные подходы к созданию эффективных каталитических композиций на основе оксидов марганца/ церия с добавкой наночастиц металлов и/или модифицированных катионами некоторых переходных металлов (Ce3+/Ce4+, Sn2+, Fe3+) для процессов окисления этанола, формальдегида и монооксида углерода. Детализируется влияние способа приготовления, природы активной фазы, распределения модифицирующих компонентов, обсуждается механизм совместного действия.
КЛ-6
Докладчик: д.х.н. Будникова Юлия Германовна
«Синтетические модели гидрогеназ как молекулярные электрокатализаторы для водородной энергетики – от молекул до материалов»
Институт органической и физической химии им. А.Е. Арбузова
Обособленное структурное подразделение ФИЦ КазНЦ РАН, Казань, Россия

Лектор отметила, что в настоящее время мы наблюдаем зарождение новой глобальной энергетической реальности. Большое внимание уделяется переходу от традиционных технологий получения и переработки тепла и электричества к новой водородной энергетике. Использование молекулярного водорода является одним из возможных путей рационального, экологически безопасного и устойчивого развития. Водород – идеальный энергетический материал, а разложение воды с использованием солнечной/электрической энергии – один из способов получения водорода. Это чистый источник энергии, поскольку при его сгорании образуется только вода. И топливные элементы на основе водорода, так называемые H2/O2 топливные элементы, в качестве побочных продуктов образуют только воду и тепло. Замена платины в водородных топливных элементах на широко распространенные металлы станет решающим шагом на пути к практическому использованию Н2 в качестве альтернативного топлива. В последнее время получено много новых интересных и многообещающих результатов с применением высокоактивных электрокатализаторов, как гомогенных, так и гетерогенных (иммобилизованных на электроде).
Комплексы переходных металлов, гетероатомные или допированные металлами наноуглеродные материалы, металлоорганические каркасы и другие производные металлов, которые в той или иной степени являются структурными или функциональными аналогами гидрогеназ, широко исследуются в качестве альтернативы катализаторам на основе драгоценных металлов. В настоящем докладе были обобщены некоторые достижения в области разработки новых электрокатализаторов для получения/окисления H2 и их применения в топливных элементах, основное внимание уделено рассмотрению каталитической активности комплексов и координационных полимеров на основе металлов подгруппы никеля.
КЛ-7
Докладчик: к.х.н. Чистяков Андрей Валерьевич
Чистяков А.В.1, Чистякова П.А.1, Николаев С.А.2, Завелев Д.Е.1, Цодиков М.В.1
«Закономерности самоконденсации этанола в присутствии гетерогенных биметаллических катализаторов»
1 − Институт нефтехимического синтеза им. А.В. Топчиева РАН, Москва, Россия
2 − Московский государственный университет имени М.В. Ломоносова, Москва, Россия

В лекции было отмечено, что этанол является одним из наиболее крупнотоннажных продуктов переработки биомассы, годовое производство которого уже сейчас превышает 120 млрд. л. Этанол в настоящее время широко используется в качестве добавки к бензину, однако он также может быть использован в качестве возобновляемого источника широкого спектра углеводородов.
Установлено, что в сверхкритическом состоянии этанол в присутствии ряда моно- и биметаллических катализаторов превращается в бутанол c выходом, практически на порядок превышающим уровень, достигнутый при превращении газо-образного этанола. Проведенные кинетические исследования показали, что в ходе превращения сверхкритического этанола отсутствуют диффузионные затруднения и реакция протекает в кинетической области; кроме того, благодаря высокой плотности флюида по сравнению с газообразным веществом, на три порядка увеличивается предэкспоненциальный множитель, что свидетельствует об увеличении числа соударений реагента с каталитической поверхностью. Наиболее высокой активностью обладают биметаллические Au-M/Al2O3 и Pd-M/Al2O3 системы, в присутствии которых конверсия этанола превышает 60% при селективности 85-90 % по образуемым линейным первичным спиртам (1-бутанол, 1-гексанол, 1-октанол).
Сопутствующим продуктом образования линейных первичных спиртов из этанола является вода, которая в силу, по-видимому, конкурентной хемосорбции на кислотных центрах катализатора ингибирует реакцию. Показано, что прокалка катализатора при температуре 500 °С позволяет восстановить кислотность поверхности катализатора. Впервые продемонстрирована возможность увеличения выхода 1-бутанола путем использования абсолютированного этанола или добавления в реактор карбоната калия для связывания образующейся воды
КЛ-8
Докладчик: д.х.н. Якубов Махмут Ренатович
«Переработка тяжелых нефтей с повышенным содержанием ванадия и никеля»
Институт органической и физической химии им. А.Е. Арбузова
Обособленное структурное подразделение ФИЦ КазНЦ РАН, Казань, Россия
В лекции было отмечено, что в настоящее время тяжелые нефти относят к нетрадиционным углеводородным ресурсам, для эффективной переработки которых необходимо совершенствование известных технологических методов и создание принципиально новых подходов. Тяжелые нефти характеризуются повышенной плотностью и вязкостью и, в большинстве случаев, высоким содержанием асфальтенов, смол и гетероатомных сера-, азот и кислородсодержащих соединений, а также металлокомплексов ванадила и никеля. Асфальтены являются наиболее тяжелыми компонентами нефти с высокой молекулярной массой, плотностью и ароматичностью, и максимальным содержанием гетероатомных компонентов и металлов. Способность асфальтенов к агрегации и выпадению при изменении внешних условий приводит к образованию отложений при добыче и транспортировке нефти.

Махмут Ренатович Якубов
В России, в основном в Волго-Уральском регионе, к настоящему времени в разработку вовлечено достаточно большое количество месторождений тяжелых нефтей с общим объемом около 5 млн т в год, в которых суммарное содержание ванадия и никеля превышает 0,03 % мас., что позволяет рассматривать их как сырьевой источник данных металлов. С другой стороны, такой уровень концентраций металлов в нефтях, наряду с повышенным содержанием серы и азота, существенно осложняет их переработку, так как деактивирует катализаторы.
