22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы

Л. Юдина.
Катализ в интересах устойчивого развития. -
Российско-Голландский семинар
Премии и награды по химии
За рубежом
Показатели цитирования журналов по катализу
Глобальную проблему - катализ в интересах устойчивого развития, обсуждали на российско-голландском семинаре, состоявшемся 22-25 июля в Доме Ученых Академгородка, около ста специалистов в данной области. В научном форуме приняли участие ученые из шести университетов Голландии, шести российских университетов, Казахского национального университета им. Аль-Фараби, одиннадцати российских научных институтов, представители промышленных компаний той и другой сторон.
Предлагаем вниманию читателей заметку корреспондента газеты "Наука в Cибири" Л.Юдиной о прошедшем Российско-Голландский семинаре.

Двусторонние семинары по катализу - традиционная форма сотрудничества академических институтов различных стран. Их основная цель - обсуждение хода совместных исследований, планирование новых перспективных проектов. Впервые Институтом катализа Сибирского отделения РАН проведен семинар с каталитиками Голландии.
Я попросила профессора Зинфера Ришатовича ИСМАГИЛОВА, заведующего лабораторией экологического катализа Института катализа СО РАН, заместителя председателя оргкомитета, "подвести базу" под обоснованность данного научного события и остановиться на некоторых наиболее значимых его моментах.
- Сегодня Голландия, видимо, наиболее развитая из стран Европы в области фундаментальных и прикладных исследований по катализу. Пожалуй, это единственное место в мире, где исследования по катализу в шести университетах объединены в "виртуальный" Институт катализа Нидерландов (NIOK).
Когда мы начинали подготовку к семинару, то исходили из положения, что у нас давние и очень хорошие отношения. Институт катализа много лет плодотворно сотрудничает с учеными Нидерландов. Налажены прочные связи с университетами Амстердама, Эйндховена, Лейдена, Твенте, технологическим университетом Дельфта и Схутским институтом катализа. В цепочку, помимо академических и образовательных учреждений, включены и Энергетический центр Нидерландов (ECN) и промышленные компании.
В общей сложности, более десяти совместных проектов поддерживалось грантами разного уровня - и нашей страны, и Голландии, и международными.
Года два тому назад возникла идея провести семинар. Наиболее важные решения были приняты во время Международного конгресса по катализу в Испании, где присутствовали Председатель научного совета по катализу, директор Института катализа СО РАН академик В.Пармон и Президент "виртуального" Института катализа Нидерландов профессор Рутгер ван Сантен, а также другие руководители научных коллективов с российской и с голландской стороны.
Следующие переговоры состоялись на европейском конгрессе по катализу в Ирландии в 2001 году: установили точную дату проведения семинара, определили тематику и круг рассматриваемых проблем, оговорили все организационные моменты. Основными организаторами семинара выступили Институт катализа СО РАН, NIOK и Научный совет по катализу РАН. Ученый секретарь NIOK, доктор Ханс Баума, вел всю подготовку с голландской стороны.
Мы особо обращали внимание на то, чтобы соблюсти "баланс сил" - то есть было примерно равное число именитых ученых и тех, кого называют молодыми и перспективными.
- Сумели реализовать все положения программы?
- Абсолютно! Семинар превратился в полнокровную научную конференцию. Его участники отмечали высокий уровень докладов, особо оживленную атмосферу постерной сессии. Несомненно, определенный вес нашему мероприятию придал тот факт, что в Новосибирск приехал президент Королевского химического общества Нидерландов, один из крупнейших химиков-каталитиков мира, профессор Рутгер ван Сантен.
- Приезд господина ван Сантена, кажется, придал особую торжественность и другому событию, отмечаемому в рамках семинара, - юбилею Новосибирского отделения Российского химического общества им. Менделеева (РХО).
- Несомненно! На это юбилейное мероприятие, посвященное пятилетию областной организации РХО, были приглашены руководители ячеек Менделеевского общества в Новосибирске, представители городских властей, вузов, промышленных предприятий. Cобравшихся приветствовал вице-президент РХО, директор Института химии твердого тела и механохимии, член-корреспондент РАН Н.Ляхов. Председатель Новосибирского отделения общества, директор Института катализа академик В.Пармон сделал основательный доклад, в котором рассказал об истории химического общества в России, о работе областного отделения Менделеевского общества, назвал его основные вехи, знаменитых химиков, которые состояли в нем. Он отметил те положительные явления, которые наблюдаются в последние годы и способствуют возрождению Российского химического общества. Докладчик рассказал о планах "сибиряков-менделеевцев", о проведенном недавно конкурсе молодых лаборантов-аналитиков. Профессор Рутгер ван Сантен, обратившись с поздравлениями к собравшимся по торжественному случаю гостям, рассказал о деятельности Королевского химического общества Нидерландов.
