22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы

К 90-летнему юбилею
 15 марта 2019 г. исполнилось 90 лет действительному члену Российской академии наук, профессору кафедры общей и неорганической химии РГУ нефти и газа им. И.М. Губкина Илье Иосифовичу Моисееву.
15 марта 2019 г. исполнилось 90 лет действительному члену Российской академии наук, профессору кафедры общей и неорганической химии РГУ нефти и газа им. И.М. Губкина Илье Иосифовичу Моисееву.
Академик И.И. Моисеев – выдающийся ученый-химик, специалист в области кинетики и металлокомплексного катализа жидкофазных органических реакций, автор двух монографий, более 600 статей в научных журналах, а также более 100 патентов и авторских свидетельств.
Профессиональная деятельность Ильи Иосифовича началась в 1952 г. в Московском проектном институте МХП СССР в должности инженера по окончании МИТХТ им. М.В. Ломоносова (специальность “Технология основного органического синтеза”). В 1958 г. в МИТХТ он защитил кандидатскую диссертацию “Исследование в области жидкофазной гидратации ацетилена (реакция М.Г. Кучерова)”.
С 1963 г. его научная деятельность связана с Институтом общей и неорганической химии им. Н.С. Курнакова РАН, где он создал и возглавил лабораторию металлокомплексного катализа.
В 1992 г. Илья Иосифович был избран действительным членом Российской академии наук. С 1994 г. он является действительным членом Academia Europaea (Лондон), с 1996 г. – Academia Scientiarum et Artium Europaea (Вена), Академии наук, искусств и литературы (Париж).
Академик И.И. Моисеев известен российской и мировой научной общественности как крупнейший ученый в области координационной химии переходных металлов, один из основоположников металлокомплексного катализа – важнейшего направления в современной химии. Он является автором пионерских исследований по катализу жидкофазных реакций соединениями переходных металлов, синтезу новых координационных соединений, конструированию эффективных каталитических систем. И.И. Моисеевым созданы основы современных промышленных процессов получения ацетальдегида из этилена, муравьиной кислоты из СО и воды, 2-этилантрахинона. Его именем названа открытая им реакция получения винилацетата окислением этилена в уксусной кислоте.
Под руководством И.И. Моисеева сделаны открытия в области нефтехимии и катализа, имеющие важнейшее фундаментальное и практическое значение. Обнаружена каталитическая активность впервые синтезированных И.И. Моисеевым гигантских кластеров палладия в практически важных реакциях окислительного ацетоксилирования, карбонилирования, гидрирования, изомеризации, диспропорционирования и др. Разработан синтез биядерных комплексов 3d-переходных металлов – аналогов активной части природных металлоферментов. Найдены пути выяснения механизма действия природных металлоферментов. Установлен механизм реакций гидропероксидного окисления антрацена, алкенов и перфторалкенов, молекулярного азота до закиси азота. Разработаны методы защиты топлив и масел от окисления с помощью металлокомплексных добавок.
В созданном академиком И.И. Моисеевым Институте фундаментальных проблем химической переработки природного газа при РГУ нефти и газа имени И.М. Губкина (2003 г.) разработаны новые эффективные катализаторы получения этилена, оксида углерода и водорода из метана. Найдена новая реакция производства моторного топлива из растительного сырья – восстановительная дегидратация спиртов.
Созданная им научная школа “Комплексы и наноразмерные структуры в катализе” подготовила и воспитала десятки кандидатов и докторов наук.
И.И. Моисеев ведет большую научно-организационную работу. Он является вице-президентом Российского химического общества им. Д.И. Менделеева, научным руководителем Института фундаментальных проблем химической переработки природного газа при РГУ нефти и газа им. И.М. Губкина; представителем Российского газового общества в Рабочем комитете Международного газового союза, членом бюро Научного совета по катализу ОХНМ РАН.
И.И. Моисеев является членом редколлегий ведущих отечественных и зарубежных журналов: Успехи химии, Кинетика и катализ, Доклады РАН, Catalysis Letters and Topics in Catalysis Mendeleev Communications, Chemical Technology, членом международного совета Международных симпозиумов по гомогенному катализу и Международных конференций по металлоорганической химии и по механизмам каталитических реакций.
За свою многолетнюю активную научную, педагогическую и общественную деятельность Илья Иосифович Моисеев отмечен российскими и международными наградами и премиями: орденом Трудового Красного Знамени (1986), орденом Почета (1999), ему присуждена премия А.П. Карпинского (1999), Государственная премия РФ (2003), премия "Триумф" (2002), Государственная премия РФ в области науки и технологии (2003), присвоены звания "Почетный работник высшего профессионального образования" (2004); "Почетный работник газовой промышленности" (2004), получена медаль Королевского химического общества "Выдающийся лектор столетия" с правом чтения цикла лекций в университетах Великобритании (2007), Орден Дружбы (2009), Премия Правительства РФ в области науки и техники (2011), Демидовская премия за выдающийся вклад в координационную химию и металлокомплексный катализ органических реакций (2012), золотая медаль им. Д.И. Менделеева за выдающиеся работы в области катализа и энергосберегающих технологий (2013).
Научный совет по катализу ОХНМ РАН и редакция Каталитического бюллетеня
сердечно поздравляют Илью Иосифовича с юбилеем, желают ему крепкого здоровья
и новых творческих достижений!
 |
Российская академия наук |
Отчет о научно-организационной деятельности в 2018 году
Секретариат Научного совета по катализу ОХНМ РАН (НСК) предлагает вашему вниманию сводный отчет о деятельности Совета и научных исследованиях в области катализа, выполненных научными коллективами под руководством членов Научного совета по катализу в 2018 году.
Отчет состоит из трех разделов:
Тексты отчетов, полученные от членов НСК и научно-исследовательских коллективов, практически не подвергнуты корректировке.
Организационная деятельность
В 2018 году в рамках научно-организационной деятельности Научного совета по катализу ОХНМ РАН (НСК) были выполнены следующие мероприятия.
Выпущены три ежеквартальных сборника «Каталитический бюллетень», содержащие оперативную информацию о важнейших результатах фундаментальных и прикладных исследований в области катализа в России и за рубежом, материалы, посвященные деятельности выдающихся отечественных и зарубежных исследователей в области катализа, дается перечень предстоящих конференций, краткие отчеты о проведенных конференциях, рабочих совещаниях и другие материалы. Членами НСК курируются журналы в области катализа: «Кинетика и катализ» (главный редактор В.Б. Казанский) и «Катализ в промышленности» (главный редактор В.Н. Пармон). Секретариат НСК ведет переписку и текущую работу с членами Научного совета по катализу ОХНМ РАН.
Под эгидой Научного совета по катализу и при активном участии его членов организованы и проведены следующие конференции:
Изданы материалы проведенных конференций.
Продолжается сотрудничество с организациями Академий наук РФ и стран СНГ, Министерствами РФ, институтами разных ведомств и другими организациями России, дальнего и ближнего зарубежья по различным вопросам научной, научно-организационной, учебно-преподавательской и общественной деятельности в области катализа.
При активном участии членов НСК проводится работа в рамках Комплексного плана научных исследований «Ресурсо- и энергоэффективные катализаторы и процессы», цель которого – повысить на международном уровне конкурентоспособность отечественной химической науки в области межотраслевых технологий, при этом применяя результаты фундаментальных исследований в конкретных направлениях для последующих исследований полного цикла.
ОСНОВНЫЕ РЕЗУЛЬТАТЫ 2018 г.
Фундаментальные исследования в области создания
новых каталитических систем и применения
физических методов для их диагностики
Новые катализаторы для кислородной и углекислотной конверсии метана на основе цеолита структуры MFI и алюмомагниевого гидроталькита
Проведены
сравнительные испытания разработанных новых каталитических материалов
для кислородной и углекислотной конверсии метана. Показано, что
природа носителя (цеолит структуры MFI, алюмомагниевый гидроталькит,
стабилизированный оксид церия, оксид неодима) существенно влияет на
стабильность Ni- и Ni–Co-катализаторов в реакции кислородной
конверсии метана и в меньшей степени – на стабильность
катализаторов углекислотной конверсии метана в синтез-газ.
Алюмомагниевый гидроталькит оказался предпочтительным носителем для
никелевого катализатора, обеспечивающим стабильно высокий выход
синтез-газа (95-99%) в процессах кислородной и углекислотной
конверсии метана, не снижавшийся на протяжении 50-60 ч. Цеолит
структуры MFI проявил себя как стабильный и селективный носитель
Ni–Co-катализаторов углекислотной конверсии метана (выход
синтез-газа 95%, не снижавшийся на протяжении
академик А.Г. Дедов, академик И.И.
Моисеев,
д.х.н., проф. А.С. Локтев, аспирант И.Е. Мухин
Российский государственный университет нефти и газа
(Национальный исследовательский университет) имени И.М.
Губкина, г. Москва
Синтез новых композиционных материалов на основе традиционных носителей и углерода
Разработано новое направление в синтезе композиционных материалов на основе традиционных носителей и углерода. Новый композит из углерод-кремнеземного материала был успешно использован в приготовлении катализаторов синтеза Фишера-Тропша, так как позволяет получить меньшие частицы оксида железа с более узким распределением по размеру, чем чистый кремнезем. Уменьшение размеров частиц оксидов железа на углерод-силикатной композитной поверхности приводит к образованию карбида Hägg (Fe5C2) в результате активации в потоке CO/H2. Композиция из углерод-силикатного композита увеличивает каталитическую активность железа при гидрогенизации СО. Другими преимуществами новых катализаторов являются подавление образования метана и более высокое значение параметра роста углеродной цепи.
академик
РАН В.В. Лунин, д.х.н. П.А. Чернавский
Московский государственный
университет имени М.В. Ломоносова,
Химический факультет, г.
Москва
Приготовление каталитически активных систем с углеродными оболочками методом искрового плазменного спекания
С использованием метода искрового плазменного спекания (ИПС) впервые получены каталитически активные системы на основе железа, кобальта, никеля и меди, инкапсулированные в каркасные структуры на основе углеродных нанотрубок. Для этого воздействию ИПС подвергали УНТ, декорированные наночастицами оксидов соответствующих металлов. В ходе обработки они восстанавливались углеродом до металлов и покрывались углеродными оболочками, в то время как УНТ уплотнялись и спекались, образуя трехмерные каркасы. Каталитические испытания продемонстрировали высокую эффективность композитов в процессе ФТ, причём наличие углеродных оболочек позволило избежать процедуры предварительного восстановления образцов.
