22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы

Постановлением Президиума Российской академии наук премия имени А.А. Баландина 2013 года присуждена члену-корреспонденту РАН Лапидусу Альберту Львовичу, доктору химических наук Усачеву Николаю Яковлевичу (Федеральное государственное бюджетное учреждение науки Институт органической химии им. Н.Д. Зелинского Российской академии наук) и доктору химических наук Третьякову Валентину Филипповичу (Федеральное государственное бюджетное учреждение науки Ордена Трудового Красного Знамени Институт нефтехимического синтеза им. А.В. Топчиева Российской академии наук) за серию работ “Теоретические основы создания каталитических процессов переработки ненефтяного сырья в углеводородные топлива и продукты для нефтехимии”.
Представленная серия работ имеет важное научное и практическое значение для решения проблем альтернативной энергетики и глобальной экологической безопасности. Авторами разработаны катализаторы нового поколения, изучены их физико-химические свойства, предложен механизм получения синтез-газа, не разбавленного азотом, изложены результаты перспективного процесса Фишера-Тропша для получения компонентов моторных топлив и ценных химических соединений на базе ненефтяного сырья, что приводит к высокотехнологичному и безопасному процессу. Серия работ А.Л. Лапидуса, Н.Я. Усачева, В.Ф. Третьякова вносит значительный вклад в теорию гетерогенного катализа и по многим позициям опережает существующий мировой уровень научных разработок.
ПОЗДРАВЛЯЕМ КОЛЛЕГ С ВЫСОКОЙ НАГРАДОЙ!
Премия имени А.А. Баландина присуждается Российской академией наук за выдающиеся работы в области катализа.
История присуждения премии:
2010 | Анаников В.П. | За работу “Катализируемые переходными металлами реакции присоединения к ацетиленовым углеводородам” |
2007 | Садыков В.А. | За серию работ “Роль дефектности и микроструктуры катализаторов окислительно-восстановительных реакций” |
2004 | Лин Г.И. | За цикл работ “Исследование механизма и кинетики каталитических превращений одноуглеродных молекул” |
2001 | Андрушкевич Т.В. | За серию работ “Гетерогенно-каталитическое окисление основных органических соединений в карбоновые кислоты: механизм, кинетика, |
1998 | Грязнов В.М. | За серию работ “Исследования механизма реакций с переносом атомов водорода на металлах, создание новых катализаторов и способов проведения этих реакций” |
1995 | Лунин В.В. | За цикл работ “Новые гетерогенные катализаторы на основе интерметаллических соединений и их гидридов” |
1993 | Миначев Х.М. | За цикл работ “Структура поверхности и каталитические свойства высокодисперсных металлнаселённых систем” |
2-я Всероссийская научная конференция
«Методы исследования состава и структуры функциональных материалов»
21-25 октября 2013 г.
Новосибирск (Россия)
21-25 октября 2013 года в Доме Ученых Сибирского отделения Российской академии наук состоялась 2-я Всероссийская научная конференция «Методы исследования состава и структуры функциональных материалов». Конференция проводилась в преддверии Международного года кристаллографии и была посвящена 100-летию со дня открытия рентгеновской дифракции, которое отмечалось в прошлом году. Основными организаторами конференции являлись Институт катализа им. Г.К. Борескова СО РАН и Новосибирский государственный университет.

Создание новых материалов и технологий – несомненный приоритет технически развитых стран, стремящихся к высокому экономическому уровню. Материаловедением считают прикладную науку о связи состава, строения и свойств материалов. Теоретической основой материаловедения являются соответствующие разделы физики и химии, при этом разработка новых методов исследования строения (структуры), состава и физико-механических свойств материалов способствует дальнейшему развитию материаловедения. Объектами исследований являются технологии получения и методы характеризации широкого спектра разнообразных функциональных материалов, в том числе сверхпроводящих и магнитных материалов, полупроводников, полимеров, а также наноматериалов, предназначенных для электроники, фотоники, информационных технологий, здравоохранения и экологии. Значение методов исследования состава и структуры функциональных материалов особенно велико для последующей оптимизации их свойств и контроля качества при их производстве.
Конференция МИССФМ-2013 собрала 168 участников из 20 городов России – как из европейской части (Москва, Санкт-Петербург, Ростов-на-Дону, Екатеринбург), так и из сибирских регионов (Томск, Улан-Удэ, Красноярск, Омск и др.). В конференции также приняли участие специалисты в области химических и физических методов исследования из Испании, Германии, США.
На конференции было представлено 17 пленарных лекций, 4 ключевых, 66 устных секционных докладов, 6 презентационных докладов спонсоров, 62 стендовых доклада, а также 13 устных докладов в секции молодых ученых. Отличительной особенностью тематики конференции МИССФМ является междисциплинарность задач исследования всего спектра характеристик состава и структуры функциональных материалов. Поскольку границы между отдельными научными дисциплинами условны и подвижны, можно с уверенностью сказать, что доклады многих участников были представлены "на стыке" наук, с использованием большого набора различных методологических приемов, над которыми работали многочисленные коллективы исследователей разных специальностей. Здесь чётко прослеживается процесс интеграции дисциплин, дающий возможность качественных прорывов в решении научных проблем.