Мировая тенденция к увеличению доли тяжелой нефти в общем балансе нефтедобычи представляет собой большой стимул для изучения возможности комбинирования имеющихся технологий переработки такой нефти. Решение о том, какой подход лучше, зависит в основном от свойств нефти и целевых показателей полученных продуктов. В связи с этим, процессы каталитической гидроконверсии для переработки тяжелой нефти приобретают большее значение, чем в прошлом. Проведение всесторонних научных исследований формирует основу для принятия решения о целесообразности интеграции различных технологических процессов для переработки тяжелых нефтей.
КЛ-9
Докладчик: д.х.н. Волошин Ян Зигфридович
Волошин Я.З.1,2,4, Бузник В.М.1, Санджиева Д.А.1, Локтев А.С.1-3, Дедов А.Г.1-3
«Электрокаталитическое и каталитическое получение водорода и синтез-газа с использованием гибридных каталитических материалов на основе клатрохелатов d-металлов»
1 – Российский государственный университет нефти и газа (национальный исследовательский университет) имени И.М. Губкина, Москва, Россия
2 – Институт общей и неорганической химии имени Н. С. Курнакова РАН, Москва, Россия
3 – Институт нефтехимического синтеза им. А.В. Топчиева РАН, Москва, Россия
4 – Институт элементоорганических соединений им. А. Н. Несмеянова РАН, Москва, Россия
В своем выступлении Я.З. Волошин отметил, что важнейшей технологической задачей для глобального будущего человечества является поиск безопасных, экологически чистых и возобновляемых источников энергии и сырья без увеличения выбросов CO2. Водород является потенциальной альтернативой углерод-содержащему топливу. Поэтому создание нового поколения каталитических материалов для высокоселективной конверсии углеродсодержащего топлива в H2-содержащие смеси продуктов их превращений (в частности, в синтез-газ), а также для получения водорода из воды – одна из важнейших задач современной водородной энергетики и химической промышленности. Использование катализаторов получения водорода из воды на основе соединений дешевых и распространенных неплатиновых d-металлов делает более доступными топливные элементы, электролизеры воды, электрохимические водородные компрессоры/концентраторы для водородной и ядерной энергетики, а также экологически чистого транспорта.

Ян Зигфридович Волошин
Квазиароматические клатрохелаты d-металлов являются синтетически-доступными соединениями, которые устойчивы в широком диапазоне редокс-потенциалов, pH и температуры. Их окислительно-восстановительные характеристики и физические свойства могут быть тонко настроены с использованием подходящих функционализирующих заместителей для достижения минимального перенапряжения реакции выделения водорода. Эти заместители с терминальными полиароматическими группами эффективно иммобилизируются за счет сильной физической адсорбции на поверхность углеродных электродных материалов. Кроме того, клатрохелаты с терминальными полярными группами адсорбируются на поверхность керамических подложек, приводя к гибридным системам, перспективным для каталитического получения синтез-газа. Ряд импрегнированных или иммобилизированных на углеродные катодные материалы клатрохелатов железа и кобальта(II) был испытан в качестве катодных электрокатализаторов получения Н2 в электролизерах воды. Их физическая адсорбция является структурно-зависимым процессом, на который влияет число терминальных (поли)арома-тических групп. Это позволило получить самоорганизованные монослои с высокой поверхностной концентрацией каталитически-активных металлоцентров.
Помимо этого, на каждой секции были представлены приглашенные устные доклады:
УДп-I-1
Докладчик: к.х.н. Бельская Ольга Борисовна
Бельская О.Б.1, Степанова Л.Н.1, Лихолобов В.А.2
«Новые подходы к синтезу катализаторов на основе слоистых двойных гидроксидов для реакций селективного гидрирования»
1 – Центр новых химических технологий ИК СО РАН, Институт катализа СО РАН, Омск, Россия
2 – Институт катализа СО РАН, Новосибирск, Россия
Доклад был посвящен теме селективного гидрирования, которое остается востребованным направлением превращения полифункциональных соединений, а разработка новых эффективных катализаторов – актуальной задачей. В качестве перспективных катализаторов аквафазного гидрирования рассмотрены системы на основе слоистых двойных гидроксидов (СДГ). Структура данных соединений, представленная положительно заряженными катионными слоями, разделёнными пространством, содержащим гидратированные анионы, позволяет не только регулировать текстурные и кислотно-основные свойства мате-риалов на основе СДГ, но и реализовать различные методы закрепления активного металла при синтезе катализаторов. Среди таких методов следует выделить интеркалирование активного металла в межслоевое пространство СДГ в составе анионных комплексов, а также введение катионов металлов в структуру гидроксидных слоев на стадии соосаждения гидроксидов.
Свойства полученных катализаторов Pd/MgAlOx, Ni(Со)/М2+AlOx рассмотрены в реакциях аквафазного гидрирования фурфурола и тринитробензойной кислоты. Показано, что в условиях аквафазных реакций использование катализатора Pd/MgAlOx, обладающего основными свойствами, позволяет не только варьировать его активность в зависимости от состояния нанесенного палладия, но и способствует нетипичному направлению превращения фурфурола через гидрирование фуранового цикла, а также возможности получения нитро-диаминобензола (продукта промежуточного гидрирования тринитробензойной кислоты) с высокой селективностью.
УДп-II-1
Докладчик: к.х.н. Денисов Сергей Петрович
Денисов С.П., Аликин Е.А. Бакшеев Е.О.
«Катализ в автомобильной отрасли. Взаимное развитие и современное состояние»
ООО «Экоальянс», Новоуральск, Россия
Докладчик напомнил, что появление двигателя внутреннего сгорания (ДВС) способствовало огромному скачку в развитии технологий человечества и росту численности населения планеты. Известно, что при сгорании топлива в цилиндрах ДВС в атмосферу выделяются вредные выбросы: СО, NOx (NO, NO2, N2O), NH3, углеводороды (порядка 600 видов), SOx, частицы сажи, CO2. Утилизация данных выбросов (кроме СО2) за счет каталитических технологий на борту автомобиля уже более 50 лет как внедрена в практику.