академик РАН В.В. Лунин, д.х.н. С.В. Савилов, к.х.н. С.А. Черняк
Московский государственный университет имени
М.В. Ломоносова,
Химический факультет, г. Москва
Совместное использование in situ ЯМР и ИК-спектроскопии для исследования молекулярно-ситовых катализаторов
Изучены механизмы каталитического действия и механизмы дезактивации активных центров молекулярно-ситовых катализаторов разного типа в ряде важнейших процессов газохимии, нефтехимии и органического синтеза, таких как олигомеризация бутенов, алкилирование анилина метанолом, синтез изопрена из изобутилена и формальдегида, конверсия этанола в бутанол, дегидрирование пропана, конверсия метанола в олефины, гидрообессеривание тиофена, эпоксидирование октена. Были установлены взаимосвязи между строением и составом активных центров и их каталитическими свойствами в данных реакциях, выбраны наиболее эффективные молекулярно-ситовые катализаторы и даны рекомендации по их дальнейшему усовершенствованию. Проведен сравнительный анализ разных in situ подходов к изучению гетерогенно-каталитических реакций. Показано, что и in situ ЯМР, и ИК-спектроскопии обладают огромным потенциалом для изучения «работающей» поверхности катализатора, идентификации продуктов уплотнения, ключевых интермедиатов, а также исследования кинетики поверхностных процессов. Важным достоинством ИК-спектроскопии является высокая чувствительность, что позволяет регистрировать сигналы от возможных интермедиатов с очень малой концентрацией. Одним из основных преимуществ спектроскопии ЯМР является возможность селективного наблюдения за несколькими ядрами раздельно. Спектроскопия ЯМР позволяет не только обнаружить, но и полностью расшифровать молекулярные структуры исследуемых соединений, отследить переход ядер из одного соединения в другое при использовании изотопной метки. Таким образом, одновременное использование обоих спектральных методов дает возможность получить максимально полную информацию не только о катализаторе, но и о химических процессах, происходящих на его поверхности.
профессор,
д.х.н. И.И. Иванова
Московский государственный университет имени
М.В. Ломоносова,
Химический факультет, г. Москва
Исследование свойств катализаторов PdNi/Al2O3 в реакции гидродехлорирования хлорбензола
Впервые исследованы физико-химические и каталитические свойства полученных методом лазерного электродиспергирования (ЛЭД) биметаллической мишени катализаторов PdNi/Al2O3 в реакции гидродехлорирования хлорбензола в паровой фазе. Методами СЭМ и ПЭМ-ЭДА установлено, что осажденные методом ЛЭД на решетку-держатель для ПЭМ частицы преимущественно включают оба металла в восстановленном состоянии с соотношением, примерно соответствующем составу исходного сплава Ni77Pd23. Методом РФЭС показано, что при осаждении на гранулы оксида алюминия никель подвергается полному окислению при контакте с носителем с образованием алюмината никеля, а палладий преимущественно (84%) остается в металлическом состоянии. В этом случае in situ восстановление обоих металлов в ячейке РФЭС-спектрометра сильно затруднено. Тем не менее, NiPd/Al2O3 более эффективен в газофазном гидродехлорировании хлорбензола при 150–350 °C по сравнению с монометаллическими катализаторами с тем же содержанием металлов (0,005 масс.%) и существенно более стабилен. Различие может быть обусловлено образованием новых активных центров в результате контакта Pd0 и NiAlOx на поверхности носителя, а также защитным действием шпинели, реагирующей с HCl и предотвращающей отравление палладия. Работа имеет важное теоретическое значение для разработки теории каталитического действия биметаллических катализаторов и практически важна с точки зрения получения эффективных катализаторов с ультрамалым содержанием благородного металла.
д.х.н. Е.С.
Локтева, к.х.н. Е.В. Голубина, д.х.н. Т.Н. Ростовщикова,
к.ф.-м.н. К.И. Маслаков, к.х.н. С.А. Николаев
Московский
государственный университет имени М.В. Ломоносова,
Химический факультет, г. Москва
д.ф.-м.н.
С.А. Гуревич, к.ф.-м.н. В.М. Кожевин
Физико-технический институт им. А.Ф. Иоффе РАН, г. С.-Петербург
А.Е. Ермаков
Институт физики металлов им. М.Н. Михеева УрО РАН, г. Екатеринбург
Новые методы синтеза титаносиликатных цеолитов со структурой MFI
Разработаны синтетические методы получения титаноси-ликатных цеолитов со структурой MFI, которые позволяют целенаправленно варьировать не только размер частиц этого молекулярного сита от 0,05 до 30 мкм, но и морфологию получаемых микрокристаллов. Применение фторсодержащих поверхностно-активных веществ таких, как перфторфенол и перфторнонановая кислота в качестве структурно-направляющих добавок при гидротермальной кристаллизации титоносиликатов позволило получить ультратонкие пластинчатые наночастицы цеолитного материала. Установлено, что разработанные методы синтеза титаносиликатов обеспечивают полное встраи-вание атомов титана в кристаллическую структуру цеолита при мольном соотношении Si/Ti в исходном геле не менее 80; при меньшем соотношении получаемые образцы содержат внеструктурный диоксид титана, что приводит к непроизводительному расходу пероксида водорода при каталитическом эпоксидировании непредельных соедине-ний.
д.х.н. Б.В. Романовский
Московский государственный университет имени М.В. Ломоносова,
Химический факультет, г. Москва
Гетерометаллические комплексы со связью Me-Me в качестве предшественников высокоселективных катализаторов деокси-генации сложных эфиров и гидрирования жирных кислот
Деоксигенация липидов растительных масел развивается с целью разработки альтернативных подходов к получению компонентов моторного и дизельного топлив. В присутствии известных промышленных катализаторов в процессе деоксигенации протекает процесс декарбоксилирования, в результате которого происходит потеря углеродной массы в результате образования С1, С2, СО и СО2.
В лаборатории Каталитических нанотехнологий ИНХС РАН совместно с лабораторией Химии обменных кластеров ИОНХ РАН разработаны каталитические системы Pt-5Sn/Al2O3 и Fe-2Sn/Al2O3 на основе биметаллических оригинальных платинооловянных и железооловянных гетерометаллических комплексов, в структуре которых есть гетерометаллическая связь металл – металл (М1 – М2), используемых в качестве предшественников. Платинооловянная система проявляет практически исчерпывающую активность и селективность в восстановительной деоксигенации сложных эфиров, включая эфиры липидной части растительных масел. В процессе деоксигенации эфиры превращаются в алифатические углеводороды с числом углеродных атомов, равным углеводородным фрагментам эфиров, и воду. Железооловянная система проявляет повышенную селективность в гидрировании жирных кислот до альдегидов, являющиеся ценными продуктами для основного органического синтеза.
Изучение структуры показало, что каталитические компоненты на поверхности присутствуют в виде нанораз-мерных гетерометаллических сплавов и разновалентных оксидов олова (Sn4+; Sn2+). На основании данных по эволюции структуры каталитических систем и квантово-химического моделирования представлены основные принципы механизма селективной деоксигенации: кислород эфиров координируется на льюи-совских центрах олова, в то время как канал восстановления реализуется на гетерометаллических кластерах PtSn3±δ. Данные квантово-химического моделирования показали, что сущест-венно понижен энергетический барьер переноса атомов Н с атомов платины на атомы олова с последующей их ассоциацией в молекулы Н2. В результате этого можно предположить снижение гидрирующей и крекирующей активности платиносодержащих компонентов при сохранении «мягкой» активности к восстановлению кислорода.
д.х.н.,
проф. М.В. Цодиков, к.х.н. А.В. Чистяков
Институт
нефтехимического синтеза им. А.В. Топчиева РАН, г. Москва
к.х.н. С.С. Шаповалов
Институт общей и неорганической химии им. Н.С.
Курнакова РАН, г. Москва
Разработка покрытия [Bi-Pb]/TiO2, обладающего фотоэлектро-каталитической активностью при облучении в видимой области спектра
В рамках разработки фоточувствительных материалов, способных к преобразованию солнечной энергии в видимой области спектра и проявляющих каталитическую активность в электрохимических реакциях с целью очистки водных сред от токсичных органических примесей, в лаборатории Каталитических нанотехнологий ИНХС РАН совместно с лабораторией Процессов в химических источниках тока в качестве фотоэлектрокатализаторов разработаны алкоксо-синтезы однофазных моно- и бикомпонентных оксидов титана состава [Bi-Pb]/TiO2. Изучено влияние полученных оксидных систем на область поглощения света. Модификация бикомпонентным модификатором оксида титана сдвигает большую часть света в видимую область 400-450 нм. Разработана лабораторная технология получения покрытия поверхности фотоанода псевдо-однофазным [Bi-Pb]TiO2 сложным оксидом, обладающим поглощением света в видимой области. Сдвиг в видимую область поглощения указывает на снижение ширины запрещенной зоны TiO2 от 3,2 до 2,7 эВ. В отличие от ранее синтезированных однофазных оксидов FexTi 1,75xO2±δ и FexZr1,75xO2±δ, также показывающих сдвиг в видимую область, но не проявляющих фотоэлектро-каталитическую активность, разработанная биметаллическая система обладает наиболее высокой каталитической актив-ностью в фотоэлектроокислении метанола, растворенного в воде. На слайде показано, что в отличие от темновых опытов, при облучении видимой областью спектра в цепи генерируется ток. Квантовый выход реакции окисления метанола приближенно на порядок выше по сравнению с активностью висмутсодержащих титанатов, полученных другими методами.
д.х.н. М.В. Цодиков
Институт нефтехимического
синтеза им. А.В. Топчиева РАН, г. Москва
д.х.н. В.А. Гринберг
Институт физической химии и электрохимии им. А.Н. Фрумкина РАН, г. Москва
Разработка гетерогенных катализаторов
Найден эффективный, дешевый и рециклизуемый гетерогенный катализатор для реакции эпоксидов с диоксидом углерода с получением циклических карбонатов.
Изучен катализ наночастицами меди в реакции образования связей углерод-углерод, углерод-сера, углерод-азот. В итоге показаны дальнейшие возможности замены катализаторов на основе палладия на катализаторы на основе меди.
академик
И.П. Белецкая
Московский
государственный университет имени М.В. Ломоносова,
Химический факультет, г. Москва
Химические и фазовые превращения в системах “Na2WO4-MnxOy – носитель” (SiO2 или Al2O3)
Методами термодесорбции кислорода, рентгеновской фотоэлектронной спектроскопии, рентгенофазового анализа in situ, а также сканирующей электронной микроскопии совместно с энергодисперсионной рентгеновской спектро-скопией исследованы химические и фазовые превращения, протекающие в NaWMn-содержащих оксидных системах, нанесенных на SiO2 и Al2O3, которые проявляют высокую эффективность как катализаторы окислительной конденсации метана. Показано, что возможность окислительно-восстано-вительного перехода Mn(2+) « Mn(3+), определяющего каталитические свойства системы и связанного с превращением MnWO4 « Mn2O3, зависит от интенсивности взаимодействия нанесенных фаз с носителем. Более высокое сродство фазы MnWO4 к SiO2 и Mn2O3 к Al2O3 определяет более высокие активность и селективность катализатора NaWMn/SiO2. Показано, что ключевым моментом для эффективного протекания каталитического процесса на катализаторах данного типа является образование расплава на основе вольфрамата натрия, сильно взаимодействующего с носителем. Приведенные результаты получены впервые и раскрывают механизм действия наиболее эффективного из известных катализатора практически важной реакции – окислительной конденсации метана в этилен.
д.х.н.