Участниками конференции был отмечен высокий уровень пленарной сессии. Первым был представлен доклад Главного научного сотрудника Института неорганической химии им. А.В. Николаева СО РАН, д. ф.-м.н.
Программа конференции включала ключевые и устные доклады, которые были представлены в узкоспециализированных секциях:
 Стендовая сессия была в большой степени посвящена работам прикладного характера на примерах конкретных систем и материалов. В результате обсуждений и дискуссий с материаловедами перед специалистами в области методов исследования были сформулированы проблемы, решение которых сможет обеспечить прогресс в разработке и синтезе функциональных материалов, в том числе наноматериалов.
Стендовая сессия была в большой степени посвящена работам прикладного характера на примерах конкретных систем и материалов. В результате обсуждений и дискуссий с материаловедами перед специалистами в области методов исследования были сформулированы проблемы, решение которых сможет обеспечить прогресс в разработке и синтезе функциональных материалов, в том числе наноматериалов.
В рамках конференции была успешно проведена секция научной молодежи, на которой аспиранты и студенты-магистранты докладывали результаты своих работ в десятиминутных докладах, выслушивали мнение коллег, отвечали на вопросы.
Выставка компаний-производителей аналитического оборудования – Intertech Corporation, Bruker, NETZSCH-Gerätebau GmbH, Technoinfo Ltd. – привлекла представителей университетов и академических институтов, которые с интересом рассматривали предложения специалистов, демонстрирующих последние достижения в области производства научно-технического и аналитического оборудования для исследования состава и структуры функциональных материалов, в том числе наноматериалов.

Для участников конференции были организованы обзорные экскурсии по двум институтам Новосибирского научного центра (Институт неорганической химии им. А.В. Николаева и Институт катализа им. Г.К. Борескова), работающим, в том числе, в области исследования свойств и строения функциональных материалов.
Конференция была поддержана Российским фондом фундаментальных исследований. Необходимо отметить поддержку молодых ученых конференции Международным центром данных по дифракции (International Centre for Diffraction Data), США. Благодаря помощи Центра российские аспиранты и студенты имели возможность участвовать в конференции, представить свои доклады, общаться со старшими коллегами, прослушать доклады высокого мирового уровня. Молодые ученые, отобранные Программным комитетом, были награждены почетными грамотами.
Участниками было принято решение о проведении следующей конференции МИССФМ через три года.
Материал подготовили
С.В. Цыбуля и Т.В. Замулина
(Институт катализа СО РАН, Новосибирск)
6-й Азиатско-Тихоокеанский
Конгресс по катализу
13-17 октября 2013 г.
Тайпей (Тайвань)
Азиатско-Тихоокеанский Конгресс по катализу проводится каждые три года (1997 г. – Корея, 2000 г. – Австралия, 2003 г. – Китай, 2006 г. – Сингапур, 2010 г. – Япония). 6-й Конгресс проходил 13-17 октября 2013 года в г. Тайпей (Тайвань). Основная тема Конгресса звучала как «Новая эра катализа: эффективность, значимость и устойчивость». В программе Конгресса были предусмотрены следующие секции:
 Делегация из Институт катализа СО РАН была довольно многочисленной: с ключевой лекцией о развитии катализа в России выступил академик В.Н. Пармон, с устными докладами выступили д.х.н. В.И. Симагина, Н.И. Кузнецова и Л.А. Исупова, к.х.н. О.П. Таран и Е.А. Козлова, Б.Л. Мороз, стендовый доклад представил В.В. Данилевич.
Делегация из Институт катализа СО РАН была довольно многочисленной: с ключевой лекцией о развитии катализа в России выступил академик В.Н. Пармон, с устными докладами выступили д.х.н. В.И. Симагина, Н.И. Кузнецова и Л.А. Исупова, к.х.н. О.П. Таран и Е.А. Козлова, Б.Л. Мороз, стендовый доклад представил В.В. Данилевич.
В научной программе Конгресса особенно широко были представлены доклады по фотокатализу, которые занимали около 30% научной программы. Профессор Kazunari Domen из Университета Токио сделал ключевой доклад по теме «Фотокатализаторы для разложения воды», в котором осветил современные взгляды на синтез ассиметричных систем для разложения воды на кислород и водород под действием видимого излучения.
 Профессор Hsisheng Teng из National Cheng Kung University в своей ключевой лекции рассказал о создании новых фотокатализаторов для разложения воды на основе графена.
В устных докладах рассматривались, в основном, композитные фотокаталитические системы для получения водорода под действием видимого излучения. Е.А. Козлова из Института катализа СО РАН сделала доклад по теме «Дизайн нанокристаллического фотокатализатора CdS/TiO2 для окисления этанола под действием видимого излучения». Данная работа была удостоена приза за лучший доклад молодых ученых.
Профессор Hsisheng Teng из National Cheng Kung University в своей ключевой лекции рассказал о создании новых фотокатализаторов для разложения воды на основе графена.