В адрес тех, кто отмечал свой пятилетний юбилей, прозвучали многочисленные поздравления.
- В рамках научных мероприятий, как правило, проводит свою "конференцию" ИНТАС...
- И это вполне оправданно - ИНТАС поддерживает многие научные проекты. Финансово помощь организации, может быть, и не очень ощутима, но она чрезвычайно важна для создания научных сетей, как говорят на Западе, "карты дорог" - то есть для взаимодействия ученых, осуществления их контактов.
Сессия ИНТАС органично влилась в тематику семинара и его заседания, проходила очень активно.
- Вы как-то очень нетрадиционно начали работу, не по расписанию: с субботнего дня.
- Да, уже в субботу заслушивали пленарные лекции, активно работали в воскресенье и так далее. Приехали очень занятые люди, ограниченные во времени. В частности, профессор Рутгер ван Сантен, который кроме выше названных позиций еще является ректором Эйндховенского университета, мог выделить из своего напряженного графика только два выходных дня.
На семинаре прозвучало 9 пленарных лекций, 25 устных и 46 постерных докладов по следующим направлениям: научные основы катализа: теория, химия поверхности; гомогенный катализ; катализ для производства энергии из возобновляемого сырья; катализ для охраны окружающей среды; кинетика и моделирование химических реакторов. Все неизменно отмечали отличные выступления академиков В.Пармона, В.Казанского, профессоров Рутгера ван Сантена и Я.Муляйна. Речь шла о развитии катализа, успехах, достигнутых на сегодня. Доклады, даже сугубо фундаментальные, предлагали выходы в промышленность. Профессор З.Исмагилов свою лекцию полностью посвятил отчету по результатам выполненных исследований по грантам Министерства науки Нидерландов - NWO. Эта организация совместно с РФФИ ежегодно объявляет конкурсы на совместные Российско-Голландские проекты по различным научным направлениям, в этом году впервые объявлен конкурс по катализу, и видимо это не случайное совпадение с проведением первого совместного семинара по катализу.
В ходе работы семинара состоялась встреча ректора НГУ чл.-корр. РАН Н.Диканского и директора ИК СО РАН академика В.Пармона с ректором Эйндховенского университета проф. Р. ван Сантеном. Они обсудили возможные новые формы сотрудничества между университетами по подготовке студентов и аспирантов.
На церемонии закрытия научного форума выступающие с той и другой стороны, подчеркивая важность состоявшейся встречи, особо обращали внимание на новые возможности расширения контактов, осуществления крупных программ и проектов.
Закрытие ознаменовалось рождением новой традиции: каждому был вручен сертификат, подтверждающий факт участия в работе семинара "Катализ в интересах устойчивого развития".
Как шутя отмечали участники встречи, оргкомитет творчески подошел к организации мероприятия, запрограммировав много приятных неожиданностей. Даже красочно оформленное приглашение, на котором соседствуют традиционные голландские тюльпаны и русские ромашки, настраивало на определенный лад.
Известнейший ученый, голландский профессор Я.МУЛЯЙН так отозвался о прошедшем научном форуме:
- Семинар самого высокого качества. Проблемы, которые обсуждались на нем, весьма актуальны, насущны. Участники были чрезвычайно активны в дискуссиях.
Абсолютно очевидно, что NIOK и Боресковский институт катализа в Новосибирске являются комплементарными, то есть дополняют друг друга.
Голландский институт NIOK, комбинация кафедр катализа университетов, занимается фундаментальными проблемами катализа больше, чем Боресковский институт. Но тем не менее, взаимодействие голландских университетов и промышленности велико.
Ваш Институт катализа имеет очень много практически важных разработок. Наиболее яркий тому пример - разработка Г.Панова по использованию закиси азота. В настоящее время в Голландии много научных групп изучают аналогичные цеолитные катализаторы. А Боресковский институт уже сделал основанный на этом катализаторе процесс использования закиси азота для получения ценных продуктов.
Есть надежда, что в исследовательской группе профессора Муляйна скоро коммерциализируют такой же катализатор для разложения закиси азота в целях защиты окружающей среды - мы работаем с подобными образцами. Думаю, что взаимодействие двух институтов катализа - голландского и русского - имеет хорошее будущее. А научные встречи, подобные этому семинару, только способствуют развитию добрых отношений.