М.Ю. Синев, к.х.н. Е.А. Пономарева,
к.х.н. З.Т. Фаттахова, к.х.н. Д.П. Шашкин,
к.х.н. В.И. Ломоносов, Ю.А. Гордиенко
Институт
химической физики им. Н.Н. Семенова РАН, г. Москва
Изучение автоколебаний скорости окисления этилена на металлическом Ni. Разработка метода твердофазного синтеза оксидных медно-цериевых катализаторов избирательного окисления СО
Детально изучены автоколебания скорости окисления этилена на металлическом Ni. Автоколебания обнаружены в интервале температур 600-800° С. Установлено, что, в противоположность известным автоколебаниям окисления этилена на Pt и Rh, автоколебания на Ni происходят в смесях с относительным избытком этилена. Колебания наблюдали в режиме непрерывной подачи газовой смеси, а также в импульсном режиме. Данные, полученные в импульсном режиме, позволили вычислить периодические изменения балансов по кислороду и углероду. В результате было установлено, что в ходе автоколебаний происходит периодическое восстановление-окисление никеля. Эти процессы сопровождались движением фронтов (волн) окисления Ni или восстановления NiO на поверхности катализатора.
Разработан новый экологически чистый метод твердофазного синтеза оксидных медно-цериевых катализаторов избирательного окисления СО в смеси с водородом методом механохимической активации (МА) из смесей порошка Cu (8 масс. %) с CеО2. Максимальная конверсия СО зависит от времени МА, составляя 98% при 165°С после 60 мин МА, и практически не отличается от каталитической активности традиционных нанесенных CuО/CeО2 катализаторов.
д.х.н.,
проф. В.Н. Корчак
Институт химической физики им. Н.Н.
Семенова РАН, г. Москва
Селективные катализаторы для реакций конденсации пропаналя
Разработаны новые селективные наноструктурированные гетерогенные катализаторы для процессов гомоконденсации пропаналя и его кросс-конденсации с этаналем и метаналем в различных соотношениях. Установлена зависимость конверсии реагентов и селективности процесса гомо/гетероконденсациии от природы катализатора и температуры. Полученные результаты подтверждают значительные потенциальные синтетические возможности реакций конденсации альдегидов, в том числе для решения экологических вопросов.
д.х.н.,
проф. В.Р. Флид, д.х.н., проф. Л.Г. Брук,
асп. Е.М. Марцинкевич
Московский технологический университет, г. Москва
Каталитические системы для аллилирования норборнадиена аллилформиатом
Разработаны новые гомогенные и гетерогенизированные каталитические системы для реакций аллилирования и гидроаллилирования норборнадиена аллилформиатом. Оба процесса рассмотрены с единых позиций, предложен механизм, предполагающий различные направления гидридного переноса в ключевых интермедиатах. С помощью дейтерированных реагентов доказано, что гидридный перенос может осуществляться с участием аллильного, норборненильного или формильного фрагментов. Во всех случаях на этой стадии происходит отрыв водорода, связанного с углеродным атомом в β-положении относительно палладия. Предложены оптимальные условия для селективного образования индивидуальных соединений, являющихся перспективными мономерами и полупродуктами в органическом синтезе.
д.х.н., проф. В.Р. Флид, д.х.н. Р.С. Шамсиев,
асп. С.А. Дураков
Московский технологический университет, г. Москва
Селективные катализаторы раскрытия циклов нафтеновых соединений
Раскрытие
циклов представляет собой одно из наиболее эффективных направлений
улучшения качества дизельного топлива и переработки тяжелых
нефтепродуктов (полицикли-ческих углеводородов). В ИОХ РАН
разработаны селективные катализаторы раскрытия циклов нафтеновых
соединений (на примере декалина) на основе нанесенных биметаллических
Pt-Ru наночастиц. Конверсия декалина
80% наблюдается уже при
проф., д.х.н. Л.М. Кустов
Институт
органической химии им. Н.Д. Зелинского РАН, г. Москва
Влияние калия на каталитические свойства Fe/C в синтезе Фишера–Тропша. Новые катализаторы метоксикарбонилирования иодбензола
Установлено влияние щелочного промотора (калия) на генезис активной фазы и каталитические свойства Fe/активированный уголь в синтезе Фишера–Тропша. Показано, что предварительное внесение калия способствует снижению среднего размера частиц магнетита с меньшим разбросом по размеру по сравнению с непромотированным катализатором. Активация катализатора в атмосфере синтез-газа приводит к полной или почти полной трансформации магнетита в карбид Хэгга. Катализаторы с предварительно внесенным калием демонстрируют увеличенную активность и селективность в отношении углеводородов С5+, меньшее образование метана.
Предложены новые катализаторы метоксикарбонилирования иодбензола: инкапсулированные в металл-органические каркасы наночастицы Pd. Изучено влияние природы MOF на морфологию и активность наночастиц Pd.
чл.-корр. РАН А.Л. Лапидус
Институт
органической химии им. Н.Д. Зелинского РАН, г. Москва
Высокоселективный катализатор гидрирования алкинов на основе интерметаллических наночастиц Pd1In1
Разработан эффективный метод синтеза нанесенных биметаллических Pd-In/Al2O3 катализаторов, активными центрами которых являются высокоупорядоченные ноночастицы интерметаллида Pd1In1. Методом ИК спектроскопии адсорбированного СО установлено, что на поверхности наночастиц атомы Pd изолированы друг от друга атомами In, образуя поверхностную структуру “single-atom” с центрами Pd1. Катализаторы показали высокую селективность в реакциях жидкофазного гидрирования замещенных алкинов. Кроме того, высокая однородность Pd1 центров обеспечивает уникальную селективность Pd-In/Al2O3 катализаторов в реакции газофазного гидрирования алкинов, что делает их перспективными для использования в процессе очистки пиролизного этилена от примеси ацетилена перед полимеризацией.
к.х.н. И.С. Машковский, ст. инж. П.В.
Марков,
к.х.н. Г.О. Брагина, к.х.н. Г.Н. Баева,
д.х.н., проф. А.Ю. Стахеев
Институт
органической химии им. Н.Д. Зелинского РАН, г. Москва
Биметаллический Pd-Cu катализатор для проведения реакции кросс-сочетания Соногаширы
Предложен биметаллический Pd-Cu/α-Al2O3 катализатор для проведения реакции кросс-сочетания Соногаширы. Иссле-дование параметров активность/селективность для серии катализаторов Pd-Cu/α-Al2O3 с соотношением Pd:Cu, варьируемым в пределах от 1:0.5 до 1:4, показало, что даже небольшого количества Cu (Pd:Cu = 1:0.5) достаточно, чтобы существенно повысить каталитическую активность, а максимальный выход продукта кросс-сочетания (85%) достигается на образце с соотношением Pd:Cu = 1:2. Исследование структуры катализаторов методами электронной микроскопии, рентгенофазового анализа и ИК-спектроскпии адсорбированного СО позволило установить, что активными центрами, на которых протекает реакция, являются наночастицы PdCu со структурой твердого раствора замещения. Сравнение каталитических свойств Pd-Cu/α-Al2O3 и механической смеси Pd/α-Al2O3+Cu/α-Al2O3 с одинаковым соотношением Pd:Cu убедительно показало, что высокая активность биметаллических Pd-Cu/α-Al2O3 катализаторов обусловлена образованием сплавных частиц Pd-Cu.
асп. А.В. Рассолов, к.х.н. Г.Н. Баева,
д.х.н., проф. А.Ю. Стахеев
Институт органической химии им.
Н.Д. Зелинского РАН, г. Москва
Формирование и трансформация моноклинной и орторомбической фаз в реакторных порошках сверхвысокомолекулярного полиэтилена
При использовании интенсивного синхронного излучения на линии “Белок” в НИЦ “Курчатовский институт” проведено рентгенодифракционное исследование реакторного порошка сверхвысокомолекулярного полиэтилена, полученного на постметаллоценовых катализаторах полимеризации и не подвергшихся никаким внешним механическим воздействиям. Обнаружено, что наряду с пиками от орторомбической фазы наблюдаются пики моноклинной фазы, стабильной только под напряжением. Это свидетельствует о развитии напряжений в процессе синтеза полимера, ответственных за формирование моноклинной фазы, которые не релаксируют при комнатной температуре. Зафиксировано постепенное исчезновение пиков при 340 К. Сравниваются результаты исследований фазового состава “virgin” частицы и таблетки, полученной из того же реакторного порошка при компактировании при комнатной температуре под давлением. Рентгенодифракционные исследования таблетки проводили и на дифрактометре D2 Phaser Bruker. Обнаружено, что параметры моноклинной фазы, образующейся при синтезе, отличаются от параметров моноклинной фазы, возникающей в таблетке в процессе компактирования. Обсуждаются механизмы образования разных моноклинных фаз в этих процессах.
чл.-корр. РАН С.С. Иванчев
Институт высокомолекулярных соединений РАН, г.
Санкт-Петербург
Создание магнитоотделяемых катализаторов конверсии инулина в маннитол
Синтезирован магнитоотделяемый Ru-содержащий катализатор (на основе магнитного кремнезема, Fe3O4-SiO2) гидролитического гидрирования инулина в маннит. Изучено влияние параметров реакции на селективность к маннитолу. При оптимальных условиях максимальная селективность к маннитолу достигла 44,3% при 100% конверсии исходного полисахарида, Катализатор показал высокую стабильность при гидротермальных условиях проведения процесса. Легкость отделения от реакционной смеси и возможность повторного использования без потери селективности и активности делают этот катализатор перспективным для практических применений в преобразовании биомассы.
к.х.н. О.В. Манаенков, асп. Е.А.
Раткевич,
к.х.н. О.В. Кислица, к.х.н. А.А. Степачева,
д.х.н., проф. В.Г. Матвеева, д.х.н., проф. М.Г. Сульман,
д.х.н., проф. Э.М. Сульман
Институт нано- и биотехнологий
Тверского государственного технического университета, г.
Тверь
Изучение окисления глюкозы в присутствии магнитоотделяемых биокатализаторов
Синтезированы магнитноотделяемые биокатализаторы для ферментативного окисления D-глюкозы до D-глюконовой кислота с высоким выходом продукта. Носитель биокатализаторов основан на кластерах наночастиц (NPC), состоящих из частиц магнетита, покрытых силикой и аминогруппами для облечения дальнейшей функционализации. Она включает модификацию глутаровым альдегидом с последующим ковалентным присоединением глюкозоксидазы (GOx) через свои аминогруппы. Биокатализаторы показали высокую относительную активность (94%). Полученный результат объясняется комбинацией двух главных факторов: расположение GОx на поверхности носителя, которая должна предотвратить денатурацию (аналогично поведению фермента в клетках), и подвижность фермента, которая должна быть сохранена при иммобилизации. Высокая стабильность синтезированных биокатализаторов на основе GOx в 10 последовательных реакциях, а также магнитное отделение, совмещенное с превосходной каталитической активностью, делают этот дизайн биокатализатора перспективным для других типов ферментативных катализаторов.
асп. Е.А. Голикова, асп. А.М. Сульман,
к.х.н. Н.В. Лакина, д.х.н., проф. Э.М. Сульман,
д.х.н., проф. В.Г. Матвеева
Институт нано- и биотехнологий
Тверского государственного технического университета, г.
Тверь
Наночастицы палладия, стабилизированные в матрице сверхсшитого полистирола – катализаторы селективного гидрирования тройной связи и кросс-сочетания Сузуки.
Синтезированы наночастицы палладия в полимерной матрице сверхсшитого полистирола марки MN270. Показано, что независимо от способа восстановления катализатора (in situ в ходе гидрирования тройной связи ацетиленовых спиртов или ex situ в токе водорода), а также от наличия в методике синтеза катализатора этапа обработки соединениями щелочных металлов, сверхсшитый полистирол обеспечивает формирование частиц палладия диаметром 3-4 нм, проявляющих высокую активность в реакциях селективного гидрирования тройной связи и кросс-сочетания Сузуки.