На протяжении истории развитие шло по пути повышения универсальности, ресурсоустойчивости и компактности систем нейтрализации. Одной из основных вех развития стало появление трехмаршрутных катализаторов (TWC) для бензиновых двигателей. Они обеспечивали одновременное окисление СО и СН и восстановление NOx, но при этом требовали точного поддержания стехиометрического соотношения топлива и кислорода. Такой технологический скачок позволил также резко повысить топливную эффективность работы двигателя, но главным его движущим мотивом все-таки было обеспечение эффективной работы катализатора очистки выхлопа. Более сложно обстоит дело с нейтрализацией выбросов дизельных двигателей. Для удаления сажи используются фильтры различных конструкций, в которых происходит каталитическое окисление сажи до CO2. Применение этих технологий, как и в случае с бензиновым двигателем, потребовало существенных доработок дизельного двигателя, также повысивших его эффективность.
Сегодня широко обсуждается достижение нулевой эмиссии токсичных веществ на транспорте, которое предполагает полный отказ от ДВС в пользу электрической энергии, а также водородной энергетики. В любом случае это будет сопряжено с развитием каталитических технологий, необходимых для экологизации новых электрогенерирующих мощностей и создания мобильных электрохимических генераторов и риформеров углеводородного топлива в водород.
Массовое применение каталитических технологий сделало автотранспорт сегодня самым большим потребителем платиновых металлов, что неизбежно ведет к их дефициту и удорожанию. Это стимулирует усилия по разработке более дешевых и высокоэффективных катализаторов. В России такие работы постоянно ведутся на ООО «Экоальянс» при активной поддержке ведущих научных организаций, прежде всего ИК СО РАН.
УДп-III-1
Докладчик: д.ф.-м.н. Емелин Алексей Владимирович
Емелин А.В., Рудакова А.В., Бакиев Т.В., Силявка Е.С., Артемьев Ю.М., Рябчук В.К.
«Гетероструктурные материалы для преобразования солнечной энергии в “солнечное топливо”»
Лаборатория «Фотоактивные нанокомпозитные материалы», СПбГУ, Санкт-Петербург, Россия
В докладе было рассказано о том, что в последнее десятилетие в области гетерогенного фотокатализа ярко проявляется тенденция по целенаправленному созданию фотокатализаторов на основе полупроводниковых гетероструктур. Такие гетероструктурные материалы способны эффективно поглощать солнечный свет и преобразовывать его энергию в энергию окислительно-восстановительных реакций.
В 2012 году коллективом под руководством доктора А.В. Емелина была предложена полностью твердотельная гетероструктура для реализации так называемой Z-схемы фотовозбуждения и разделения зарядов. В такой гетероструктуре разница между потенциалами восстановления и окисления существенно больше, чем у ее отдельных компонентов, что позволяет инициировать редокс процессы с большим значением |∆G|.
В докладе были представлены результаты сравнительных исследований двойных и тройных гетероструктур на основе компонентов CdS/WO3/TiO2 и BiVO4/CuBi2O4/TiO2, их фотоэлек-трохимического поведения и спектрального отклика. В частности, рассмотрен вопрос о том, как метод формирования гетероструктур определяет характер фотовозбуждения и разделения зарядов в гетеропереходах, задавая тем самым поведение гетероструктур в фотопроцессах.
УДп-IV-1
Докладчик: к.т.н. Верниковская Надежда Викторовна
«Математическое моделирование промышленных каталитических процессов»
Институт катализа СО РАН, Новосибирск, Россия
Новосибирский государственный технический университет, Новосибирск, Россия
Докладчик отметила, что оптимальной стратегией разработки промышленных каталитических процессов и реакторов является подход, сочетающий в себе экспериментальные работы и математическое моделирование. Экспериментальные исследования дают информацию о закономерностях протекания отдельных составляющих процесса, позволяют получить корректные значения параметров модели и критерии для проверки корректности модели. Математическое моделирование обеспечивает перенос информации между отдельными составляющими процесса, её анализ и синтез.
Каталитические системы имеют сложное многоуровневое строение. Полноценный анализ химико-технологических систем возможен только с привлечением метода математического моделирования. В настоящее время все чаще возникает необходимость не последовательного, а одновременного (совместного) учета в модели процессов, протекающих на разных пространственно-временных уровнях. Использование таких моделей позволяет найти режимы, способные интенсифицировать промышленный каталитический процесс и получить выигрыш в технологических показателях процесса, создать качественно новый процесс, объяснить явления, которые невозможно объяснить с помощью математических моделей, построенных на основе иерархического подхода. В работе были представлены результаты математического моделирования различных процессов, для каждого из которых были определены оптимальные параметры.
Помимо основной научной программы, в рамках Конгресса был проведен ряд сателлитных мероприятий, каждое из которых имело свою актуальную научную направленность и собрало большое число заинтересованных участников.
Отвечая на современный тренд к развитию «Зеленой химии» и снижению «углеродного следа», был проведен круглый стол «Химические технологии снижения “углеродного следа”».
Модераторами выступили:
академик РАН Бухтияров Валерий Иванович,
Институт катализа СО РАН, Новосибирск, Россия;
д.х.н. Носков Александр Степанович,
Институт катализа СО РАН, Новосибирск, Россия;
Краева Екатерина Анатольевна,
журнал «The Chemical Journal», Москва, Россия
Основные тематические направления Круглого стола:
 экономические и правовые аспекты «углеродного следа»;
экономические и правовые аспекты «углеродного следа»;
 технологические проблемы снижения «углеродного следа» в
технологические проблемы снижения «углеродного следа» в
o нефтехимии и нефтепереработке;
o азотной промышленности;
o черной металлургии и алюминиевой промышленности;
 проблемы развития водородной экономики.
проблемы развития водородной экономики.