"Наука в Сибири" N30 (август 2002 г.
E.V. Murphree Award in Industrial & Engineering hemistry
Sponsored by ExxonMobil Research & Engineering Co. and ExxonMobil Chemical Co.
The work of many scientists can only be appreciated by colleagues in the same arcane specialty. The results of George R. Lester's labors, on the other hand, are familiar to hundreds of millions of people worldwide. He is considered to be one of the fathers of the catalytic converter, which destroys pollutants in automotive exhaust.
Born in War Eagle, W.Va., in 1934, Lester grew up in a coal camp. His father worked in the coal mine but was determined that his children would not follow him into the profession. Lester's opportunity to escape was provided by a tuition-free education at Berea College, where he earned a B.A. in chemistry in 1954. He continued his studies at the University of Kentucky, Lexington, earning an M.S. in 1956 and a Ph.D. two years later, both in physical chemistry.
Lester then joined Universal Oil Products as a senior research chemist. By 1974, he was manager of applied catalysis for the UOP Research Center. In 1976, he was promoted to the position of director of material science at the Signal UOP Research Center.
Eight years later, Lester returned to the lab as a research fellow with the company, which by then was called AlliedSignal Research & Technology. In this role, Good says, he studied the mechanisms by which catalysts become deactivated and designed catalysts that were less vulnerable to these processes.
He served as a senior research fellow-the company's highest technical position-from 1991 until his retirement from the firm in 1996. During the course of his career, he authored 45 patents.
Lester defied the constraints of middle age and took up flying when he turned 50. Since then he has racked up 1,300 hours of flight time, and he now pilots his own airplane.
The award address will be presented before the Division of Industrial & Engineering Chemistry. - Sophie Wilkinson
ACS Award for Creative Research in Homogeneous or
Heterogeneous Catalysis
Sponsored by Shell Oil Foundation
Jack H.Lunsford doesn't hesitate when asked about his start in science. "I had a good friend who lived a few doors away and he had a chemistry set," Lunsford recalls. "And in those days, chemistry sets were fun," he adds chuckling. "I really enjoyed working through the experiments and just generally playing around with it."
An excellent high school chemistry teacher furthered Lunsford's interest in science. He graduated from Texas A&M University in 1957 with a bachelor's degree and from Rice University in 1962 with a Ph.D., both in chemical engineering.
Lunsford served as a first lieutenant in the Air Force from 1962 to 1965 and then joined the chemistry faculty at Sam Houston State College. In 1966, he came to Texas A&M University as an assistant professor of chemistry and moved through the academic ranks, attaining the position of professor in 1971. In 1999, he was named distinguished professor of chemistry.
Researchers in heterogeneous catalysis have long recognized Lunsford's contributions to that field. He is well known for his insightful work on zeolites, for example. Using NMR and IR spectroscopy and catalytic probe reactions, Lunsford's research group deepened understanding of the nature of acidity and catalytic sites in zeolites. And the group's work demonstrated the important role of extra framework aluminum in zeolite-supported catalytic reactions.
Lunsford, 65 and now semiretired, is perhaps best known for his contributions in the area of methane activation. The pioneering work focused on oxidative coupling of methane to form ethane and subsequently ethylene. Through research published in a number of scientific papers, Lunsford elucidated the reaction mechanism demonstrating the role of surface-formed methyl radicals.
Catalytic applications of electron spin resonance spectroscopy and related techniques is another area in which Lunsford's work is well known. He and his students devised procedures to use the spectroscopic methods for investigating paramagnetic oxygen ions such as O-, O2-, and O3- and hydrocarbon radicals involved in catalytic reactions.
Bruce C. Gates, a professor of chemical engineering and materials science at the University of California, Davis, remarks that "no one has for so long so consistently produced thoughtful, innovative, and reliable research in heterogeneous catalysis. Jack's work places catalytic chemistry squarely in the center of chemistry".
The award address will be presented before the Division of Colloid & Surface Chemistry. - Mitch Jacoby
George A. Olah Award in Hydrocarbon or Petroleum Chemistry
Sponsored by the George A. Olah Endowment
Gary B. McVicker of ExxonMobil Research & Engineering Co. is being honored for his exemplary career in developing an understanding of the catalytic transformations of petroleum-derived hydrocarbons.