к.х.н. Л.Ж.
Никошвили, асп. Н.А. Немыгина,
магистр Т.Е. Худякова, н.с. И.Ю.
Тямина,
к.х.н. А.В. Быков,д.х.н., проф. Э.М. Сульман
Институт нано- и биотехнологий
Тверского государственного технического университета, г.
Тверь
Никелевый катализатор, синтезированный методом осаждения в полимерной матрице, в реакции деоксигенирования жирных кислот в сверхкритических условиях
Исследовано деоксигенирование стеариновой кислоты в сверхкритическом гексане с использованием никель-содержащего катализатора, нанесенного на сверхсшитый полистирол гидротермальным методом. Показано, что гидротермальный синтез приводит к образованию на внутренней поверхности сверхсшитого полистирола тонкого слоя равномерно распределенных наночастиц гидроксида никеля со средним размером 5 нм. Изучение деоксигенирования стеариновой кислоты в сверхкритическом гексане показало, что при проведении некаталитического процесса основными продуктами являлись С10-С12 углеводороды. При использовании никелевого катализатора селективность сдвигалась в сторону образования С16-С18 углеводородов.
к.х.н. А.А.
Степачева, асп. А.А. Маркова,
к.х.н. А.В. Быков, к.х.н. А.И.
Сидоров,
д.х.н., проф. М.Г. Сульман, д.х.н., проф. В.Г. Матвеева,
д.х.н., проф. Э.М. Сульман
Институт
нано- и биотехнологий Тверского государственного технического
университета, г. Тверь
Новый катализатор для полимер-электролитных топливных элементов
Разработан новый эффективный катализатор для полимер-электролитных топливных элементов (ПЭТЭ) – комплекс никеля (II) с 1,4-диаза-3,7-дифосфациклооктановым лигандом [Ni(PPy2Np-Tol2)2]2+2[BF4]- на углеродной подложке Vulcan XC-72, применение которого в качестве анода ПЭТЭ позволяет достичь плотности мощности 14.66 мВт см–2, что превосходит показатели аналогов, содержащих неблагородные металлы.
академик
РАН О.Г. Синяшин, д.х.н., проф. А.А. Карасик, д.х.н. М.К. Кадиров
Институт органической и физической химии им. А.Е. Арбузова ФИЦ КазНЦ РАН, г. Казань
Синтез бензимидазол-пиридиламидных пинцетных комплексов циркония (IV) и гафния (IV)
Синтезированы новые бензимидазол-пиридиламидные пинцетные комплексы циркония (IV) и гафния (IV), являющиеся селективными катализаторами конверсии углекислого газа СО2 до метана СН4. Показано, что каталитическая активность полученных комплексов в данном процессе значительно превышает каталитическую активность известных мировых аналогов.
д.х.н., проф. РАН Д.Г. Яхваров, асп.
З.Н. Гафуров,
д.х.н., проф. А.А. Карасик, академик РАН О.Г.
Синяшин
Институт органической и физической химии им. А.Е. Арбузова ФИЦ КазНЦ РАН, г. Казань
Интенсификация процессов окисления алкиларенов до гидропероксидов соединениями непереходных металлов I и II групп
Впервые при изучении каталитического распада гидропероксидов изопропилбензола, этилбензола исследованы этилгексаноаты металлов I и II групп, не дающие самоассоциатов в растворах, в отличие от нафтенатов. На основании исследования механизма и кинетики брутто- и радикального распада ГПК в присутствии соединений металлов подгруппы цинка показано, что каталитический эффект этилгексаноатов уменьшается в ряду Cd(ЭГ)2 > Zn(ЭГ)2 > Hg(ЭГ)2. На основании параметров разложения ГПК в присутствии металлсодержащих катализаторов установлено и квантово-химически подтверждено, что в промежуточных активированных комплексах ROOH…MeLn помимо водородных связей образуется связь металла с кислородом гидропероксида.
к.х.н. Н.М. Нуруллина, к.х.н., проф.
Н.Н. Батыршин,
д.х.н., проф. Х.Э. Харлампиди
Казанский
национальный исследовательский технологический университет,
г. Казань
Кумольная технология получения оксида пропилена
Разработаны рецептура и условия приготовления нанодисперсных молибденовых катализаторов. Найдены оптимальные условия проведения каталитического эпоксиди-рования пропилена гидропероксидом изопропилбензола с использованием нанодисперсного молибденового катализатора. Наработана партия каталитических систем длительного пробега на пилотной установке. Отработана стадия эпоксидирования пропилена гидропероксидом изопропилбензола. Проведено разделение эпоксидата на легкую и тяжелую части.
к.т.н. Н.П. Мирошкин, к.т.н. А.А. Гайфуллин,
д.т.н., проф. Э.А. Каралин,к.т.н. Г.Г. Елиманова,
к.т.н. С.Н. Тунцева, д.х.н., проф. Х.Э. Харлампиди
Казанский университет
Первые примеры синтеза диамантана гидроизомеризацией гексациклических димеров норборнадиена под действием железосодержащей ионной жидкости
Впервые установлено, что железосодержащая ионная жидкость состава [BMIM]+-[Fe2Cl7]– (BMIM-Cl – хлорид 1-бутил-3-метилимидазолия) является эффективным катализатором скелетной изомеризации гидрированных гексациклических димеров норборнадиена в диамантан с выходами 78-93%. Реакция проходит в мягких условиях (50°С, 8 ч), при мольном соотношении [C14H18] : [BMIM-Fe2Cl7] = 1 : 1÷3. Образование диамантана (C14H20) является неожиданным, так как в молекуле углеводородов (С14H18) содержится меньше водорода, чем в диамантане. Очевидно, что донором водорода служит ионная жидкость, которая в ходе реакции выполняет три функции, играя роль гидрирующего, изомеризующего агента и растворителя.
д.х.н. Р.И. Хуснутдинов, к.х.н. Р.И. Аминов,
д.х.н., проф. РАН В.А. Дьяконов, чл.-корр. РАН У.М. Джемилев
Институт нефтехимии и катализа УФИЦ РАН, г. Уфа
Цеолитные катализаторы FeHY-mmm, NiHY-mmm, HY-mmm, NaY-mmm в селективной функционализации диамантана
С использованием микро-, макро- и мезопористых цеолитных катализаторов FeHY-mmm, NiHY-mmm, HY-mmm, NaY-mmm разработаны эффективные методы прямого селективного амидирования, галогенирования и алкоксилирования диамантана – второго представителя гомологического ряда алмазоподобных углеводородов. Выходы амидов, хлор-, бром- и алкоксипроиз-водных диамантана в условиях 150° С, 5 ч достигают 85%. Цеолитные катализаторы FeHY-mmm, NiHY-mmm, HY-mmm, NaY-mmm не теряют активности при повторном использовании в течение 4-5 циклов.
д.х.н. Хуснутдинов Р.И., к.х.н. Щаднева Н.А.,
к.х.н. Байгузина А.Р., м.н.с. Маякова Ю.Ю.,
д.х.н., проф. РАН В.А. Дьяконов, чл.-корр. РАН У.М. Джемилев
Институт нефтехимии и катализа УФИЦ РАН, г. Уфа
Промышленно перспективный метод синтеза 2,3-диалкилхинолинов с использованием цеолитных катализаторов
Разработан перспективный для промышленного использования гетерогенно-каталитический способ синтеза важных 2,3-диалкилхинолинов реакцией анилина с алифатическими альдегидами С3-С5 в присутствии иерархических цеолитных катализаторов. Наиболее эффективны каталитические системы, созданные на основе гранулированного цеолита Y высокой степени кристалличности с микро-, мезо- макропористой структурой в H-форме (Ymmm). Способ позволяет получать 2,3-диалкилхинолины с селективностью до 76% при конверсии анилина до 99%.
д.х.н. Н.Г. Григорьева, к.х.н. С.А. Костылева,
аспирант А.Р. Гатаулин,
д.х.н. Б.И. Кутепов,
д.х.н., проф. РАН В.А. Дьяконов, чл.-корр. РАН У.М. Джемилев Институт нефтехимии и катализа УФИЦ РАН,
г. Уфа
Первые примеры синтеза циклических спиросочлененных азатрипероксидов с участием лантанидных катализаторов
Разработан эффективный метод синтеза практически важных спиросочлененных азатрипероксидов каталитической рецикли-зацией гептаоксаспирокарбоциклов с помощью первичных ариламинов, катализируемой Sm(NO3)3·6H2O в условиях (20°С, 6 ч, 5 мол.% катализатора). Выходы соответствующих азатрипероксидов составляют 84‒92%. Полученные азатрипероксиды перспективны в качестве противомалярийных и противо-опухолевых препаратов.
к.х.н. Махмудиярова Н.Н., аспирант
Ишмухаметова И.Р.,
д.х.н. Ибрагимов А.Г., д.х.н., проф. РАН В.А. Дьяконов,
чл.-корр. РАН У.М. Джемилев
Институт нефтехимии и катализа УФИЦ РАН, г. Уфа
Механизм действия биметаллических каталитических систем на основе металлоценовых комплексов и алюминийорганических соединений в реакциях хемо- и стереоконтролируемого построения новых С-Н, С-С и металл-С связей
Обнаружены новые биметаллические Zr, Al гидридные комплексы, обладающие высокой аффинностью по отношению к метилалюмоксану – активатору реакции полимеризации алкенов. Квантово-химическое исследование процесса показало предпочтительное участие моделей МАО с фрагментами цепочек (MeAlO)n или/и гексагонов со звеном (MeAlO)3. Найденные новые гидридные кластеры могут выступать в качестве зондов для изучения структуры активаторов реакций типа Циглера-Натта.
д.х.н. Халилов Л.М., д.х.н. Парфенова Л.В.,
к.х.н. Ковязин П.В., к.х.н. Тюмкина Т.В.,
аспирант Исламов Д.Н., д.х.н., проф. РАН В.А. Дьяконов,
чл.-корр. РАН У.М. Джемилев
Институт нефтехимии и катализа УФИЦ РАН, г. Уфа
Изучение реакций гидрирования иминов и енаминов при катализе наночастицами никеля, нанесенными на активированный уголь, цеолит или катионообменную смолу
Изучена реакция гидрирования иминов и енаминов в соответствующие вторичные и третичные амины при катализе наночастицами никеля, нанесенными на активированный уголь или цеолит в реакторе вытеснения. Установлено, что процесс протекает при атмосферном давлении водорода и температуре 140-200°С с образованием вторичных и третичных аминов, соответственно, с выходами до 98%.
Исследована кинетика процесса гидрирования дициклопенталиена при температуре 80-120°С в реакторе вытеснения при катализе наночастицами никеля, нанесенными на катионообменную смолу.
Разработан непрерывный способ получения вторичных алифатических аминов с выходом до 97% селективным гидрированием нитридов при катализе наночастицами никеля, нанесенными на цеолит при температуре 200-220°С.