В устных докладах рассматривались, в основном, композитные фотокаталитические системы для получения водорода под действием видимого излучения. Е.А. Козлова из Института катализа СО РАН сделала доклад по теме «Дизайн нанокристаллического фотокатализатора CdS/TiO2 для окисления этанола под действием видимого излучения». Данная работа была удостоена приза за лучший доклад молодых ученых.
Участникам Конгресса была предложена разнообразная культурная программа: экскурсия по городу Тайпей, посещение музеев, поездка на Тихий океан и в деревню Chiufen.
Материал подготовила
Е.А. Козлова
(Институт катализа СО РАН, Новосибирск)
Carbon Nanotube Transistors Could Help Displays Flex
Researchers use a springy ion gel to help carbon nanotube tran-sistors stretch further than previous devices
To make future displays that roll, bend, and stretch, electronics makers need the circuits that control the pixels to be elastic. In particular, they need flexible transistors. Now researchers have built super stretchy transistors from carbon nanotubes (Nano Lett. 2014, DOI: 10.1021/nl403941a).
Michael S. Arnold, a materials scientist at the University of Wisconsin, Madison, thinks carbon nanotubes have amazing properties that make them ideally suited for elastic electronics. “They are some of the best conductors of charge ever discovered—much faster than silicon or organic electronics,” he says. They’re also chemically and mechanically robust: The nanomaterials don’t oxidize when exposed to air, and meshes of the nanotubes can stretch, fold, and withstand tremendous strain without damage.
Given this great stretchiness and conductivity, companies are already looking into using these materials to make flexible electrodes for touchscreens. Making stretchable transistors is harder, Arnold says. Transistors have more parts, and all of them have to stretch in unison. If they cannot move in sync, electrical contacts break, and the transistor can no longer switch on and off.
One particularly tricky transistor part is the dielectric, an insulating material that’s usually made out of unyielding inorganic oxides. The dielectric layer helps ensure that a transistor doesn’t leak current when it is in its off state. It basically ensures the transistor stays off and doesn’t waste power. Feng Xu, a researcher in Arnold’s lab, had the idea to use an ion gel—a squishy polymer embedded with ions—as the dielectric. Ion gels have been used in carbon nanotube transistors before (Nano Lett.2013, DOI: 10.1021/nl3038773). That previous work took advantage of the gels’ compatibility with printing-based fabrication methods, not their ability to stretch.
To make the stretchy transistors, the researchers first constructed a nanotube mesh by dripping a solution of semiconducting nanotubes onto a glass slide, spreading out the solution, and letting the organic solvent evaporate. Next the team stretched a sheet of clear, rubbery polydimethylsiloxane and pressed it onto the glass slide to pick up the carbon nanotube film. The researchers patterned gold electrodes onto the sheet while it was stretched. They then drop-cast the ion gel onto the nanotube mesh surface.
When the team let the rubber substrate relax, the nanotubes and the gold electrodes wrinkled and buckled. This pre-stretching enabled the finished devices to expand without breaking when strained, Arnold says.
The Wisconsin researchers put their stretchy transistors through electrical and mechanical tests. The devices had a high electron mobility, a measure of how fast electrons can move through the transistor, and a large on-off ratio, a measure of the difference in conductance between its on and off states. To test the devices’ physical resilience, the scientists pulled the transistors sitting on polydimethylsiloxane so that the devices were 57% longer than their resting length. After more than 1,000 pulls, the transistors showed no signs of degradation in electrical performance. The previous record for carbon nanotube transistors was a 20% stretch (Nat. Mater. 2013, DOI: 10.1038/nmat3572).
The devices exhibit good stretchiness, says Young Hee Lee, a researcher at Sungkyunkwan University, in South Korea, who developed the previous record-holding stretchy transistors. But he says the devices still need improvements before they are ready for commercialization. For example, transistor arrays for commercial organic light-emitting diode (OLED) displays will need to switch on and off faster than the devices reported, Lee says.
Arnold is continuing to develop his team’s transistors for use with OLED displays. He wants to incorporate a more elastic dielectric that better matches the other ingredients—it’s the ion gel that breaks down when it’s stretched past 57%, not the electrodes or the nanotubes.
Metal-Organic Framework Used For Chiral Chromatography
A column loaded with a MOF can separate enantiomers of pharmaceuticals via high-performance liquid chromatography.
Researchers have demonstrated that a chiral metal-organic framework (MOF) can separate enantiomers of drug molecules using traditional chromatography (Anal. Chem. 2013, DOI: 10.1021/ac403674p). Although the study marks a new application for the materials, some experts warn that the MOFs may not outperform standard materials used for chiral separations.
Separating mixtures of enantiomers is important in the pharmaceutical industry because enantiomers of drug molecules may have different biological effects. Currently, chemists isolate enantiomers using high-performance liquid chromatography (HPLC) through columns packed with a stationary phase that includes chiral materials. As the mixture moves through the column, each enantiomer interacts differently with the stationary phase, causing the molecules to travel at different speeds through the column. As a result, one compound will flow off of the column earlier than the second one.