К участию в заседании Круглого стола были приглашены представители государственных органов, российские компании в области нефтехимии, нефтепереработки, азотной промышленности, ведущие ученые и специалисты.
Доклады, представленные на круглом столе:
1. Маганов Наиль Ульфатович,
генеральный директор ПАО «Татнефть»
«Направления низкоуглеродного развития ПАО “Татнефть”»
2. Грачев Андрей Владимирович, руководитель практики Стратегические инновации, ПАО «Сибур»
«Направления декарбонизации нефтехимических компаний»
3. д.т.н. Капустин Владимир Михайлович, член экспертного Совета по технологическому развитию нефтегазовой отрасли при Минэнерго России
«Низкоуглеродные моторные топлива и компоненты»
4. чл.-корр. РАН Максимов Антон Львович, директор Института нефтехимического синтеза РАН
«Выделение и химическая переработка диоксида углерода в процессах производства крупнотоннажной продукции: проблемы и направления перспективных исследований»
5. д.э.н. Жуков Станислав Вячеславович, заместитель директора Института мировой экономики и международных отношений им. Е.М. Примакова РАН (ИМЭО РАН)
«Механизм трансграничного углеводородного регулирования и “Зеленая таксономия” Евросоюза»
6. к.э.н. Масленников Александр Оскарович, старший научный сотрудник Центра энергетических исследований ИМЭО РАН
«Требования к корпоративной информации по углеродному следу в рамках системы ЕС по торговле квотами на выбросы СО2»
7. Краева Екатерина Анатольевна, главный редактор журнала «The Chemical Journal»
«Зеленая сделка: лидеры и аутсайдеры»
В ходе прошедшей с большим успехом Молодёжной школы по катализу «Физико-химические методы исследования – ключ к пониманию принципов каталитического действия» участникам школы, в основном студентам, аспирантам и молодым специалистам, было представлено четыре пленарные лекции (30 мин.) и 25 устных докладов (15 мин.) по направлениям:
 Современные методы исследования строения и свойств функциональных материалов на нано- и макроуровне
Современные методы исследования строения и свойств функциональных материалов на нано- и макроуровне
 Возможности применения методов исследования in situ
Возможности применения методов исследования in situ
 Квантовохимические методы исследования механизмов каталитических реакций
Квантовохимические методы исследования механизмов каталитических реакций
 Физико-химические методы исследования катализаторов и их активных центров
Физико-химические методы исследования катализаторов и их активных центров
Пленарные лекции на Молодежной школе:
ПЛм-1
Докладчик: к.ф.-м.н. Бугаев Арам Лусегенович
Бугаев А.Л.1,2, Усольцев О.А.1, Скорынина А.А.1, Солдатов А.В.1
«Синхротронное излучение для диагностики активных центров катализаторов при реалистичных технологических условиях»
1 – МИИ ИМ ЮФУ, Ростов-на-Дону, Россия
2 – ЮНЦ РАН, Ростов-на-Дону, Россия
В лекции было рассказано о том, что активные фазы в катализаторах формируются непосредственно в условиях реакции. Поэтому ключевым шагом к пониманию взаимосвязей между структурой и каталитическими свойствами являются исследования в режиме operando, т.е. непосредственно в ходе работы катализатора при реалистичных технологических условиях. Уникальный инструментарий для проведения operando исследований предоставляют сегодня современные источники синхротронного излучения. В докладе был представлен обзор современного состояния в области синхротронного излучения и основанных на его использовании экспериментальных методик, широко используемых для исследования катализаторов.
ПЛм-2
Докладчик: к.х.н. Стонкус Ольга Александровна
«Современные методы просвечивающей электронной микроскопии для изучения структуры катализаторов от нано- до атомного уровня»
Институт катализа СО РАН, Новосибирск, Россия
В лекции было показано, что современные гетерогенные катализаторы состоят из дисперсных частиц, сформованных в гранулы или закрепленных на пористых носителях. Высокая величина удельной поверхности катализаторов обеспечивает высокую активность катализаторов при меньшей загрузке активного компонента. Это достигается за счет уменьшения размера кристаллитов катализатора до нано- и субнаноразмерного уровня. Для создания новых высокоактивных катализаторов и улучшения существующих необходимо понимание принципов функционирования катализатора, строения активного центра и роли носителя. Наиболее полную информацию о строении катализаторов получают, используя широкий комплекс физико-химических методов. Среди них незаменимым является метод просвечивающей электронной микроскопии (ПЭМ), позволяющий наблюдать морфологию и структуру катализаторов на нано- и атомном уровне.
Были продемонстрированы возможности использования различных методов ПЭМ для исследования оксидных и нанесенных металл-оксидных и металл-углеродных катализаторов, содержащих формы активного компонента различной дисперсности. Были представлены результаты, полученные на традиционном электронном микроскопе JEOL JEM-2010 с разрешением 1.4 Å и на современных электронных микроскопах JEOL JEM-2200FS и Thermo Fisher Scientific THEMIS Z, обладающих разрешениями 1 и 0.6 Å, соответственно.
ПЛм-3
Докладчик: к.ф.-м.н. Медведев Михаил Геннадьевич
«Молекулярное моделирование – “микроскоп”, в котором видно, как взаимодействуют отдельные молекулы»
Институт органической химии им. Н.Д. Зелинского РАН, Москва, Россия
Лектор рассказал, что ещё с начала 20-го века было разработано большое количество методов, позволяющих оценить молекулярное строение соединения (инфракрасная (ИК) спектроскопия, рамановская спектроскопия, рентгеноструктурный анализ, масс-спектрометрия, спектрометрия ядерного магнитного резонанса (ЯМР), ультрафиолетовая спектроскопия (УФ), спектроскопия кругового дихроизма (КД) и другие). Однако ни один из перечисленных выше методов не позволяет проследить эволюцию молекулярной системы в ходе её химического превращения – это можно сделать только с помощью методов молекулярного моделирования, которые используют законы квантовой физики (иногда существенно упрощённые) для предсказания физических свойств – например, энергии или экранирования ядер электро-нами, – любой заданной конфигурации атомов (состояния молекулярной системы). Имея возможность рассчитывать энергии разных конфигураций атомов, можно построить энергетические профили для альтернативных механизмов химического превращения, на основании которых можно определить, идёт ли реакция термодинамически, оценить её скорость, рассчитать вклады различных механизмов, предсказать соотношения продуктов. То есть, по большому счёту, предсказать, как будет вести себя изучаемая система в действительности, причём не феноменологически, а имея детальное представление о том, как реакция протекает на молекулярном уровне. Таким образом, можно сказать, что молекулярное моделирование является уникальным микроскопом, в котором можно разглядеть, как двигаются атомы в ходе химической реакции.