д.х.н., проф. Ю.В. Попов
Волгоградский государственный технический университет, г. Волгоград
Катализаторы асимметрического синтеза
В результате проведенных исследований впервые разработан новый малостадийный и атом-экономный способ для получения P,N-ферроценилазиновых лигандов. Применение (metal-free) SNH кросс-сочетаний позволяет увеличить выходы и энантиомерную чистоту планарно хиральных ферроценов (L1, L2), а также избежать использования в синтезе палладиевых катализаторов и галогенированных азинов. Сопоставление с литературными данными по реакциям асимметрического аллильного алкилирования, восстановления карбонильных соединений, [3+2]-циклоприсоединения, присоединения диэтилцинка к альдегидам показывает, что предложенные лиганды обладают вполне сравнимыми характеристиками, а в некоторых случаях превосходят таковые у подобных коммерчески доступных соединений.
академик РАН О.Н. Чупахин
Институт органического синтеза им. И.Я. Постовского УрО РАН,
г. Екатеринбург
Исследование механизмов каталитических реакций на основе нового метода анализа дифференциальной селективности
Продолжены исследования механизмов каталитических реакций, базирующиеся на новом методе анализа дифференциальной селективности, использующем фазовые траектории реакций. В реакции прямого арилирования гетероароматических аренов показано, что ключевая стадия С-Н активации ароматического субстрата имеет ярко выраженную обратимость, что исключает гипотезу ее механизма, включающую протекание реакции карбопалладирования. Совокупность полученных экспериментальных данных (дифференциальная селективность конкурентных реакций арилгалогенидов, конкурентных реакций индолов, дифференциальная селективность по региоизомерным С2 и С3 продуктам) указывает на протекание С-Н активации по механизму электрофильного замещения.
Установлена анионная природа активных специй Pd(0), участвующих в активации арилирующего субстрата, и соединений Pd(+2), участвующих в активации ароматического субстрата.
Полученные в ходе изучения реакции Мицороки-Хека с использованием ангидридов ароматических кислот в качестве арилирующих реагентов данные о закономерностях дифференциальной селективности позволили получить первые прямые доказательства реализации исключительно гомогенного механизма катализа без участия формирующихся в системе наноразмерных частиц палладия, выполняющих роль «резервуара» растворимых каталитически активных комплексов.
Все результаты были получены в реальных каталитических условиях без использования каких-либо модельных экспериментов.
д.х.н., проф. А.Ф. Шмидт, к.х.н. А.А. Курохтина,
асп. А.А. Ларина, асп. Е.В. Ярош, асп. Н.А. Лагода
Иркутский государственный университет, г. Иркутск
Исследование свойств палладиевых катализаторов в гидрировании 2-этил-9,10-антрахинона. Закономерности влияния состава каталитических систем на основе Со(асас)2∙nН2Ои AlEt3 или AlEt2(OEt) на их активность в реакции гидрировании бензола
На основе совокупности результатов кинетического и физико-химического исследования установлено влияние состава каталитической системы, размера частиц и фосфорного модификатора на свойства палладиевых катализаторов в гидрировании 2-этил-9,10-антрахинона. Показано, что в зависимости от природы восстановителя (H2, AlEt3) образующиеся кристаллиты палладия наряду с гидрированием 2-этил-9,10-антрахинона в значительной мере катализируют различные побочные процессы: преимущественно реакцию гидрогенолиза (восстановитель H2) или гидрирование ароматических колец 2-этил-9,10-антрагидрохинона (восстановитель AlEt3). Побочный процесс гидрогенолиза 2-этил-9,10-антрагидрохинона наиболее эффективно протекает под действием звездоподобных кристаллитов палладия размером около 127 нм. Гидрирование ароматических колец 2-этил-9,10-антрагидрохинона эффективно ускоряют электронодефицитные малые кластеры палладия диаметром 1.5-2.0 нм. Обнаружен промотирующий эффект элементного фосфора на селективность палладиевых катализаторов в синтезе пероксида водорода антрахиноновым методом. Модифицирование белым фосфором повышает селективность палладиевых катализаторов по пероксиду водорода с 69 до 95%. Рассмотрены основные факторы, позволяющие регулировать селективность палладиевых катализаторов в получении пероксида водорода антрахиноновым методом.
Установлены основные закономерности влияния состава каталитических систем на основе Со(асас)2∙nН2О (n = 0, 0.5 и 2) и AlEt3 или AlEt2(OEt) на их количественные каталитические характеристики в гидрировании бензола. Показано, что наиболь-шую активность в гидрировании бензола (TOFС6Н6 ≈ 5.5 мин–1 при Т = 120 °С и РН2 = 15 бар) проявляет система Со(acac)2 – AlEt3 при отношении Al/Ni = 4. Для системы Со(acac)2 – AlEt2(OEt) частота оборотов не превышает 3.0 мин-1 при Т = 120°С и РН2 = 15 бар в широком интервале отношений Al/Со = 4 ÷ 8. Получены значения параметров уравнения Аррениуса гидрирования бензола для систем Со(асас)2 – Red (Red =AlEt3; AlEt2(OEt)). Среднее значение эффективной энергии активации соответствует 11.1 ккал/моль. Данные кинетических исследований, ЭПР спектроскопии, электронной микроскопии высокого разрешения и рентгеновского микроанализа показывают, что активность в катализе гидрирования аренов в системах Со(acac)2 – AlEt3 и Со(acac)2 – AlEt2(OEt) проявляют наночастицы, содержащие кобальт в нулевой степени окисления.
д.х.н., проф. Ф.К. Шмидт
Иркутский государственный университет, г. Иркутск
Механизм катализа супероснованиями фундаментальных реакций ацетилена
В Иркутском институте химии им. А.Е. Фаворского СО РАН (совместно с Иркутским государственным университетом) квантово-химическими методами (B2PLYP/6-311+G**//B3LYP/6-31+G*) изучены механизмы катализа фундаментальных атом- и ресурсосберегающих реакций ацетилена, приводящих к практически важным продуктам. Анализировались суперосновные каталитические системы типа КОН/ДМСО, которые на много порядков ускоряют реакции нуклеофильного присоединения к тройной связи, а также присоединение ацетиленовых карбанионов к карбонильной группе. Показано, что пентасольватная модель, в которой супероснование представлено комплексами KOH·5ДMСО (KOBut·5DMSO), хорошо описывает элементарные стадии указанных реакций и последующие превращения, в том числе процессы самоорганизации сложных молекул с участием ацетилена и кетонов. Установлен механизм катализа однореакторной каскадной сборки 6,8-диоксабицикло[3,2,1]октанов (базовой структуры феромонов группы фронталина), циклопентенолов и фуранов (прекурсоров лекарств) из кетонов и ацетилена. Проведено сравнение энергетических профилей реакций построения указанных гетероциклов. Диастереоселективность сборки циклопентенолов определяется низким активационным барьером внутримолекулярного винилирования в ключевом интермедиате.
академик РАН Б.А. Трофимов, д.х.н., проф. Н.М. Витковская,
д.х.н., проф. В.Б. Кобычев, д.х.н. Е.Ю.
Шмидт, к.х.н. В.Б. Орел
Иркутский институт химии им. А.Е. Фаворского СО РАН, г. Иркутск
Исследование гиперполяризации спинов ядер анса-аминоборанов на основе о-фенилена
Способность фрустрированных льюисовых пар активировать молекулярный водород представляет значительный интерес для катализа без использования металлов. Активация Н2 является также ключевым элементом индуцированной параводородом поляризации ядер (ИППЯ), одного из методов гиперполяризации ядерных спинов, используемых для усиления сигнала в ЯМР и МРТ на несколько порядков величины. В работе показано, что анса-аминобораны (ААБ) на основе о-фенилена могут создавать гиперполяризацию спинов ядер через обратимое взаимодействие ААБ с параводородом при комнатной температуре. Поляризация спинов гетероядер полезна для многих приложений ЯМР и МРТ, так как в отличие от ядер 1Н гетероядра имеют широкий диапазон химических сдвигов и большие времена релаксации и могут выступать в качестве меток, не содержащихся в исследуемом объекте или организме. В работе впервые показано спонтанное формирование гиперполяризации ядер 15N фрагмента N-H аддукта ААБ с параводородом для семейства соединений ААБ. Этот процесс эффективен в высоких магнитных полях спектрометров ЯМР и МР томографов (7 Тл) и обеспечивает 350-кратное усиление сигнала ЯМР ядер 15N. Различные эффекты гиперполяризации наблюдаются для различных структур AAБ и в широком температурном диапазоне. Спонтанная гиперполяризация несколько меньшей величины наблюдается также для ядер 11B.
д.х.н., проф. И.В. Коптюг, К. Сорочкина,
В.В. Живонитко, К. Черниченко
Международный томографический центр СО РАН, г. Новосибирск
Исследование фотокаталитических систем с металл-органическим каркасом методом ЭПР
Методом электронного парамагнитного резонанса (ЭПР) изучена структура активного центра фотокаталитической системы на основе металл-органического каркаса Co@NH2-MIL-125(Ti), эффективной в генерации водороды из воды под воздействием видимого света. Показано, что активным центром данного композита является обменно-связанный димер Co(II)-Ti(III), образующийся в реакционной смеси под действием фотооблучения на длительных временах.
Методом ЭПР показано, что направленное введение пар ионов Fe(III) в металл-органический каркас (МОК) MIL-53(Al) приводит к образованию кластеров Fe(III)-Fe(III), структура и магнитные свойства которых близки к таковым в природном ферменте метанмонооксигеназе, являющейся эффективным катализатором конверсии метана в метанол. Данное исследование является яркой демонстрацией возможности мимикрирования каталитических функций биологических ферментов с помощью МОК.
д.ф.-м.н., проф. РАН М.В. Федин, к.ф.-м.н. С.Л. Вебер
Международный томографический центр СО РАН,
г. Новосибирск
Наноразмерные частицы металлов, выполняющие функции физико-химических нанороботов
В рамках методологии познания наноразмерного мира изучены специфические физические и химические свойства (функции) дисперсных частиц металлов подгруппы железа (Fe, Co, Ni). Частицы с набором таких функций названы полифункциональными наноразмерными системами (ПНС). Показано, что они способны управлять многостадийными технологиями синтеза различных нано-продуктов (НП). Феномен ПНС состоит в том, что в процессе синтеза по многостадийной технологии она представляет из себя лабильную реакционноспособную систему, перестраивающуюся в унисон с последовательностью стадий нано-технологии. ПНС в таком состоянии названы физико-химическими нанороботами (ФХНР). Предложена, научно обоснована и реализована в виде действующей модели система, способная выполнять работу ФХНР. Она представлена и изучена в виде нанодисперсной никелевой частицы. Продемонстрирован набор нанотехнологий ряда материалов.
чл.-корр. РАН Р.А. Буянов, академик РАН В.Н. Пармон,
к.х.н. И.В. Мишаков
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Разработка блочных катализаторов получения синтез-газа на пористых носителях (никелевая лента, фехралевая сетка, пеноячеистые материалы)
Разработана методика приготовления и приготовлена серия блочных катализаторов парциального окисления легких алканов на основе никельсодержащих металлических лент, фехралевых сеток, пеноячеистого материала с нанесенным активным компонентом NiO-MgO для получения и использования синтез-газа в газотурбинных установках. Экспериментально доказано, что введение инициирующих добавок синтез-газа в камеру сгорания горелочного модуля и, следовательно, в камеру сгорания природного газа в газотурбинной установке является эффективным способом снижения эмиссии оксидов азота и углерода и открывает перспективы создания низкоэмиссионных газотурбинных установок.