Bo Tang at Shandong Normal University in China, and his colleagues wondered if a chiral MOF could be used as a stationary phase. MOFs are three-dimensional structures easily assembled using metals and ligands. When chemists use chiral ligands, they produce MOFs with chiral pores. Since individual enantiomers would have different affinities for these asymmetric pores, Tang thought that the chiral MOFs could be used to separate the isomers.
The researchers synthesized a MOF using zinc and a chiral salen ligand functionalized with pyridine. They packed crystals of the MOF into an HPLC column and used the column to separate enantiomers of ibuprofen, phenylethylamine, and benzoin. While the column easily separated those three mixtures, it could not separate enantiomers of ketoprofen and naproxen because the molecules are larger than the diameter of the MOF’s pores.
Though using MOFs for chiral separations is a new application of the materials, says Yoshio Okamoto, an emeritus professor at Nagoya University in Japan and a researcher at Harbin Engineering University in China, wonders if this MOF offers any advantage over the ubiquitous polysaccharide columns currently used in chiral separations. He also notes that, like ketoprofen and naproxen, many drug molecules are larger than the approximate 9.8 Å pore size of this MOF. Yu Ma, a researcher who worked on the project, says the pore size can easily be changed by varying the metal and ligand used to make the MOF.
Engineering Titanium Dioxide To Respond To Visible Light
Photocatalysis: Gently depositing nitrogen atoms on the surface of TiO2 makes the material active across a broader range of wavelengths of light.
Many scientists view titanium dioxide as an attractive, low-cost photocatalyst for a variety of applications, including water purification, water splitting, and solar power. But there is one snag: The material catalyzes reactions only in response to ultraviolet light. Now, researchers in Singapore have found a way to dope TiO2 with nitrogen so that it responds to visible light, drastically increasing its activity in sunlight (J. Phys. Chem. C 2013, DOI: 10.1021/jp408798f).
A material’s photoactivity depends in part on its band gap, the energy needed to kick electrons from a nonconductive state to a conductive one. TiO2 has a band gap of 3 eV, which corresponds to energies of photons in the UV range. Doping TiO2 with nitrogen lowers the band gap below 2 eV, making the material photoactive with visible light. However, up to now, the doping methods used, such as magnetron sputtering and high-energy ion bombardment, have created defects in the bulk TiO2, which reduced the photocatalytic efficiency of the material.
A team of researchers from the Institute of Materials Research & Engineering (IMRE) and the National University of Singapore used a different source of nitrogen atoms that sent low-speed beams of atoms at the TiO2. Because these nitrogen atoms did not penetrate beyond the TiO2 surface, the researchers could replace the top layer of the material with nitrogen and create a defect-free surface, says Junguang Tao, a physicist at IMRE.
The team measured the photocatalytic activity of TiO2 samples with and without a nitrogen-doped surface. The doped TiO2 showed photoactivity when illuminated with visible light, unike the undoped TiO2. What’s more, the surface-doped TiO2 showed greatly enhanced photoactivity under UV illumina-tion, unlike TiO2 doped using previous methods.
Clear Solar Cells Power Up Windows
Materials: Islands of perovskite make light-colored solar cells that are efficient, semitransparent
The vast real estate of windows in office buildings and skyscrapers could be a fruitful field for harvesting solar energy—if light-weight solar cells could be made with high enough efficiency and aesthetic appeal. Now researchers at Oxford University report sem-itransparent solar cells that might do the trick (ACS Nano 2013, DOI: 10.1021/nn4052309).
For use in windows, solar cells need to absorb enough light to produce sufficient energy but also let enough visible light through to be transparent. Organic photovoltaic materials can absorb infrared light and pass visible light, but they have low energy-conversion efficiencies. On the other hand, inorganic semiconductors, such as amorphous silicon, absorb visible light strongly. So films of these materials must be thin to be transparent, thus decreasing the amount of photons they capture. They also tend to give windows a brownish or reddish tint, which architects dislike.
The Oxford team, led by physicist Henry J. Snaith, made solar cells using perovskites. This class of crystalline materials has recently grabbed the attention of photovoltaics researchers. That’s because perovskites have properties similar to inorganic semiconductors, including high sunlight-to-electricity conversion efficiencies.
To make their semitransparent cells, the researchers first deposited a film of the perovskite CH3NH3PbI3-xClx onto glass coated with fluorine-doped tin oxide. They made the film by mixing methylammonium iodide and lead chloride and spin-coating the solution along with a solvent, such as dimethylsulfoxide, onto the glass. They then heated the resulting film to temperatures ranging from 90 to 130 °C.
As the solution cooled, it formed droplets on the glass surface. As the solvent evaporated, the droplets yielded islands of crystalline material with empty spaces in between. The islands absorb photons and convert them to electrons, while the empty spaces let light pass through. The result was a transparent solar cell with a neutral, grayish tint that is more desirable for use in windows.
As the transparency of the film increases, energy-conversion efficiency decreases. The most transparent films, which let through about 30% of incoming light, converted light to electricity with efficiencies of 3.5%. The darkest films, only 7% transparent, had efficiencies near 8%. Snaith says the ideal coating would let through about half the light and have a 5% conversion efficiency. “We think there’s a lot of scope to improve it further,” he says. He’s formed a company, Oxford Photovoltaics, that hopes to commercialize a device by 2017.