The vibrant nature of McVicker's work results from its being first-rate, rigorous, and well-done science. His pioneering research in the use of "smart" molecules to probe the properties of acid catalysts is accepted as definitive and has been adopted by many of his industrial and academic colleagues in their work. His early and still widely quoted papers on model compound studies of catalytic reforming of hydrocarbons clearly delineated the synergistic interaction of metal and acid sites in the bifunctional catalytic reaction pathways.
His landmark 1983 paper on isobutane conversion over both amorphous and zeolitic solid acids was the first to report that different catalytic functions are required for the initiation and propagation steps in paraffin cracking and isomerization reactions. McVicker was also among the first to identify the important role extra-framework alumina plays in promoting parafrin cracking over zeolitic solids.
In addition, McVicker, 60, has made outstanding contributions to other hydrocarbon conversion technologies. Notably, he worked on the development of platmum-iridium bimetallic reforming catalysts that were crucial to the phaseout of lead from gasoline in the U.S. His studies on staged metal and acid catalyst systems, which provided guidance for the optimization of each catalyst function, formed the basis for markedly improving the yield of high-octane motor gasoline. He continues to work on catalytic systems for producing reformulated gasoline.
McVicker is recognized as a leader in the team that developed a second-generaton Pt/KL n-hexane aromatization catalyst that exhibits a 100-fold lifetime improvement and incremental increases in both benzene yield and selectivity. McVicker recognized the need to enhance both platinum dispersion within the zeolite channels and the crystallinity and morphology of the KL zeolite support.
More recently, McVicker, has pioneered a new metal-catalyzed technology that selectively opens naphthene rings present in hydrotreated mid-distillate feed streams. The novel chemistry of this approach enhances both the yield and cetane value of the diesel fuel product without causing extensive loss in molecular weight via dealkylation of pendant ring substituents.
Born in Iowa, MeVicker received a B.S. in chemistry and mathematics from Upper Iowa University in 1963 and a Ph.D. in inorganic chemistry in 1968 from the University of Wyoming. He has been employed by ExxonMobil Research & Engineering Co. since then and is the author of nearly 50 technical papers and over 50 U.S. patents in a variety of areas. He also serves as a mentor for younger members of the technical staff and received the prestigious ExxonMobil Golden Tiger Award for such efforts.
Over the years, McVicker has received numerous other honors from his colleagues. He has served as director of the Catalysis Society of New York and is a director-at-large and vice president for the North American Catalyst Society. He received the 1995 Award for Excellence in Catalysis from the Catalyst Society of Metropolitan New Yark and the 2000 F. G. Ciapetta Lectureship Award from the North American Catalyst Society. He also serves on the editorial board of the Journal of Catalysis and chaired the 1994 Gordon Conference on Catalysis.
The award address will be presented before the Division of Petroleum Chemistry. - David Hanson
C&EN / January 21, 2002
Gabor Somorjai Receives National Medal of Science
Gabor Somorjai, Professor of Chemistry at the University of California, Berkeley was among a group of 15 recipients of the US National Medal of Science. This is the highest award for science and is presented by President Bush. As Rita Colwell, director of the National Science Foundation, said in 1998 "These are superstars in their respective fields. They've contributed a lifetime of stunning discoveries. We can only recognize them once with a science medal, but we applaud them daily for their continual contributions to humankind, to the reservoir of scientific knowledge and for the impact they have on the students they mentor and educate along the way." He has also been named University Professor at Berkeley. He becomes only the 23rd individual in the entire University of California system to be honored with this prestigious title. Previous holders of this distinction include Glenn T. Seaborg and Melvin Calvin.
Israel Wachs Receives 2001 Clean Air Excellence Award
Professor Israel Wachs of Lehigh University's Chemical Engineering Department has received a 2001 Clean Air Excellence Award. The EPA 2001 Clean Air Excellence Awards program honors outstanding, innovative efforts that help to make progress in achieving cleaner air. The research, sponsored by Georgia-Pacific Corp., has provided the pulp industry with a potentially profitable and innovative third alternative method of processing their waste gases. Using a new process and catalyst developed at Lehigh, the methyl alcohol and mercaptans can be converted to formaldehyde, a building-block chemical used for the adhesives, which find application in the plywood industry. [See www.pollutionengineering.com or N. Moretti's article in Pollution Engineering, Jan. 2002, pp 24-28]. The waste gases are simply processed through a plant, which is similar in design to a conventional formaldehyde plant that utilizes commercial-grade methyl alcohol as a feed material. The novel environmentally benign process was conceptually developed and experimentally proven on a laboratory scale (see US Patent Nos. 5,907,066 and 6,198,005 Bl to I.E. Wachs/Lehigh University). The pilot plant studies were performed at Georgia-Pacific's Brunswick, GA pulp mill on the real industrial waste streams.