академик РАН В.Н. Пармон, д.т.н. В.А. Кириллов
Институт катализа им. Г.К.
Борескова СО РАН, г. Новосибирск
Твердофазные топливные гидридсодержащие композиции с высокой энергетической плотностью для получения водорода в газогенераторах
Изучены твердофазные водородгенерирующие композиции на основе боргидрида натрия и соединений кобальта. Показано, что в процессе прессования не происходит существенных фазовых перестроек. Определены основные факторы, влияющие на скорость газогенерации: давление прессования таблетки, природа кобальтового соединения, его растворимость в воде, предварительное восстановление катализатора, способность к дегидратации солей кобальта под давлением. Впервые продемонстрировано, что при добавлении твердофазной гидридсодержащей композиций с низкотемпературным боридом кобальта в воду выделяется водород, содержащий только пары воды, поэтому без дополнительного увлажнения и очистки он может подаваться в анодное пространство топливного элемента. Установлено, что замена воды на концентрированные растворы серной и соляной кислот приводит к присутствию диборана в водородсодержащем газе, а также примесей оксидов серы и хлороводорода, соответственно. Водородгенерирующие композиции на основе боргидрида натрия и кобальтового катализатора прошли успешные испытания на Сарапульском радиозаводе, которые показали, что содержание водорода составило 7,6 масс.% с учетом добавленной воды. Результаты исследований легли в основу Заявки № 2018112837 (09.04.2018) на патент РФ. Ведутся переговоры по возможности коммерциализации разработки с представителями Росатом-ТВЭЛ, АО «Радий» и ООО «Эй Ти Энерджи».
к.х.н. О.В. Нецкина, , к.х.н. О.В. Комова,
к.х.н. А.М. Озерова, д.х.н. В.И.
Симагина
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Каталитическая система для энантиоселективного бензильного гидроксилирования арилалканов пероксидом водорода на основе хиральных аминопиридиновых комплексов марганца
Разработана каталитическая система для энантиоселективного бензильного гидроксилирования арилалканов пероксидом водорода на основе хиральных аминопиридиновых комплексов марганца, позволяющая получать 1-арилалканолы с выходом до 45% и оптической чистотой до 89% ее в среде фторированных спиртов. Столь высокие выходы целевого хирального продукта были ранее недостижимы вследствие быстрого дальнейшего окисления первоначально образующегося спирта в кетон. Установлена причина значительного повышения выхода целевого продукта в среде фторированных спиртов – образование водородных связей между растворителем (сильным донором водородных связей) и первоначально образовавшимся 1-арилалканолом, приводящее к подавлению дальнейшего окисления 1-арилалканола в кетон и в итоге к преимущественному окислению арилалкана (имеющего более прочную С–Н связь) в хиральный целевой продукт.
д.х.н., проф. РАН К.П. Брыляков, д.х.н., проф. Е.П. Талзи,
к.х.н. Р.В. Оттенбахер, Т.В. Рыбалова
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Разработка синтетических подходов к получению и использованию в каталитических процессах полифторарил-дифторборанов (ArFBF2), обладающих Льюисовой кислотностью
Разработаны синтетические подходы к получению и использованию в каталитических процессах полифторарилдифторборанов (ArFBF2), обладающих Льюисовой кислотностью. Показано, что полученные соединения растворимы и устойчивы в ряде органических растворителей (углеводороды, хлоруглеводороды). Установлено, что полученные соединения являются мягкими кислотами Льюиса и могут находить применение в селективных кислотно-катализируемых процессах с участием высокореакционноспособных и/или полифункциональных субстратов. Изучена зависимость каталитических свойств от природы заместителей при атоме бора, показано, что варьирование структуры заместителя позволяет управлять кислотными свойствами борорганического соединения.
д.х.н., проф. РАН Н.Ю. Адонин, к.х.н. С.А. Приходько,
М.М. Шмаков
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Структурные исследования полиядерных каркасных металл-содержащих силоксанов и гермоксанов как предшественников эффективных катализаторов гомогенного окисления
С использованием оборудования Курчатовского источника синхротронного излучения (НИЦ “Курчатовский институт”, Москва) проведены рентгеноструктурные исследования и получены кристаллические структуры для обширной серии новых каркасных полиядерных металлоорганосилоксанов и гермоксанов. Проанализированы факторы, влияющие на ядерность и геометрию кластерного остова в зависимости от условий синтеза. Детально охарактеризованы 5-, 6-, 7-, 8- и 11-ядерные кластеры. Впервые получен рекордный по ядерности триметаллический каркас состава Cu42Ge24Na4 (без учета легких атомов). Следует отметить, что соединения данного класса с трудом образуют крупные качественные монокристаллы из-за высокой степени структурной лабильности, склонности к разупорядочению и включению в кристаллическую структуру сольватных молекул. Поэтому использование рентгеновского синхротронного излучения в таких исследованиях является особенно важным и ценным. Для всех новых кластерных соединений данного класса подтверждена высокая каталитическая активность в реакциях окисления алканов пероксидом водорода (в частности, циклогексана в циклогексанол и циклогексанон). Для одного из 6-ядерных фенилмедьсилсесквиоксанов именно по результатам рентгеноструктурного исследования выявлена крайне необычная реакция спонтанного окисления (гидроксилирования) метильной группы в геминальный диол в координированном лиганде – неокупроине.
к.х.н. А.Н. Биляченко, д.х.н. М.М. Левицкий, д.х.н. Е.С. Шубина
Институт элементоорганических соединений им. А.Н. Несмеянова РАН, г. Москва
д.х.н. В.Н. Хрусталев
Российский университет дружбы народов, г. Москва
д.х.н. Г.Б. Шульпин
Институт химической физики им. Н.Н. Семенова РАН, г. Москва
П.В. Дороватовский, д.ф.-м.н. Я.В. Зубавичус
НИЦ «Курчатовский институт», г. Москва
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Синтез и реакционная способность бифункциональных катализаторов на основе пероксовольфраматных комплексов в процессах “зеленой химии”
Синтезирован ряд бифункциональных каталитических систем на основе тетраядерных пероксополиоксокомплексов вольфрама, включающих четвертичные аммониевые катионы, содержащие алкильные заместители с различной длиной цепи, а также содержащие одновременно алкильные и арильные заместители. Наиболее перспективные катализаторы протестированы в реакциях окисления пероксидом водорода: октена-1, изоамилового спирта и N-фосфонометилиминодиуксусной кислоты в условиях межфазного катализа. Изучена формальная кинетика указанных реакций, определены кажущиеся константы реакций и порядки по субстрату, окислителю и катализатору. Показано, что при проведении реакций окисления в условиях межфазного катализа в присутствии бифункциональных катализаторов на основе тетраядерных пероксокомплексов вольфрама, содержащих четвертичные аммониевые катионы, можно достигать высоких выходов продуктов: гептановой кислоты – 97-99%, изо-валериановой кислоты – 95-96%, N-оксида-N-фосфонометилиминодиуксусной кислоты – 92-93%.
д.т.н. З.П. Пай, П.В. Бердникова,
Д.Ю. Ющенко, Н.В. Селиванова,
к.х.н. А.М. Бескопыльный
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Полупроводниковые катализаторы и материалы для процессов фотокаталитического и плазмофотокаталитического окисления летучих соединений
Синтезированы высокоактивные композиционные фотокатализаторы на основе нанокристаллического диоксида титана, нанесенного на поверхность цеолита, обладающие высокой адсорбционной способно стью. Использование цеолита в качестве носителя препятствует спеканию частиц TiO2 при высокотемпературной обработке и обеспечивает высокий уровень начальной адсорбции загрязнителей и их быстрое удаление из газовой фазы, а также препятствует интенсивному накоплению промежуточных продуктов неполного окисления в газовой фазе, значительно снижая общий уровень опасности по сравнению с TiO2
асп. Н.С. Ковалевский, к.х.н. М.Н. Люлюкин,
к.х.н. Д.С. Селищев, д.х.н., проф. РАН Д.В. Козлов
Институт катализа им. Г.К. Борескова
СО РАН, г. Новосибирск
Изучение влияния нанесения соединений никеля и цинка на фотоэлектрохимические характеристики фотоэлектродов
Было показано, что нанесение на поверхность фотоэлектрода Cd0.3Zn0.7S/FTO соединений никеля и цинка позволяет увеличить плотность тока короткого замыкания в 12 раз, КПД преобразования энергии света в электрическую энергию – в 10 раз, время жизни электронов – в 37 раз. Выявлена корреляция между каталитической активностью фотокатализаторов и эффективностью преобразования энергии видимого излучения в электрическую энергию.
асп. Д.В. Марковская, к.х.н. Е.Н. Грибов,
к.х.н. Е.А. Козлова, д.х.н., проф. РАН Д.В. Козлов
Институт катализа им. Г.К. Борескова
СО РАН, г. Новосибирск
Исследование механизма реакции окисления пропилена в акролеин
Разработан метод идентификации поверхностных интермедиатов, заключающийся в проведении реакции при пониженных температурах, в условиях, когда продукты реакции не десорбируются в газовую фазу, а остаются на поверхности катализатора. С использованием этого метода была впервые экспериментально определена последовательность стадий реакции окисления пропилена в акролеин на Bi-Mo катализаторе и установлено, что присоединение кислорода к пропилену предшествует отрыву второго атома Н. Измерены энергия активации, кинетический изотопный эффект реакции, исследовано влияние давления кислорода. С использованием метода сверхмалых конверсий (≤ 0.01%) впервые установлено, что ключевым промежуточным продуктом реакции окисления пропилена является аллиловый спирт. Предложен механизм, объясняющий все особенности протекания реакции окисления пропилена в акролеин. Новые представления о механизме могут быть востребованы для совершенствования катализаторов окисления пропилена в акролеин.
д.х.н., проф. Г.И. Панов, к.х.н. Е.В. Староконь,
к.х.н. М.В. Парфенов, к.х.н. В.И. Соболев,
к.х.н. Л.В. Пирютко, студент Б. Вэй,
д.х.н. А.С. Харитонов
Институт катализа им. Г.К. Борескова
СО РАН, г. Новосибирск
Коллоидные катализаторы окисления воды
Коллоидные катализаторы окисления воды до диоксида, стабильные при хранении и в условиях реакции, разработаны на основе гидроксидов Co(III), Mn(III), Fe(III). Стабилизация коллоидов декстрированным крахмалом позволяет остановить процесс старения гидроксида на стадии образования первичных частиц (около 2-3 нм по данным ПЭМ). Исследования катализаторов методом динамического светорассеяния и моделирование методом молекулярной механики показали, что они имеют структуру типа ядро-оболочка, где гидроксидное ядро стабилизируется молекулами крахмала (около 5-7 нм). Коллоидные катализаторы высокоэффективны в реакции окисления воды одноэлектронным окислителем Ru(bpy)33+ при рН 7-10. Изучено влияние рН, концентрации катализатора и природы буфера на выход кислорода. Максимальные выходы составили 72, 53 и 78%, а число оборотов 7.8; 54 и 360 для Fe-, Mn- и Co-содержащих катализаторов, соответственно. Синтезированные катализаторы представляют интерес для исследований кинетики и механизма реакции окисления воды методом остановленного потока, а также в качестве предшественников для закрепления наноразмерных частиц гидроксидов на различных носителях с целью разработки биомиметических систем искусственного фотосинтеза.