Snaith says the next step is to determine the stability of the perovskite material. A practical solar cell should function for several years. But even if it stops generating electricity, the window containing the solar cell should maintain its color and transparency for at least a decade.
The new solar cell is “fantastic technology,” says Yang Yang, head of the organic electronic materials and devices group at the University of California, Los Angeles. Yang, who is also pursuing transparent solar cells for windows, says there are challenges to commercialization for perovskites, such as the use of lead and the cells’ sensitivity to moisture. But he finds the materials promising: “The rapid progress of efficiency is unprecedented in solar-cell technology.”
A Control Knob To Add C–N Bonds
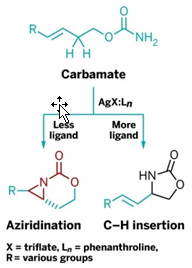 Simple catalyst modifications orchestrate amine formation reactions
Simple catalyst modifications orchestrate amine formation reactions
A new technique makes it possible to add carbon-nitrogen bonds at different spots in some organic compounds by making simple changes to a catalyst. The technique controls how and where amine formation reactions occur in a compound by altering the catalystligand ratio in a silver-based catalytic system (J. Am. Chem. Soc. 2013, DOI: 10.1021/ja406654y).
Nitrogen-containing groups such as amines are crucial in many pharmaceuticals and other biologically active molecules. Researchers have long used transition-metal catalysts to introduce amines at reactive C–H sites or C=C sites. But the ability to favor one reaction over the other was previously possible only by changing reagent combinations.
Studies suggest that silver catalyst complexes undergo unique alterations in geometry when their composition changes in certain ways. Jennifer M. Schomaker and coworkers at the University of Wisconsin, Madison, wondered whether that feature of silver-based complexes could alter their reactive properties and, thus, be used to control where C–N bonds formed.
They now report that a silver triflate catalyst with a phenanthroline ligand in a 4:5 catalyst-ligand ratio catalyzed almost exclusively the addition of aziridine (a three-membered ring with a nitrogen) to the site of a C=C group in carbamate compounds. But changing the catalyst-ligand ratio to 1:3 transformed the reaction predominantly to amine formation at a C–H bond in the same carbamates.
Schomaker suspects that cutting the catalyst-ligand ratio congests the catalyst’s local steric environment, making the C–H reaction more likely and vice versa. Her group is working on extending this silver-based catalysis technique to chemoselective amination of other organic compounds.
Jennifer L. Roizen, an organic chemist at Duke University, comments that “other examples where ligand stoichiometry is linked to or causes indirect or direct changes in reactivity or chemoselectivity are few and far between.”
Chemical & Engineering News
Научно-технологический симпозиум
«Нефтепереработка: катализаторы и гидропроцессы»
(20-22 мая 2014 г., Санкт-Петербург)
http://conf.nsc.ru/STS
Научно-технологический симпозиум «Нефтепереработка: катализаторы и гидропроцессы» будет проведен 20-22 мая 2014 года в городе Пушкин (пригород Санкт-Петербурга).
Современные каталитические гидрогенизационные технологии в настоящее время являются важнейшим фактором повышения экономической, энергетической и экологической эффективности процессов нефтепереработки в России, улучшения качества производимых топлив, а также расширения сырьевой базы этих производств. Ключевым звеном прогресса в этой области являются разработка и применение новых катализаторов для осуществления гидропроцессов. Российская наука накопила значительный и вполне конкурентоспособный на мировом уровне потенциал в этой области, актуальный для российских нефтеперерабатывающих предприятий.
Задачей симпозиума является организация обмена информацией и мнениями между научными специалистами и представителями промышленных компаний, специализирующихся в области современных технологий переработки нефти, по вопросам новейших достижений в сфере разработки новых катализаторов и процессов для нефтепереработки, а также тенденций технологического развития этих отраслей в России и мире.
IV Всероссийская научная молодежная школа-конференция
«Химия под знаком СИГМА:
исследования, инновации, технологии»
(12-18 мая 2014 г., Омск)
http://conf.nsc.ru/chemsigma2014/ru
IV Всероссийская научная молодежная школа-конференция «Химия под знаком СИГМА: исследования, инновации, технологии» состоится 12-18 мая 2014 г. в городе Омске. В рамках школы-конференции традиционно будут представлены пленарные лекции ведущих ученых-химиков, устные и стендовые доклады молодых ученых, специалистов и преподавателей по современным направлениям фундаментальных и прикладных исследований в области химии и химической технологии.
Третья всероссийская (с международным участием) научная конференция
«Успехи синтеза и комплексообразования»
21-25 апреля 2014 г., Москва
http://www.conferencerudn.com
Третья всероссийская научная конференция «Успехи синтеза и комплексообразования», организованная Российским университетом дружбы народов, будет проведена в Москве с 21 по 25 апреля 2014 г.