Club/Society News
The Michigan Catalysis Society announces that Thomas J. Pinnavaia, the University Distinguished Professor, Michigan State University, has been selected as the winner of the 2002 Giuseppe Parravano Memorial Awards. Professor Pinnavaia gave the award lecture at the 24th annual spring symposium of the Michigan Catalysis Society: "Mesostructured Aluminosilicate Catalysts with Improved Accessibility, Acidity and Hydrothermal Stability". The 24th annual spring symposium organized by the Michigan Catalysis Society was held on Wednesday, May 15 at the Kettering University in Flint, Michigan. The symposium featured the Parravano award and the F.G. Ciapetta Lectureship in Catalysis Award Lecture by Dr. John Monnier, Research Fellow, Eastman Chemical Company, on the "Roles of Alkali and Halide Promoters for the Ag-Catalyzed Epoxidation of Butadiene". Presentations on the fundamentals of catalytic materials preparation, characterization, and mechanistic studies were given by students from Michigan State University, The University of Michigan, as well as researchers at the Dow Chemical Company. Another featured topic in this symposium was the lean NOx treatment for vehicles using plasma catalytic process. Researchers from Ford Research Labs and Delphi Research Labs discussed their recent findings on this challenging problem.
The Catalysis Division, The Canadian Society.
Michael Baird is this year's CIC Catalysis Award Winner. He will receive a rhodium-plated silver medal and travel expenses to present the Award Lecture at the Canadian Symposium on Catalysis. Prof. Baird was born in Hamilton, Ont., and received an Hon. B.Sc. from McMaster University in 1962, an M.A. and a Ph.D. from the University of Toronto in 1963 and 1965, respectively. After two formative and extremely productive postdoctoral years with the late Geoffrey Wilkinson, at Imperial College, London, Mike returned to Canada in 1967 to a position at Queen's University. Here he has remained except for six-month in 1975 as a Humboldt Fellow in the laboratory of E.O. Fisher at the Technische Universitat, München. He is currently Professor of Chemistry at Queen's, and was awarded the Queen's University Prize for Excellence in Research in the Physical and Applied Sciences in 1998. His research over the years has revolved around organotransition metal chemistry and catalysis, although he has on occasion delved into bioinorganic, medicinal and, currently, fullerene chemistry. He has worked with over fifty graduate students on research which has resulted in more than 200 publications and three patents, and was also awarded the Chemistry Departmental Student Council Prize for Excellence in Teaching in 1989 and 1994. He was elected a Fellow of the C.I.C in 1978, and won the Alcan Lecture Award of the C.I.C. in 1986.
The Catalysis Division of the Chemical Institute of Canada announces that Professor Warren Piers, Department of Chemistry, University of Calgary has been awarded the 2002 Canadian Catalysis Lectureship Award. In addition, Professor Harold Kung, Department of Chemical Engineering, Northwestern University (Evanstown, Ill) has been awarded the 2002 Cross-Canada Catalysis Lectureship Award. These awards are sponsored by the Canadian Catalysis Foundation, and consist of an honorarium and a travel grant to cover the costs of giving a series of lectures at universities and research institutes in Canada. The 17th Canadian Symposium on Catalysis will be held in conjunction with the Canadian Society of Chemistry meeting in Vancouver on June 1-5, 2002. The conference will cover most aspects of heterogeneous and homogeneous catalysis, with a strong focus on environmental issues. www.nacatsoc.org
The North American Catalysis Society-June, 2002
Iron-peroxide complex seen in lipoxygenase structure
The first X-ray crystal structure of the purple oxidized form of lipoxygenase supports the hypothesis that an iron(III)-peroxide complex is an intermediate in the reaction that the enzyme catalyzes, researchers at the University of Toledo report [J. Am. Chem. Soc., 123 10814 (2001)]. Lipoxygenases, which contain a nonheme iron coractor, catalyze the peroxidation of polyunsaturated fatty acids. Previous X-ray structures have been of "resting" forms of the enzyme, with its cofactor in the iron(II) state. Now, chemists Max O. Furik Jr., Ewa Skrzypczak-Jankun, and coworkers have obtained a crystal structure of the enzyme's purple active iron(III) form complexed to a lipid hydroperoxide. Although purple lipoxygenase is extremely sensitive to light and heat, the researchers were able to determine its structure by carrying out their experiments in the dark. The structure shows that the peroxide forms a covalent complex with iron via the peroxy group and occupies the sixth ligand position in the iron coordination sphere. The lipoxygenase that the Toledo team has been studying is from soybeans and differs in some significant ways from a lipoxygenase from rabbits, the researchers note. The factors that determine the enzymes' regio- and stereoselectivity likely will turn out to be different in the plant and mammalian cases, they suggest.