д.х.н., проф. РАН О.П. Таран
Институт
химии и химической технологии ФИЦ КНЦ СО РАН, г. Красноярск
асп. А.С. Чикунов, к.х.н. В.Л. Кузнецов
Институт катализа им. Г.К. Борескова
СО РАН, г. Новосибирск
Оптимизация процесса получения L-арабинозы и D-галактозы кислотно-каталитическим гидролизом арабиногалактана
Проведена экспериментальная и математическая оптимизация процесса получения востребованных в медицине и фармакологии моносахаридов L-арабиноза и D-галактоза кислотно-каталитическим гидролизом арабиногалактана, выделенного из древесины лиственницы. Впервые сопоставлена активность в процессе гидролиза растворенных (0,1 М 2SO4 и 0,1 M HCl) и твердых кислотных (Amberlyst-15, кислотно-модифицированные Сибунит и SBA-15) катализаторов в интервале температур 90–150°С. Установлено, что константы скорости реакции гидролиза увеличиваются в ряду катализаторов: Сибунит (150°С) < SBA-15 (150°С) < Amberlyst-15 (150°С) < 0,1 М H2SO4 (130°С) < 0,1 M HCl (130°С). При этом значения энергии активации реакции образования арабинозы (65-91 кДж/моль) ниже, чем галактозы (112-140 кДж/моль). Установлены оптимальные режимы процесса гидролиза арабиногалактана в присутствии твердого катализатора SBA-15, при которых достигается его 100% конверсия: температура 150°С и продолжительность 4,5 ч. В присутствии сернокислотного катализатора полная конверсия арабиногалактана достигается при 124°С в течение 4,8 ч. Результаты исследования свидетельствуют о перспективности применения твердых кислотных катализаторов в процессе гидролиза арабиногалактана до L-арабинозы и D-галактозы.
к.х.н. О.В. Яценкова, м.н.с. А.М.
Скрипников,
д.х.н., проф. Б.Н. Кузнецов
Институт химии и химической технологии
СО РАН, г. Красноярск
Разработка метода получения формоустойчивых аэрогелей из микрофибриллярной целлюлозы для приготовления фотокатализаторов
Разработан упрощенный метод изготовления формоустойчивых аэрогелей из микрофибриллярной целлюлозы, получаемой из волокон сочетанием интенсивной механической обработки с замораживанием-размораживанием.Аэрогели служат в качестве матрицы для диоксида титана, наносимого методом направленного золь-гель синтеза, при котором процесс проводится в неводном растворе с добавлением ограниченного количества воды, сорбируемого гигроскопичной целлюлозой. Прекурсор после введения вступает в моментальные реакции гидролиза и конденсации в местах ее локализации, что приводит к преимущественному осаждению диоксида титана на микрофибриллах. Показано, что целлюлоза приводит к значительному понижению температуры перехода оксида металла из аморфного состояния в кристаллическое. В частности, наноразмерный анатаз удается получить при температуре, не превышающей 100°С. Полученные фотокатализаторы показали высокую фотокаталитическую активность в модельных экспериментах с метиленовым голубым.
чл.-корр. РАН Ю.А. Щипунов, к.х.н. И. В. Постнова,
к.х.н. В. Е. Силантьев, инженер-технолог О. Н.
Хлебников
Институт химии ДВО РАН, г. Владивосток
Разработка и усовершенствование промышленных катализаторов и технологий
Разработка высокоэффективного нанесенного титанмагниевого катализатора ИКТ-8-12С и успешные опытно-промышленные испытания этого катализатора в производстве полиэтилена
На основании научных результатов, полученных в Институте катализа при исследовании процессов формирования нанесен-ных титанмагниевых катализаторов полимеризации этилена с контролируемой структурой и морфологией, разработана новая модификация высокоэффективного нанесенного титанмагниевого катализатора ИКТ-8-12С для производства полиэтилена (ПЭ) различных марок суспензионным методом. Отработана технология приготовления этого катализатора. Наработана укрупненная опытная партия катализатора ИКТ-8-12С, которая успешно испытана на промышленной линии в производстве ПЭ в ПАО «Газпромнефть Салават»; при этом наработано около 5 тысяч тонн ПЭ двух марок, в том числе наиболее востребованной трубной марки бимодального полиэтилена (ПЭ-100). По результатам испытаний показана возможность замены импортного катализатора марки Z-501, используемого на производстве ПЭ в ПАО «Газпромнефть Салават», на отечественный катализатор ИКТ-8-12С.
д.х.н. Т.Б. Микенас, к.х.н. В.Е. Никитин,
к.х.н. М.А. Мацько, д.х.н. В.А. Захаров
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Катализатор изомеризации легких алканов
Предложен способ получения цеолитсодержащего катализатора путем введения нанопорошков металлов с сохранением их свойств, позволяющий целенаправленно изменять структурные и кислотные свойства цеолита, повышать изомеризующую активность катализатора и выход целевого продукта. Синтезирован цеолит структурного типа MFI с силикатным модулем 40, проведено его модифицирование наноразмерным порошком никеля ([Ni] =0,5 масс.%) с последующей механической обработкой катализатора. Показано, что механообработка приводит к уменьшению размеров частиц цеолита и нанопорошка никеля с частичной его миграцией с поверхности в поры цеолита, а также к снижению удельной поверхности, пористости и кислотности катализатора. Установлено отсутствие перехода частиц никеля в окисленное состояние при получении Ni-содержащего цеолитного катализатора и его механообработанных образцов. После проведения механической обработки катализатора повышается в 1,5-2 раза селективность образования изоалканов – высокооктановых компонентов бензинов, и снижается в 1,3-1,5 раза образование побочных газообразных продуктов в модельной реакции конверсии н-гексана. Предварительная механическая обработка цеолитных катализаторов, как способ их модификации, отличается простотой аппаратурного оформления и отсутствием вредных сточных вод.
д.х.н.,
проф. А.В. Восмериков, к.х.н. Л.М. Величкина
Институт химии нефти СО РАН, г. Томск
Новый композитный катализатор для получения дизельного топлива. Каталитический синтез сверхдлинных углеродных нанотрубок
Разработан и внедрен в производство новый композитный катализатор для прямого получения дизельного топлива из синтез-газа под маркой D1. Разработка внедрена на катализаторной фабрике ООО «ИНФРА» в г. Троицке, г. Москва. Подана заявка на изобретение.
Впервые получены сверхдлинные двухстенные углеродные нанотрубки в непрерывном каталитическом процессе в реакторе с плавающим катализатором. Нанотрубки синтезированы как в виде бобин углеродного коттона, так и в виде нити, намотанной на барабан.
д.х.н. В.З. Мордкович, к.х.н. Л.В. Синева,
к.т.н. А.Р. Караева, к.х.н. Н.В. Казеннов,
к.х.н. С.А. Урванов, к.т.н. К.О. Грязнов,
асп. Е.Ю. Асалиева,
асп. Е.А. Жукова
Технологический институт сверхтвердых и новых углеродных материалов, г. Москва, г. Троицк
MXene serves as support material for single-atom catalysts
Simple procedure yields catalyst that outperforms reference noble-metal catalysts
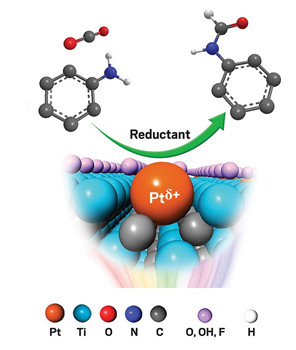
A simple preparation procedure converts ultrathin sheets of titanium carbide to a material that supports isolated platinum atoms, yielding a highly active catalyst that uses CO2, a greenhouse gas, to make valuable organic compounds (J. Am. Chem. Soc. 2019, DOI: 10.1021/jacs.8b13579). A common procedure for preparing MXenes, a family of 2-D metal carbides and nitrides, from mixed-metal carbide starting materials yields a related carbide dotted with individual titanium vacancies. Chen Chen of Tsinghua University and coworkers proposed that treating the defective material with a platinum salt would be an easy way to pin isolated platinum atoms across the MXene surface. So the researchers prepared the material, confirmed its structure, and tested its ability to catalyze reactions. They found that it was especially effective at mediating formylation of many types of amines with CO2 under mild conditions. For example, exposing aniline to CO2 at atmospheric pressure in the presence of the catalyst and a silane reductant produced N-phenyl formamide (shown) with a yield and selectivity of nearly 100%, far higher than that obtained using reference platinum catalysts. Analyses show that a small positive charge on the platinum atom (Ptδ+) contributes to catalytic performance by facilitating adsorption of reagent molecules.
Perovskites catalyze aldehyde alkylations
Organolead halides of solar-cell fame function as active photocatalysts in organic synthesis

Mention perovskites to C&EN readers, and many of them will think of solar cells—and for good reason: organolead halide compounds with the perovskite crystal structure and ABX3 stoichiometry have been the superstars of low-cost photovoltaics for a few years running. A new study shows the perovskites have other tricks up their sleeves. A team led by San Diego State University chemists Xiaolin Zhu and Yong Yan reports that methylammonium lead tribromide and the cesium analog, two of the most studied solar-cell perovskites, double as highly active photocatalysts for organic synthesis (J. Am. Chem. Soc. 2019, DOI:10.1021/jacs.8b08720). The researchers used standard methods to prepare the low-cost nanocrystal catalysts and explored their reactivity under blue-light illumination in tests with 2-bromoacetophenone and octanal. The reactions generated a mixture of products, including the aldehyde α-alkylation product (shown), other C-coupling products, and dehalogenated acetophenone. By tuning reaction conditions, the team was able to boost the selectivity of the industrially important α-alkylation reaction to 96%. The perovskites are 1,000 times as active as some iridium- and ruthenium-based catalysts and only a fraction of the cost, Yan points out.
Strained reagent opens up new cross-coupling reactivity
The twist on the Suzuki-Miyaura reaction offers a simple route to trisubstituted cyclobutanes
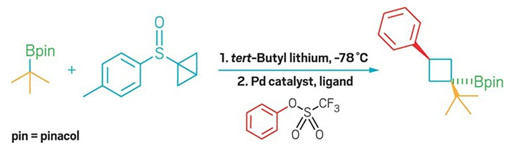
The Suzuki-Miyaura transformation is a classic way to form carbon-carbon bonds. In the reaction, a palladium catalyst typically couples two classes of molecules, aryl halides and aryl boronate complexes. Now researchers have taken a new approach to the traditional cross-coupling, with a method in which palladium reacts with the boronate compound not at the boron atom but at the neighboring carbon-carbon σ bond (Nat. Chem. 2018, DOI: 10.1038/s41557-018-0181-x). The result is a route to trisubstituted cyclobutanes valued by drug designers.
Led by the University of Bristol’s Varinder K. Aggarwal, the team accesses this novel mode of reactivity by using a strange-looking, bicyclobutyl sulfoxide reagent that exists as a bench-stable solid. Because of the strain placed on it, the cyclobutane’s middle carbon-carbon bond is weakened so it doesn’t act like a normal sigma bond, Aggarwal explains. At first, the reagent “seemed so unusual and esoteric,” he says, “but it’s actually easy to make and it does all this beautiful chemistry.”