Международная научно-техническая конференция
Нанотехнологии функциональных материалов
24-28 июня 2014 г., Санкт-Петербург
http://www.spbstu.ru/conference/2014/nfm.asp
Международная научно-техническая конференция «Нанотехнологии функциональных материалов» будет проходить 24-28 июня 2014 г. в Санкт-Петербурге. На конференции будут представлены и обсуждены новейшие научные результаты фундаментальных исследований и практических достижений в области разработки новых наноструктурированных металлических и керамических материалов с высоким уровнем эксплуатационных свойств, а также технологии их производства.
|
Materials Challenges In Alternative & Renewable Energy 2014 (MCARE 2014) Clearwater, FL, USA |
http://ceramics.org/meetings/ |
|
International Conference on Nanotechnology, Nanomaterials & Thin Films for Energy Applications London, UK |
|
|
15th Netherlands' Catalysis and Chemistry Conference (NCCC XV) Noordwijkerhout, The Netherlands |
|
|
3rd International Conference on Green Polymer Chemistry 2014 Cologne, Germany |
|
|
Всероссийская конференция с международным участием “Современные достижения химии непредельных соединений: алкинов, алкенов, аренов и гетероаренов” Санкт-Петербург, Россия |
http://ftacademy.ru/international/conference/events/ |
|
VIII Всероссийская конференция с международным участием молодых ученых по химии “Менделеев-2014” Санкт-Петербург, Россия |
|
|
Х Международная научно-практическая конференция “Нанотехнологии – производству 2014” Фрязино, Московская обл., Россия |
|
|
Asymmetric Organocatalysis; Challenges and Innovations Cambridge, UK |
https://www.soci.org/General-Pages/Display-Event.aspx? |
|
4th International Congress on Green Process Engineering Sevilla, Spain |
|
|
XIII Международный Конгресс “Биомасса: топливо и энергия-2014 ” Москва, Россия |
http://www.biotoplivo.ru/kongress_biomassa_toplivo_i_energija |
|
2014 Spring International Conference on Chemical Engineering (CEN-S) Shanghai, China |
|
|
III Всероссийская научная конференция с международным участием “Успехи синтеза и комплексообразования” Москва, Россия |
|
|
6-я Международная конференция “Платиновые металлы в современной индустрии, водородной энергетике и в сферах жизнеобеспечения будущего” Тель Авив, Израиль |
|
|
IV Всероссийская конференция “Каучук и Резина – 2014: традиции и новации” Москва, Россия |
tkonikova@mail.ru |
|
International Conference on BioMass Florence, Italy |
|
|
IV Всероссийская научная молодежная школа-конференция “Химия под знаком СИГМА: исследования, инновации, технологии” Омск, Россия |
|
|
II Всероссийская молодежная научная конференция с международным участием “Экологобезопасные и ресурсосберегающие технологии и материалы” Улан-Удэ, Республика Бурятия |
|
|
1st EFCATS-CNRS European Summer School on Catalyst Preparation (CatPrep 2014) Ardeche area (France) |
|
|
Научно-технологический симпозиум “Нефтепереработка: катализаторы и гидропроцессы” Пушкин (Санкт-Петербург), Россия |
|
|
IV Научно-практическая конференция “Современные тенденции и технологии добычи, производства, переработки и использования угольных и углеводородных топлив в промышленности и энергетике” Алушта, Украина |
|
|
7th International Conference “Inverse Problems: Modeling and Simulation” Fethiye, Turkey |
|
|
7th Tokyo Conference on Advanced Catalytic Science and Technology (TOCAT7) Kyoto, Japan |
|
|
XXV Российская конференция по электронной микроскопии (РКЭМ-2014) и 2-я Школа молодых ученых “Современные методы электронной и зондовой микроскопии в исследованиях наноструктур и наноматериалов” Черноголовка, Россия |
|
|
1st International Conference on Mechanics of Composites (MECHCOMP2014) Long Island, NY, USA |
|
|
16-я Международная Конференция “Материалы, методы и технологии” Елените, Болгария |
http://www.sciencebg.net/ru/conferences/ |
|
International Conference on Catalysis (ICC 2014) Beijing, China |
http://www.engii.org/workshop/ICC2014June/ |
|
16th Nordic Symposium on Catalysis Oslo, Norway |
|
|
4th International Colloids Conference “Surface Design and Engineering” Madrid, Spain |
|
|
21st World Petroleum Congress Moscow, Russia |
|
|
20th World Hydrogen Energy Conference 2014 (WHEC 2014) Gwangju, Korea |
|
|
38th International Symposium on Environmental Analytical Chemistry (ISEAC38) Lausanne, Switzerland |
|
|
13th International Conference on MicroREaction Technology (MRET13) Budapest, Hungary |
|
|
22nd European Biomass Conference and Exhibition (EU BC&E 2014) Hamburg, Germany |
|
|