ПЛм-4
Докладчик: д.х.н. Яхваров Дмитрий Григорьевич
«Электрохимические методы генерирования и активации металлоорганических катализаторов для процессов олигомеризации и полимеризации этилена и получения новых материалов»
Институт органической и физической химии им. А.Е. Арбузова, ФИЦ КазНЦ РАН, Казань, Россия
Химический институт им. А.М. Бутлерова, КФУ, Казань, Россия
Как было отмечено лектором, применение физико-химических методов в современной химии для генерирования новых органических и элементоорганических соединений становится все более и более популярным в течение последних лет. Основное внимание уделяется созданию новых энергосберегающих и экологически безопасных технологий получения практически значимых и востребованных химических соединений, катали-заторов и новых материалов на их основе. В лекции были рассмотрены недавно разработанные при использовании электрохимических методов процессы генерирования и активации никельорганических сигма-комплексов – активных катализаторов процессов гомо- и кросс-сочетания органических галогенидов, олигомеризации и полимеризации этилена, получение фосфорорганических соединений из элементного (белого) фосфора, включая процессы электрохимического генерирования и использования в органическом синтезе новых, ранее считавшихся нестабильными, соединений, таких как фосфиноксид Н3РО, а также новые методы получения магнитоактивных соединений с электрохимически переключаемым спиновым состоянием металла в молекуле и наноразмерных катализаторов
22 сентября прошли заседания еще двух круглых столов: Круглый стол «Исследовательская карьера: от молодого ученого до лидера проекта»
Модераторы:
д.х.н. Козлов Денис Владимирович,
Институт катализа СО РАН, Новосибирск, Россия
д.х.н. Локтева Екатерина Сергеевна,
МГУ имени М.В. Ломоносова, Химический факультет, Москва, Россия

Участники круглого стола «Исследовательская карьера: от молодого ученого до лидера проекта»
Основные тематические направления Круглого стола:
• Образование-наука-бизнес-индустрия, их место в подготовке исследователей;
• Этапы подготовки и карьерные траектории: когда и где начинается и развивается карьера?
• Где искать и как готовить лидеров проектов? Как стать успешным лидером проекта?
Круглый стол «Катализ в добыче и переработке тяжелой и нетрадиционной нефти»
Модераторы:
к.х.н. Варфоломеев Михаил Алексеевич,
Казанский (Приволжский) федеральный университет, Казань, Россия
к.х.н. Казаков Максим Олегович,
Институт катализа СО РАН, Новосибирск, Россия
к.х.н. Шляпин Дмитрий Андреевич,
Центр новых химических технологий ИК СО РАН, Омск, Россия

Участники круглого стола «Катализ в добыче и переработке тяжелой и нетрадиционной нефти»
Основные тематические направления Круглого стола:
• Современные достижения в области каталитических технологий добычи и переработки тяжелой и нетрадиционной нефти;
• Опыт российских нефтяных компаний по развитию добычи и переработки тяжелой нефти. Проблема импортозамещения;
• Будущее каталитических технологий переработки тяжелой и нетрадиционной нефти в условиях энергетического перехода.
На закрытии были подведены итоги Конгресса, вручены дипломы и памятные сувениры за лучшие устные и стендовые доклады.
Лучший устный доклад 1 степени:
к.х.н. Сидоренко Александр Юрьевич
«Контроль стереоселективности в SO3H-катализируемой реакции Принса-Риттера для синтеза 4-амидотетрагидро-пирановых соединений»
Институт химии новых материалов НАН Беларуси, Минск, Беларусь
Лучший устный доклад 2 степени:
к.х.н. Буруева Дудари Баировна
«Исследование рутениевых катализаторов M2RuxCe2-xO7
(M – La, Nd, Sm) методом ИППЯ»
Международный томографический центр СО РАН, Новосибирск, Россия;
Новосибирский государственный университет, Новосибирск, Россия
Лучший устный доклад 3 степени:
к.х.н. Борецкая Августина Вадимовна
«Реальный фазовый состав промышленных оксидов алюминия и воспроизводимость катализаторов на их основе»
Казанский (Приволжский) федеральный университет, Химический институт им. А.М. Бутлерова КФУ, Казань, Россия
Лучший стендовый доклад 1 степени:
к.х.н. Красников Дмитрий Викторович
«Генератор искрового разряда как настраиваемый источник катализатора для аэрозольного синтеза однослойных углеродных нанотрубок с заданными характеристиками»
Сколковский институт науки и технологии, Москва, Россия
Лучший стендовый доклад 2 степени:
Емельянов Михаил Алексеевич
«Комплекс кобальта в качестве донора водородной связи для фиксации углекислого газа в циклические карбонаты в мягких условиях»
Институт элементоорганических соединений
имени А.Н. Несмеянова Российской академии наук (ИНЭОС РАН), Москва, Россия
Лучший стендовый доклад 3 степени:
Голубь Федор Сергеевич
«Получение водорода из муравьиной кислоты на Pd-катализаторах, нанесенных на азотсодержащие углеродные материалы»
Институт катализа СО РАН, Новосибирск, Россия
Награждение лучших докладчиков на Молодёжной школе по катализу «Физико-химические методы исследования – ключ к пониманию принципов каталитического действия»
Лучший устный доклад 1 степени:
Глыздова Дарья Владимировна
«Гидрирование ацетилена на катализаторе Pd-Zn/Сибунит: влияние растворителя»
Центр новых химических технологий ИК СО РАН, Омск, Россия
Лучший устный доклад 2 степени:
Шмаков Михаил Михайлович
«Сравнение методов установления силы кислотности арилдифторборанов – мягких гомогенных кислот Льюиса»
Институт катализа СО РАН, Новосибирск, Россия
Лучший устный доклад 3 степени:
Белик Юлия Алексеевна
«Новый способ приготовления фотокаталитических систем на основе оксидов/силикатов висмута»
Национальный исследовательский Томский государственный университет, Томск, Россия
Для спонсоров Конгресса была организована выставка-презентация рекламных проспектов, информационных материалов, баннеров компаний, которая прошла с 20 по 24 сентября в холле первого этажа комплекса УНИКС. Организаторы благодарят спонсоров за поддержку мероприятия.