C&EN / November 12, 2001
Visible-light photocatalyst splits water
Sunlight-driven electrolysis of water to form O2 and H2 has been a longtime goal of inorganic photochemists, with the idea of efficiently producing hydrogen to power fuel cells. Transition-metal oxide semiconductors used as catalysts for this process generally absorb UV light, which accounts for only 4% of incoming sunlight, so the reactions aren't very efficient. Thus the greater goal has been to develop photocatalysts that absorb in the visible region, which is less energetic but accounts for about 43% of sunlight. In the latest step in that direction, Zhigang Zou and Hironori Arakawa of the National Institute of Advanced Industrial Science & Technology in Tsukuba, Japan, and coworkers report the solid-state synthesis of indium tantalum oxide semiconductors doped with nickel, In 1-x NixTaO4(x=0-0.2) [Nature, 414, 625 (2001)]. Nickel reduces the band-gap energy of the catalyst enough to push light absorption into the visible region but maintain the 1.23 eV needed to split water. The photocatalyst efficiency is less than 1%, however, which will need to be improved considerably to make the process practical.
New ligands for asymmetric catalysis
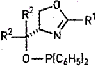
Metal complexes with simple phosphinite-oxazoline ligands are proving to be promising enantioselective catalysts. Ligands of the general structure shown can be prepared in a few steps from (S)-serine methyl ester. Chemists Chris Richards of Queen Mary University of London, and Geraint Jones of Cardiff University, in Wales, have explored the use of phosphinite-oxazolines as ligands in palladium-catalyzed allylic subsritutions [Tetrahedron Lett., 42, 5553 (2001)].With R2=H, they obtained the highest enantiomeric excesses (ee) when R1 is a relatively small ferrocenyl or phenyl group. At the University of Basel, in Switzerland, Andreas Pfaltz and Jorg Blankenstein are researching the same type of compounds as ligands for iridium-catalyzed enantioselective hydrogenation of alkenes [Angew. Chem.Int.Ed., 40, 4445 (2001)]. Their best results (up to 98% ee for unfunctionalized alkenes) were obtained with R1 = ferrocenyl or 3,5-bis(tert-butyl) phenyl and R2 = isopropyl, isobutyl or benzyl. "The modular, construction and the facile synthesis of these ligands should make it possible to tailor their structure for other substrates and other metal-catalyzed reactions," the Basel chemists write.
Simple setup yields 'nano-onions'
A simple new method yields high-quality "nano-onions", according to professor Gehan Amaratunga, research associate Manish Chhowalla, and coworkers in the department of engineering at the University of Cambridge [Nature, 414,506 (2001)]. The carbon particles could be useful as lubricants, Chhowalla says. The spherical nanoparticles, which range from 25 to 30 nm in diameter, have a C60 core surrounded by several carbon layers. Unlike other methods for generating carbon nanomaterials, this method does not require a vacuum system. The nanoparticles are generated by an arc discharge between two graphite electrodes submerged in water. The arc discharge is initiated by contacting a 5-mm pure graphite anode with a 12-mm cathode and generating a discharge voltage of 16 to 17Vand a discharge current of 30 amp. The nano-onions float on the water's surface, whereas other products sink to the bottom of the beaker. The production method could be adapted for industrial use by increasing the size of the apparatus, increasing the arc current, chilling and circulating the water, and automatically replenishing the consumable graphite anode, according to the researchers.
C&EN / December3, 2001
Siymyx furthers Dow,Rhodia collaborations
Symyx Technologies, which specializes in high-throughput combinatorial chemistry research, is expanding collaborarions with Dow Chemical and Rhodia. Symyx and Dow first teamed up in 1999, and the relationship has so far yielded two polyolefin catalysts that Dow is developing. Under the new agreement, Dow will have rights to market materials discovered by the companies and will pay Symyx royalties. Similarly, Symyx extended its collaboration with Rhodia in research related to specialty chemicals.