VIII Международная научная конференция “Кинетика и механизм кристаллизации. Кристаллизация как форма самоорганизации вещества” Иваново, Россия |
|
|
Международная научно- техническая конференция “Нанотехнологии функциональных материалов” С.-Петербург, Россия |
|
|
11th International Symposium on Systems with Fast Ionic Transport Gdansk, Poland |
|
|
International Conference “Modern Physical Chemistry for Advanced Materials” (MPC’14) Kharkiv, Ukraine |
|
|
Симпозиум по нанофотонике Киев, Украина |
admini@inphyschem-nas.kiev.ua |
|
15th International Conference on Theoretical Aspects of Catalysis (ICTAC15) London, UK |
|
|
13th International Conference on Inorganic Membranes (ICIM 2014) Brisbane, Australia |
|
|
11th International Symposium “Scientific Bases for the Preparation of Heterogeneous Catalysts” Louvain-la-Neuve, Belgium |
|
|
19th International Symposium on Homogeneous Catalysis (ISHC-XIX) Ottawa, Canada |
|
|
11th Conference on Solid State Chemistry (SSC 2014) Trenčianske Teplice, Slovakia |
|
|
XXV EUCHEM Conference on Molten Salts and Ionic Liquids Tallinn, Estonia |
|
|
Workshop “Fundamentals and Applications of Cerium Dioxide in Catalysis” Udine, Italy |
|
|
XII International Conference on Nanostructured Materials (NANO 2014) Moscow, Russia |
http://www.nano2014.org/ |
|
XXVI International Conference on Organometallic Chemistry (ICOMC 2014) Sapporo, Japan |
|
|
7th International Conference on Technological Advances of Thin Films & Surface Coatings (Thin Films 2014) Chongqing, China |
|
|
9th Liquid Matter Conference Lisbon, Portugal |
|
|
41st International Conference on Coordination Chemistry (ICCC-41) Singapore |
|
|
International Bunsen Discussion Meeting on Surface-Enhanced Spectroscopies (SES 2014) Chemnitz, Germany |
|
|
XXIV International Conference on Raman Spectroscopy (XXIV ICORS) Jena, Germany |
|
|
21st International Congress of Chemical and Process Engineering (CHISA 2014) Prague, Czech Republic |
|
|
8th International Conference on Environmental Catalysis Asheville, North Carolina, USA |
|
|
SETCOR International Conference on Smart Materials and Surfaces (SMS 2014) Bangkok, Thailand |
|
|
5th EuCheMS Chemistry Congress Istanbul, Turkey |
|
|
7th International Congress on Biocatalysis Hamburg, Germany |
|
|
23rd International Symposium on Chemical Reaction Engineering (ISCRE 23) Bangkok, Thailand |
|
|
International Summer School on Photovoltaics and New Concepts of Quantum Solar Energy Conversion (Quantsol) Hirschegg, Austria |
|
|
6th International FEZA Conference “Porous Systems: From Novel Materials to Sustainable Solutions” Leipzig, Germany |
|
|
IV International Workshop on Transition Metal Clusters Novosibirsk, Russia |
|
|
III международная конференция стран СНГ “Золь-гель синтез и исследование неорганических соединений, гибридных функциональных материалов и дисперсных систем” Суздаль, Россия |
|
|
9th Conference on Sustainable Development of Energy, Water and Environment Systems (SDEWES) Venice – Istanbul |
|
|
BIT's 5th Annual Global Congress of Catalysis 2014 (GCC-2014) Qingdao, China |
|
|
4th International Conference “Nanomaterials: Applications & Properties’ 2014” Alushta, Crimea, Ukraine |
|
|
XXI International Conference on Chemical Reactors (CHEMREACTOR-21) Delft, The Netherlands |
|
|
12th International Conference on Fundamental and Applied Aspects of Physical Chemistry (Physical Chemistry 2014) Belgrade, Serbia |
|
|
II Российский конгресс по катализу “РОСКАТАЛИЗ” Самара, Россия |
|
|
3rd International Conference on Polymer Processing and Characterization (ICPPC-2014) Kottayam, Kerala, India |
|
|
International Conference “Photocatalysis for Energy” (PHOTO4E) Lyon, France |
|
|
7th Green Solvents Conference Dresden, Germany |
|
|
9-я Международная конференция “Углерод: фундаментальные проблемы науки, материаловедение, технология” Троицк (Москва), Россия |
|
|
2015 |
|
|
18th International Conference on Composite Structures (ICCS18) Lisbon, Portugal |
|
|
45th World Chemistry Congress (IUPAC-2015) Busan, Korea |
|
|
XII European Congress on Catalysis (EuropaCat XII) Kazan, Russia |
|
|
European Symposium on Chemical Reaction Engineering (ESCRE 2015) Fürstenfeldbruck, Germany |
|
|
2016 |
|
|
16th International Congress on Catalysis (ICC 16) Beijing, China |
|
ПРИГЛАШЕНИЕ К ПУБЛИКАЦИИ СТАТЕЙ
Вышел первый номер электронного научного журнала «Технологии добычи и использования углеводородов»
Он открывается интервью с тремя ключевыми фигурами проекта.