Золотые партнеры Конгресса: ПАО «Газпром нефть» (Санкт-Петербург), ООО «Реолгрейд сервис» (Кольцово).
Спонсоры Конгресса: ООО «ФизЛабПрибор» (Москва), ООО «Сервис-центр «ХромоСиб» (Омск), ООО «Глювекс (Москва), ООО «Сайтегра (Москва), ООО «НКЦ «ЛАБТЕСТ» (Москва), ООО «Сигм плюс инжиниринг» (Москва), ООО «Элемент» (Екатеринбург), ООО «НПФ «Мета-хром» (Йошкар-Ола).
Информационные партнеры Конгресса: Журналы «Кинетика и катализ», «Катализ в промышленности», «Журнал прикладной химии», «Лаборатория и производство», «Нефть. Газ. Новации», а также информационное агентство «Девон».
После окончания Конгресса для участников была организована экскурсия в древний город Болгар. Болгар был столицей Волжской Булгарии – одного из ранних государственных объединений Восточной Европы. Болгарский историко-архитектурный комплекс – это самый северный в мире памятник средневекового мусульманского зодчества, включен в список всемирного наследия ЮНЕСКО. Болгар – святое для поволжских татар место. Здесь в 922 г. был принят ислам в качестве официальной государственной религии
Следующий конгресс планируется провести в 2024 году.
Материал подготовили:
Д.В. Козлов, Д.А. Шляпин, А.С. Аникина, С.С. Логунова
(ИК СО РАН, Новосибирск)
фото: А.Р. Иммен
How to optimize precious metal usage in catalytic converters
A study that tracks structural changes that deactivate and regenerate catalysts suggests ways to improve these important auto parts
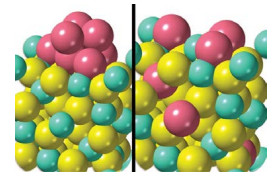
Catalytic converters may become deactivated by a reaction between rhodium nanoparticles and an alumina support (left) that forms inactive rhodium aluminate (right).
Catalytic converters efficiently strip pollutants and smog-forming compounds from engine exhaust by reacting these species on the surface of precious metals such as rhodium. The expensive metal may last longer and catalyst makers may be able to use it more sparingly thanks to a study that details atomic-level changes that deactivate and reactivate the catalytic metal (Chem. Mater. 2022, DOI: 10.1021/acs.chemmater.1c03513). Gasoline engine emissions are scrubbed by a three-way catalyst (TWC), which takes its name from the system’s ability to scrub three pollutants. TWCs oxidize hydrocarbons and carbon monoxide and reduce nitrogen oxides. Modern TWCs rely on rhodium nanoparticles typically supported on alumina. Earlier studies showed that exposure to oxidizing exhaust gases at high temperature can deactivate the catalyst and that reducing conditions can help restore its activity. But details of these processes have remained elusive. So Cheng-Han Li and Joerg R. Jinschek of the Ohio State University and coworkers at Ford Motor scrutinized TWCs using atomic-resolution microscopy, X-ray spectroscopy, and other methods. They exposed the catalysts to high temperatures and typical exhaust streams, which vary from oxygen rich to oxygen poor during normal engine operation. They showed that oxidizing conditions can cause rhodium nanoparticles to dissolve into the support and form rhodium aluminate, a catalytically inactive material. Reducing conditions help reverse the process. The study suggests that rhodium usage can be optimized by chemically anchoring the nanoparticles more tightly and by using alloys that resist dissolution.
New enzyme catalyzes biaryl cross-coupling reactions
Researchers engineered an artificial P450 enzyme to selectively catalyse the formation of a key motif
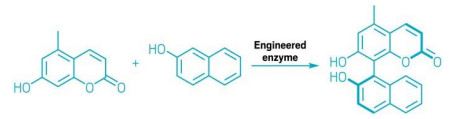
Using directed evolution, researchers have developed an enzyme that catalyses the formation of biaryl bonds (Nature 2022, DOI: 10.1038/s41586-021-04365-7). This new enzyme makes carbon-carbon bonds between aromatic moieties in a very selective manner, providing a new tool for the preparation of chiral ligands, pharmaceuticals, and materials.
Traditionally, chemists have synthesized biaryl bonds using metal-catalyzed reactions, such as Suzuki and Negishi cross-couplings. However robust, these processes require additional prefunctionalization steps to yield the target molecule. “It’s still challenging to make sterically hindered biaryl bonds,” explains lead author Alison R. H. Narayan, a chemist at the University of Michigan. Other structures, like electron deficient aromatic rings, also pose problems. “We envisioned a biocatalytic alternative could solve both issues and become a viable alternative for synthetic chemists,” she adds. The team used directed evolution, a technique that intentionally mutates the genes for a parent enzyme in successive rounds to evolve new catalytic functions.
The team explored different enzymes from secondary metabolic pathways involved in natural product formation to find a starting point. “Sometimes, these proteins already forge molecules that resemble our targets,” explains Narayan. “It’s all about finding a hint of reactivity, even 0.1%. Then it’s often possible to further optimize,” she adds. In this case, the team identified a cytochrome P450 enzyme that naturally catalyzes dimerization of coumarin into biaryls in Aspergillus fungi, and they engineered it to couple a broader range of substrates.