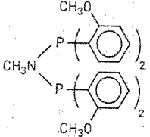
Trimerization catalyst boost
New ethylene trimerization catalyst systems based on diphosphine ligands offer an unprecedented combination of selectivity and activity, according to chemist Duncan F. Wass and coworkers at BP in the U.K. and the U.S. The team showed that, on activation with an alkyl aluminoxane, chromium complexes of ligands that bear ortho-methoxy-substituted aryl groups (such as the one shown) produce 1-hexene of almost 100% purity [Chem. Commun., 2002, 858]. 1-Hexene is an important comonomer for the production of linear low-density polyethylene. The purity of the olefin is a key factor in the process. "The higher selectivity of our systems is significant since it is difficult and costly to separate the various hexene isomers," Wass says. "Achieving more than 99.9% purity is virtually impossible by distillation." He points out that, at a given pressure, the new catalysts are some two orders of magnitude more productive than previous systems. The group hypothesizes that the potential for ortho substituents on the ligands to act as pendant donors and therefore increase the coordinative saturation of the chromium center is a critical factor in boosting catalyst performance.
C&EN / April22, 2002
Self-assembled carbon nanotubes
Self-assembled, honeycomb networks of carbon nanotubes for use in nanotechnology can be grown on oxidised silicon wafers, at around 8000C. The process uses a tunable chemical vapour deposition, with ferrocene (FeC10H10) and xylene (C8H10) as catalyst and carbon, precursors (catalyst: hydrocarbon ratio >0.2g/ml) see Figure. Prolonged deposition thickens the walls of the honeycombs, gradually filling the central holes and finally forming a packed him of vertically aligned carbon nanotubes (Z Zhang, B Wei & P M Ajayan, Chem Common 2002, 962).
Unprecedented telomerisation catalyst
The first reported monocarbene Pd(0) alkene complex (dmi)Pd0(dvds), available by treatment of the Pd(0) diallyl ether (dae) complex pd(dae)3 with dimesitylimidazol-2-ylidene carbene (dmi) in 1,1,3,3-tetramethyl-1,3-divinyl-disiloxane (dvds), at -300C, exhibits unprecedented high catalyst productivities and selectivities for a number of industrially important telomerisation reactions (R. Jackstell, M. G. Andreu, A. Frisch, K. Selvakumar, A. Zapf, H. Klein, A. Spannenberg, D. Rottger, O. Briel, R. Karch S. M. Beller, Angew Chem Int Ed Eng 2002,41.986). For example, yields and chemoselectivities greater than 98% and 99%, respectively, are observed for the (dmi)Pd0(dvds)-catalysed telomerisation of 1,3-butadiene with methanol, to give the 1-substituted 2,7-octadiene and a catalyst turnover number in excess of 98000 (900C, 1mol% NaOH, MeOH:butadiene ratio is 2: 1) see Scheme
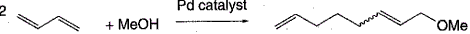
NO remuval trom exhaust gases
Increasingly stringent environmental legislation to reduce gaseous pollutants, especially nitrogen oxides from exhaust gases, requires the development of new catalysts. Medium-pore zeolites containing transition-metal ions (for example, CeO2-ZSM-5) are very active in such selective catalytic reduction (SCK) reactions, using various hydrocarbon reductants. The major problems in using these catalysts are the deactivatlon caused by the segregation of the active metal oxide from the framework, and coke formation. The presence of water at higher temperatures also results in a decrease in catalytic activity due to delumination. No such problems are encountered with 15wt% CeO2-H ferrite catalysts (surface area 128m2/g), prepared by physically mixing the components. Very high NO removals (to N2), up to 85%, are observed (for example, from a synthetic exhaust gas of composition - 1000ppm NO, 1200ppm C3H6, 10vol%, O2, 10vol% H2O and balance N2) over a broad temperature range (150-6000C). Furthermore, unlike other systems, the presence of water actually retards and reduces coke formation, as well as improving NO removal.
N2O emission control
In related studies, silver-nanoparticles supported over cobalt oxide (Co3O4) are highly active and stable catalysts for N2O decomposition to N2 and O2 (>70% conversion at 250*C), even in the presence of excess oxygen and steam. This makes an attractive end-of-pipe solution to reduce N2O emissions (L Yan, X Zhang. T Ren. H Zhang, X Wang & J. Suo, Chem Commun 2002, 860).
Phase-transfer catalyst recovery
Finally phase-transfer catalysis is a technique for conducting reactions between reaction partners located in separate, contiguous immiscible phases, employing a phase-transfer catalyst (PTC) such as a quaternary salt. After reaction is complete, the moderately expensive and mildly toxic catalyst must be separated from the product and recycled. This is usually accomplished by one of four separate methods: extraction, distillation, adsorption or binding to an insoluble support. Extraction is probably the most common method used to separate a PTC in commercial processes. Though water soluble, many PTCs need large amounts of wash water to be removed from the organic phase. This requires the evaporation of large amounts of water to concentrate the catalyst for recycling, or results in the loss of catalyst plus treatment and disposal of many litres of water per kg of product produced.