Starting from the bicyclobutane precursor, the researchers could form bicyclobutyl boronate intermediates that could react with a variety of coupling partners to form trisubstituted cyclobutane products (example shown). These products contain substitution patterns similar to those made via [2+2] cycloaddition reactions, without that transformations’ requirement that one partner contain an electron-withdrawing substituent.
Boston College’s James P. Morken, whose group has expanded the Suzuki-Miyaura reaction to work with vinyl boronate complexes, says the new reactivity is intriguing. “It will be exciting to see what other types of strained, or even unstrained, ring systems engage in this type of reaction,” he says.
Aggarwal says they’re eager to explore other classes of bicyclobutane compounds in a range of reactions.
Catalyst chirality switched with a flash of light
Molecular motor and chiral ligand combined to create photoresponsive catalyst
Ultraviolet light can switch the catalyst between two
different forms.
Cells have a lot of control over enzymes. They can modify an enzyme to be more or less active, or to change the reaction that it catalyzes. But chemistry in the lab is different. “Normally when we design a ligand or catalyst we design it to do one specific transformation,” explains Ben Feringa of the University of Groningen, who won the 2016 Nobel Prize in Chemistry. “What we wanted to do is make an adaptive catalyst.”
To do that, Feringa’s group has combined a molecular motor with an existing asymmetric catalyst. The result is a catalyst that can switch its chirality, and as a result the stereochemistry of its products, with a flash of light (J. Am. Chem. Soc.2018, DOI: 10.1021/jacs.8b10816).
The switchable catalyst features what is known as axial chirality. This type of chirality is often used in asymmetric catalysis and results from the nonplanar arrangement of groups around an axis. Feringa’s molecule contains a biphenol group with axial chirality and a rigid core that rotates when hit with ultraviolet light. Because of the tight conformational coupling of these two elements, switching the helicity around the axis also changes the chirality of the biphenol. The biphenol coordinates with a zinc ion to form the active catalyst.
As a proof of principle, the chemists tested the catalyst in an organometallic 1,2-addition reaction to produce different stereoisomers depending on which form of the catalyst was used.
The catalyst is “a real achievement in the dynamic coupling of several types of convoluted chiralities,” says Nicolas Giuseppone, an organic chemist at the University of Strasbourg.
“It nicely demonstrates that switchable chiral catalysts can be obtained by the designed integration of functional units with well-defined stereochemical properties,” adds Alberto Credi of the University of Bologna. “Such a modular approach could in principle be applied to a wealth of biaryl structures,” he adds, suggesting this could lead to various chiral switches that could control different processes.
Feringa agrees. The dream, he says, is adaptive systems that could be switched from one reaction to another using an external signal. But, he admits, “I don’t know where the most useful applications will be.”
Chemical & Engineering News
|
April
8-10, 2019 Applied Catalysis & Chemical Engineering Dubai, United Arab Emirates |
https://www.eigenscientificgroup.com/conference/catalysis-2019 |
|
April
9-12, 2019 26th Croatian Meeting of Chemists and Chemical Engineers (26HSKIKI) Šibenik, Croatia |
http://www.26hskiki.org/en |
|
24-25
апреля 2019 г. IX Всероссийская конференция «Каучук и Резина-2019: традиции и новации» Москва, Россия |
www.rubberconference.ru |
|
24-26
апреля 2019 г. Юбилейная научная конференция «XXI век: Химия в жизнь» Омск, Россия |
omsk-glyzdova@mail.ru science@ihcp.ru |
|
May
5-8, 2019 4th Green & Sustainable Chemistry Conference Dresden, Germany |
https://www.elsevier.com/events/conferences/green-and-sustainable-chemistry-conference |
|
May
13-15, 2019 Conference “Frontiers in Catalysis and Chemical Engineering” (EURO CAT 2019) London, UK |
https://www.longdom.com/eurocat |
|
May
13-17, 2019 International Symposium on Green Chemistry (ISGC) La Rochelle, France |
https://www.isgc-symposium.com |
|
May
15-17, 2019 5th International Conference on Polygeneration (ICP 2019) Fukuoka, Japan |
http://therme.mech.kyushu-u.ac.jp/ICP2019/ICP2019.html |
|
May
27-31, 2019 E-MRS Spring meeting – Symposium H: “Materials for applications in photocatalysis and photoconversion” Nice, France |
https://www.european-mrs.com/materials-applications-photocatalysis-and-photoconversion-emrs |
|
27-31
мая 2019 г. XI Всероссийская научная конференция с международным участием и школа молодых ученых «Химия и технология растительных веществ» Сыктывкар, Россия |
http://chemi.komisc.ru/ru/conference |
|
28-30
мая 2019 г. VII Международная научно-техническая конференция «Альтернативные источники сырья и топлива» (АИСТ-2019) Минск, Беларусь |
http://aist.ichnm.by/ |
|
28-30
мая 2019 г. Научно-техническая конференция молодых специалистов «Авиационные двигатели и силовые установки» Москва, Россия |
engineconf@ciam.ru
+7 (495) 362 3912 |
|
June
2-6, 2019 12th Natural Gas Conversion Symposium San Antonio, Texas, USA |
https://www.aiche.org/conferences/natural-gas-conversion-symposium/2019 |
|
June
2-6, 2019 14th International Symposium on Macrocyclic and Supramolecular Chemistry (ISMSC2019) Lecce, Italy |
https://ismsc2019.eu |
|
June
11-13, 2019 23rd Annual Green Chemistry & Engineering Conference and 9th International Conference on Green and Sustainable Chemistry Reston, VA (USA) |
http://gcande.org |
|
June
16-20, 2019 6th Nano Today Conference Lisbon, Portugal |
https://www.elsevier.com/events/conferences/nano-today-conference |
|
June
17-20, 2019 3rd International Conference on Applied Surface Science Pisa, Italy |
https://www.elsevier.com/events/conferences/international-conference-on-applied-surface-science |
|
June
18-21, 2019 1st Summer School “Catalysis: from understanding to applications” Albi, France |
https://schoolcat2019.sciencesconf.org |
|
June
19-23, 2019 22nd International Conference on Chemical Thermodynamics in Russia (RCCT 2019) St. Petersburg, Russia |
http://rcct2019.spbu.ru |
|
June
24-28, 2019 5th EuChemS Inorganic Chemistry Conference Moscow, Russia |
http://www.igic.ras.ru/docs/news/tcirkulyar_evrohim_2019.pdf |
|
June
26-30, 2019 6th European Conference on Environmental Applications of Advanced Oxidation Processes (EAAOP-6) Portoroz-Portorose, Slovenia |
http://eaaop6.ki.si |
|
1-4
июля 2019 г. VII Всероссийская школа-конференция молодых ученых «Органические и гибридные наноматериалы» Иваново, Россия |
oyarm@mail.ru
conf_nanomaterials@mail.ru тел. (495) 647-59-27, доб. 141 |
|
1-5
июля 2019 г. XIII Международная школа молодых ученых и специалистов имени А.А. Курдюмова «Взаимодействие изотопов водорода с конструкционными материалами» Саров, Россия |
https://ihism.org |
|
July 5-12, 2019 50th General Assembly & 47th IUPAC World Chemistry Congress Paris, France |
https://www.iupac2019.org |
|
July
8-11, 2019 14th International Conference on Materials Chemistry (MC14) Birmingham, UK |
http://www.rsc.org/events/detail/31760/14th-international-conference-on-materials-chemistry-mc14 |
|
July
15-19, 2019 Catalysis Fundamentals and Practice Summer School University of Liverpool, UK |
https://www.liverpool.ac.uk/chemistry/events/catalysis-summer-school-2019 |
|
July
17-19, 2019 Energy Security and Chemical Engineering Congress 2019 (SChE 2019) Penang, Malaysia |
http://esche.ump.edu.my/index.php/en |
|
July
22-27, 2019 Summer School “Making Business with Green Chemistry & Sustainable Energy” Sarteano, Siena province, Italy |
www.eric-aisbl.eu/sarteano |
|
July
26-28, 2019 Mendeleev 150: 4th International Conference on the Periodic Table endorsed by IUPAC Saint Petersburg, Russia |
http://mendeleev150.ifmo.ru |
|
August
4-8, 2019 36th International Conference of Solution Chemistry Xining, China |
http://icsc2019.csp.escience.cn/dct/page/1 |
|
August
18-23, 2019 European Congress on Catalysis (14th EuropaCat) Aachen, Germany |
www.europacat2019.eu |
|
September
2-6, 2019 5th International Conference “Catalysis for Renewable Sources: Fuel, Energy, Chemicals” (CRS-5) Crete, Greece |
http://conf.nsc.ru/CRS5 |
|
September
2-6, 2019 1st International Conference on Noncovalent Interactions (ICNI) Lisbon, Portugal |
http://icni2019.eventos.chemistry.pt |
|
4-6
сентября 2019 г. V Всероссийская научно-практическая конференция молодых ученых и специалистов «Материалы и технологии XXI века» Бийск, Россия |
post@frpc.secna.ru kubasov_artem@mail.ru 8 962 804 9888 |
|
September
9-11, 2019 Euro Conference on Catalysis & Chemical Engineering Advancements 2019 Rome, Italy |
https://scientonline.org/catalysis-conferences/index.php |
|
September
11-14, 2019 7th International Conference on Semiconductor Photochemistry Milano, Italy |
https://www.sp7.unimi.it |
|
September
23-27, 2019 5th International Congress on Catalysis for Biorefineries (Catbior 2019) Turku / Åbo, Finland |
http://www.catbior2019.fi |
|
1-4
октября 2019 г. XXIII Всероссийская конференция (с международным участием) и II Всероссийская молодежная школа-конференция «Рентгеновские и электронные спектры и химическая связь» Воронеж, Россия |
https://rescb.ru |
|
October
7-11, 2019 XI International Conference “Mechanisms of Catalytic Reactions” (MCR-XI) Sochi, Russia |
http://conf.nsc.ru/mcr2019/en/ |
|
7-11
октября 2019 г. XII Всероссийская школа-конференция молодых ученых «Теоретическая и экспериментальная химия жидкофазных систем» (Крестовские чтения 2019) Иваново, Россия |
http://krestov.isc-ras.ru/ |
|
October
10-13, 2019 III International School-Conference “Applied Nanotechnology & Nanotoxicology” (ANT-2019) Sochi, Russia |
http://conf.nsc.ru/ant-2019/en/ |
|
October
20-23, 2019 5th International Symposium on Innovative Materials and Processes in Energy Systems (IMPRES2019) Kanazawa, Japan |
http://www.knt.co.jp/ec/2019/impres2019/index.html |
|
June
28 – July 3, 2020 The World Conference on Carbon (CARBON 2020) Kyoto, Japan |
http://www.tanso.org/carbon2020 |
|
July
5-9, 2020 48th World Polymer Congress (MACRO2020) Jeju Island, Korea |
http://www.macro2020.org |
|
August
16-21, 2020 12th Triennial Congress of the World Association of Theoretical and Computational Chemists (WATOC 2020) Vancouver, Canada |
http://watoc2020.ca |