The team created over 2,000 variants of the natural enzyme and screened for those that could catalyze a cross-coupling reaction to produce chiral biaryl compounds. They used the best-yielding enzymes for successive rounds of engineering to improve yield, site selectivity and stereoselectivity. The team’s final enzyme gave a 92-fold improvement in yield.
“The catalytic and selective construction of carbon-carbon bonds is one of the most important tasks in organic chemistry,” says Ania Fryszkowska, a biocatalysis expert at Merck & Co. “Nature generates molecular complexity with ease and elegance, avoiding protecting groups and oxidative state readjustments, which is usually unachievable using traditional chemistry,” she adds.
Fryszkowska highlights how forming certain biaryl bonds is hard with the currently available tools: “It needs chiral ligands, protecting groups, auxiliary moieties,” she says. Biocatalysis offers a simple synthetic approach, which is typically safer and greener, too, since it minimizes the number of purifications and isolations along the way.
Further engineering resulted in an enzyme that offers unprecedented atroposelectivity—a preference between stereoisomers with axial chirality—in their target compound (shown). “This is a unique case in biocatalysis,” says Fryszkowska.
Atroposelectivity is key to preparing chiral ligands, which are used in asymmetric catalysis, and to synthesize certain commercial drugs like the antibiotic vancomycin and the antimalarial ancistrocladine.
By comparing the sequence of the engineered enzyme with other natural proteins, Narayan’s team has already found other promising leads: “We discovered a treasure trove of enzymes that have impressive cross-coupling activity on different classes of substrates, some with sufficient activity that engineering is not required,” says Narayan. “This panel of enzymes offers a solid starting point for others interested in planning this transformation into a synthesis, as biaryl bond formation is a bread-and-butter transformation.”
Zeolite intermediates offer new possibilities in catalysis
Amidst a well-known zeolite phase transformation, researchers have found active species that accelerate acid-catalyzed reactions
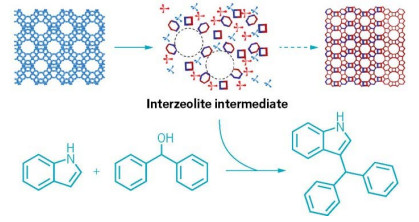
By stopping a well-known zeolite phase transformation before it completes, researchers can use the active intermediates (top, center) to accelerate acid-catalyzed reactions such as a Friedel-Crafts alkylation (bottom).
To make stable zeolites—porous inorganic materials used as industrial catalysts—researchers often convert the zeolites’ internal structure, transforming looser phases into more stable, denser forms. Now, chemists have caught these zeolites mid-transformation and discovered that the resulting intermediates supercharge three reactions important in industry (J. Am. Chem. Soc. 2022, DOI: 10.1021/jacs.2c00665). The process is highly tunable, giving chemists more control over the properties of their catalysts.
“Interzeolite transformation is a well-known process in the field,” says Javier García Martínez, whose lab at the University of Alicante conducted the study with collaborators at the National University of Colombia. “We decided to stop the process at different times and test the catalytic activity of the intermediates,” he says.
García Martínez’s team used three established methods to obtain the interzeolite transformation intermediates (ITIs): using an organic template, a surfactant, or a combination of both strategies. All are standard procedures for manufacturing mesoporous zeolites on industrial scales. The combination method creates a competition between the organic template and the surfactant, transforming zeolites considerably more slowly, which could be an advantage for controlling and monitoring the formation of ITIs, García Martínez says.
The researchers tried out the ITIs as catalysts in three popular acid-catalyzed reactions, including a Friedel-Crafts alkylation, a Claisen-Schmidt condensation, and polystyrene cracking. Overall, the turnover rate of the new catalysts was up to six times as fast as commercial zeolites.
Because the materials aren’t given time to fully set into the new structure, their pores are larger, thus allowing molecules to easily penetrate the porous network. Plus, the ITIs’ strongly acidic groups are more exposed, accelerating the acid catalysis, García Martínez says. “This is exactly why the ITIs showcase great activity.”
“Some people had tried to engineer similar catalytic materials, but this process looks more reproducible,” says Raúl Lobo, an expert in zeolites and materials engineering at the University of Delaware who was not involved in the study. Lobo points to the advantage of the improved control over the transformation and the uniformity of the new catalysts’ pores.
Matteo Cargnello, who studies materials for catalysis at Stanford University, says, “This synthesis could be easily adapted to large scales.” Companies could consider the added cost of the surfactant if their productivity increases, he adds.
In the meantime, García Martínez and collaborators have applied for a patent on the technology. “The possibilities of processing polystyrene into hydrocarbons have already attracted a few suitors,” he says.