Главный редактор журнала Генеральный директор ОАО «Зарубежнефть» С.И. Кудряшов в обращении к читателям подчеркивает, что новый «журнал ставит своей целью формирование инновационной среды нефтегазовой отрасли в виртуальном пространстве. Издание призвано осуществлять информационную поддержку новых механизмов взаимодействия бизнеса, науки и государства в рамках Технологической платформы «Технологии добычи и использования углеводородов», образованной по решению Правительственной комиссии по высоким технологиям и инновациям».
Председатель редакционного совета журнала академик РАН, директор Института проблем нефти и газа РАН А.Н. Дмитриевский миссию редакционного совета видит в том, «чтобы сделать доступными для широкого круга специалистов новейшие научно-технические и технологические решения, обеспечивающие эффективность нефтегазодобычи в стране».
Заместитель председателя редакционного совета журнала «Технологии добычи и использования углеводородов», директор НП «Национальный институт нефти и газа», д.х.н., профессор, первый проректор по стратегическому развитию РГУ нефти и газа им. И.М. Губкина М.А.Силин, считает, что «электронный журнал – это новая форма, которая позволяет оперативно открыть дискуссию по любому вопросу и помогает выполнить основную функцию технологических платформ (ТП) – создать площадку общения науки, бизнеса и государства».
С материалами первого номера можно ознакомиться на странице «Журнал» в главном меню портала www.tp-ning.ru (все материалы размещены в формате pdf).
Журнал будет выходить ежеквартально и скоро станет ВАКовским.
Journal of Environmental Chemical Engineering
(выходит с июня 2013 года в издательство Elsevier)
http://www.journals.elsevier.com/journal-of-environmental-chemical-engineering
The Journal of Environmental Chemical Engineering (JECE) publishes full length original research papers, short communications, review papers, perspectives and letters to the Editor. Papers are welcome which apply chemical engineering principles to understand important environmental processes or that develop/optimize novel remediation processes.
The Journal of Environmental Chemical Engineering provides a forum for the publication of original research on the development of alternative sustainable technologies focusing on water and wastewater treatment and reuse; treatment, reuse and disposal of waste; pollution prevention; sustainability and environmental safety; recent developments on green chemistry; alternative methods of remediation of environmental accidents including but not limited to oil spills in water bodies and nuclear accidents.
JECE calls for papers that cover the following fields:
Physico-chemical processes:
Adsorption/biosorption, ion exchange, membrane processes, magnetic separation, particle separation, phase separation, multiphase extraction, thermal/evaporative processes
Advanced oxidation processes:
Heterogeneous catalysis, UV/H2O2, Fenton oxidation, ozonation, sonolysis, electrochemical treatment, wet air oxidation
Nanomaterials for environmental and chemical applications:
Adsorbents, catalysts, and nanocomposites
Sustainable technologies:
Water reclamation and reuse, carbon capture, renewable energy and energy recovery, waste minimization, treatment, resource recovery
JECE also covers the following fields:
Editors:
Prof. Despo Fatta-Kassinos (University of Cyprus, Nicosia, Cyprus)
Prof. Yunho Lee (Gwangju Institute of Science & Technology, Korea)
Prof. Teik-Thye Lim (Nanyang Technological University, Singapore)
Prof. Eder Claudio Lima (Federal University of Rio Grande do Sul, Brazil)
ACS Photonics
(журнал издается с января 2014 года)
http://pubs.acs.org/journal/apchd5
The American Chemical Society launches a new journal, ACS Photonics. The monthly, online-only journal will focus on research in the field of photonics, the study of the interaction of light with matter.
Published as soon as accepted and summarized in monthly issues, ACS Photonics will publish Research Articles, Letters, Perspectives, and Reviews, to encompass the full scope of published research in this field. Among the areas the journal will cover are:
Editor: Prof. Harry A. Atwater (California Institute of Technology)
Новые гранты для молодых ученых, работающих в области зеленой химии
В рамках пятилетней программы, организованной ЮНЕСКО и компанией ФосАгро при поддержке Международного союза теоретической и прикладной химии, молодым ученым (до 35 лет) будут присуждаться гранты до 30 тыс. долларов для выполнения проектов в области зеленой химии, рассчитанных на один год. Каждый год будет объявляться новый конкурс. Заявки на получение грантов этого года принимаются до 28 февраля 2014 г.
PhosAgro/ UNESCO/ IUPAC Partnership in Green Chemistry for Life
Green chemistry has become a target for cutting-edge research into sustainable technologies. These may reduce (or eliminate) the production and use of hazardous substances in mining and in the design, manufacture and application of chemical products, and may also lead to energy savings and a better environment and health.
Research in green chemistry and allied areas in biochemistry, geochemistry, biotechnology, ecology and healthcare give young scientists ample opportunity to demonstrate their inventiveness and provide important input to sustainable development. With this in mind, the Green Chemistry for Life Project was launched in 2013 by UNESCO’s International Basic Sciences Programme (IBSP) and PhosAgro, the largest producer of phosphate-based fertilizer in Europe, in close cooperation with the International Union of Pure and Applied Chemistry (IUPAC).
Over the course of 5 years, the project will offer research grants of up to US$30,000 to scientists aged 35 years or less with an innovative research project that respects the 12 principles of green chemistry, to help them implement their project.