Chemical & Engineering News
|
2022 г. XIV Конференция «Металлургия цветных, редких и благородных металлов» в рамках XII Международного конгресса и выставки «Цветные металлы и минералы» Красноярск, Россия |
https://nfmsib.ru/ |
|
April
20-22, 2022 Faraday Discussion – Photoelectron Spectroscopy and the Future of Surface Analysis London, United Kingdom |
https://www.rsc.org/events/detail/45900/ photoelectron-spectroscopy-and-the- future-of-surface-analysis-faraday-discussion |
|
28-29
апреля 2022 г. VIII Международная Российско-Казахстанская научно-практическая конференция «Химические технологии функциональных материалов» Алматы, Казахстан (видеоконференция на платформе Zoom) |
http://www.kaznu.edu.kz/ru/25415/page |
|
May
16-20, 2022 International Symposium on Green Chemistry (ISGC 2022) La Rochelle, France |
https://www.isgc-symposium.com/ |
|
May
25-27, 2022 Polymers-2022 New Trends in Polymer Science: Health of the Planet, Health of the People Turin, Italy |
https://polymers2022.sciforum.net |
|
June
6-8, 2022 19th Nordic Symposium on Catalysis Espoo, Finland |
https://19nsc.fi/ |
|
June
6-10, 2022 11th European Conference on Solar Chemistry and Photocatalysis: Environmental Applications (SPEA11) Turin, Italy |
https://www.spea11.unito.it/ |
|
June
27-29, 2022 Annual Meeting of German Catalysts Weimar, Germany |
https://dechema.de/en/katalytiker2022.html |
|
June
27-30, 2022 19th International Symposium on Relations between Homogeneous and Heterogeneous Catalysis (ISHHC 19) University of Oslo, Norway (Digital conference) |
https://www.mn.uio.no/kjemi/english/ research/groups/catalysis/ishhc19/ |
|
27
июня – 3 июля 2022 г. 16-е Совещание с международным участием «Фундаментальные проблемы ионики твердого тела» (ФПИТТ-2022) Черноголовка, Московская область, Россия |
https://fpssi16.altes.su |
|
1-3
июля 2022 г. VI Всероссийский научный симпозиум «Физикохимия поверхностных явлений и адсорбции» г. Плёс, Ивановская обл., Россия (без оргвзноса) |
ads-cat@yandex.ru
https://yadi.sk/i/miR6VnX4EqXtqw 8-905-059-40-24 |
|
July
3-8, 2022 12th Triennial Congress of the World Association of Theoretical and Computational Chemists (WATOC 2020) Vancouver, Canada |
http://watoc2020.ca |
|
July
3-6, 2022 International Conference of the International Association for Spectral Imaging (IASIM 2022) Esbjerg, Denmark |
https://2020.iasim.net/ |
|
July
11-13, 2022 1st Forum of Young Researchers on Heterogeneous Catalysis (YOURHETCAT 2022) Szeged, Hungary |
https://www.catalysis.hu/yourhetcat-2022/ |
|
11-13
июля 2022 г. Кузнецовские чтения - 2022. VI семинар по проблемам химического осаждения из газовой фазы ИНХ СО РАН, Новосибирск |
http://www.niic.nsc.ru/science/conferences-inx/ 909-conferences-2022/3565-kuznetsovskie-chteniya-2022 |
|
July
17-22, 2022 28th IUPAC Symposium on Photochemistry Amsterdam, Netherlands |
https://photoiupac2022.amsterdam |
|
July
18-22, 2022 2nd International Conference on Noncovalent Interactions (ICNI 2021-2022) Strasbourg, France |
http://icni2021.unistra.fr/ |
|
July
18-22, 2022 26th IUPAC International Conference on Chemistry Education (ICCE 2020) Cape Town, South Africa |
https://iupac.org/event/26th-iupac-international-conference-on-chemistry-education/ |
|
July
24-28, 2022 84th Prague Meeting on Macromolecules – Frontiers of Polymer Colloids Prague, Czech Republic |
https://www.imc.cas.cz/sympo/84pmm/ |
|
July
24-29, 2022 9th Tokyo Conference on Advanced Catalytic Science and Technology (TOCAT9) Fukuoka, Japan |
https://tocat.catsj.jp/9/ |
|
July
24-29, 2022 XXII International Symposium on Homogeneous Catalysis (XXII ISHC) Lisbon, Portugal |
https://xxii-ishc.events.chemistry.pt/ |
|
July
25-27, 2022 2nd International Conference “Materials and Nanomaterials” (MNs-22) Rome, Italy |
https://nanomaterials-europe.eu/ |
|
July
31 – August 4, 2022 15th International Conference on Catalysis in Membrane Reactor (ICCMR-15) Tokyo, Japan |
http://iccmr15.org/index.html |
|
August
28 – September 2, 2022 44th International Conference on Coordination Chemistry Rimini, Italy |
https://www.iccc2022.com/ |
|
August
28 – September 3, 2022 6th International Conference “Catalysis for Renewable Sources: Fuel, Energy, Chemicals” (CRS-6) Bar, Montenegro |
http://conf.nsc.ru/CRS6/en |
|
September
5-9, 2022 9th IUPAC International Conference on Green Chemistry (ICGC-9) Athens, Greece |
https://greeniupac2022.org/ |
|
5-9
сентября 2022 г. X международная конференция им. В.В. Воеводского (VVV-2022) Новосибирск, Россия |
www.vvv2022.com |
|
September
19-23, 2022 7th International Conference on Structured Catalysts and Reactors (ICOSCAR7) St. Petersburg, Russia |
http://conf.nsc.ru/ICOSCAR7
|
|
September
21-23, 2022 2nd International Conference on Unconventional Catalysis, Reactors & Applications (UCRA 2022) Warwick, United Kingdom |
www.ucra2022.org |
|
25
сентября – 1 октября 2022 г. IV Съезд аналитиков России Москва, Россия |
analystscongress@geokhi.ru |
|
26-30
сентября 2022 г. XII Международная конференция “Химия нефти и газа” Томск, Россия |
http://petroleum-chemistry.ru/ |
|
31
октября – 2 ноября 2022 г. Всероссийская научно-техническая конференция «Проблемы науки. Химия, химическая технология и экология» Новомосковск, Россия (без оргвзноса) |
gez75@yandex.ru
301665 Тульская область, г. Новомосковск, ул. Дружбы, д. 8 Новомосковский институт (филиал) ФГБОУ ВО «Российский химико-технологический университет имени Д.И. Менделеева» |
|
2023 г. VI Германо-Российский семинар «Связь между модельным и реальным катализом» |
https://catalysis.ru/block/index.php?ID=2&SECTION_ID=16 |
|
2023
г.
IV Международный симпозиум «Прикладные нанотехнологии и нанотоксикология» |
https://catalysis.ru/block/index.php?ID=2&SECTION_ID=16 |
|
2023
г.
XII Международная конференция «Механизмы каталитических реакций» |
https://catalysis.ru/block/index.php?ID=2&SECTION_ID=16 |
|
August
27 – September 1, 2023 15th European Congress on Catalysis (EuropaCat 2023) Prague, Czech Republic |
https://www.europacat2023.cz/ |