American researchers (X. Xie, J. S. Brown, P. J. Joseph, C. L. Liotta & C. A. Eckert, Chem Commun 2002, 1156) have discovered that CO2 can be used to enhance the environmentally benign, and allow efficient recovery of PTCs by aqueous extraction. This method can alter the distribution of PTCs so dramatically that even in dilute solutions they can be separated selectively from an organic reaction mixture, with typically less than 55% of the water required in a traditional extraction. At pressures less than 6000kPa, CO2, drives the widely used PTC benzyl triethylammonium bromide into the aqueous phase, altering the distribution coefficient by about 200-fold.
Chemistry&Industry-1 July 2002
Fe catalyses chlorophenol destruction fast
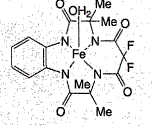
A new catalytic method has been described for the rapid degradation of chlorophenols, a class of compounds widely found in certain pesticides and wood preservatives (S Gupta et al. Science 2002, 296, 326). The combination of a macrocyclic iron complex (1) and excess hydrogen peroxide degraded 99.5% of an alkaline (pH10) aqueous solution of tri- and pentachlorophenol, within less than ten minutes (see Figure). Most of the chlorine ended up as inorganic chloride. Some still remained bound in the form of chlorinated maleic and malonic acids, but these by-products could be further destroyed at neutral pH, albeit at a slower rate. Catalyst: substrate ratios as little as 1:2000 were possible. Neither dioxmes or other toxic by-products were detected. The low toxicity and high efficiency of the iron catalyst makes this procedure extremely attractive for treating chlorinated environmental pollutants in waste streams.
Chemistry&Industry-1 August 2002
Предлагаем вниманию наших читателей показатели цитирования иностранных журналов по катализу, опубликованные в Journal of Catalysis и CATTECH.
Определения:
Импакт-фактор данного журнала можно выразить в виде дроби, где в числителе стоит количество статей опубликованных в первый и второй годы, а в знаменателе стоит количество ссылок, сделанных в третий год, на статьи, опубликованные в первый или второй годы. Так, если на каждую статью, опубликованную в 1997 или 1998 году, ссылались только один раз в 1999, импакт-фактор журнала будет равен 1.
Полупериод цитирования журнала - это период времени, выраженный в годах (отсчитывая назад от текущего года), в течение которого цитируемые статьи были опубликованы, и начиная с которого была сделана половина всех ссылок в текущем году. Иногда этот период называется "показатель устаревания", и указывает на время, в течение которого публикация сохраняет свою значимость. Следует отметить, что значимость может ограничиваться возрастом журнала или же изменением его названия.
Cattech v. 4, N2, 2000
ИМПАКТ-ФАКТОРЫ И ПОЛУПЕРИОД ЦИТИРОВАНИЯ (CATTECH)
| Impact factor | Citation half-time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Journal | 1995 | 1996 | 1997 | 1998 | 1999 | 1995 | 1996 | 1997 | 1998 | 1999 |
| Catalysis Reviews | 7.111 | 6.286 | 7.870 | 8.850 | 6.056 | >10 | 9,7 | >10 | >10 | >10 |
| Advances in Catalyst | 5.583 | nbsp; | 11.00 | 17.800 | 6.000 | >10 | >10 | >10 | ||
| Topics in Catalysis | 1.635 | 2.436 | 3.845 | 26 | 2.4 | 2.7 | ||||
| Chemical Communications | 3.107 | 3.200 | 3.407 | 3.477 | 6.5 | 6.3 | 6.3 | 6.0 | ||
| Journal of Physical Chemistry | 3.395 | 3.366 | 3.392 | 4.173 | 3.265 | 6.3 | 6.3 | 6.3 | 8.6 | 2.1 |
| Journal of Catalysis | 2,974 | 3.635 | 3,452 | 2.997 | 2.939 | 8.8 | 8.7 | 8.7 | 8.6 | 8.7 |
| Applled Catalysis B Environmental | 5.541 | 3.542 | 2.705 | 3.133 | 2.597 | 2.5 | 3.1 | 3.6 | 4.0 | 3.8 |
| Microporous Materials | 3.017 | 2.275 | 2.292 | 1.763 | 2.195 | 2.1 | 2.5 | 2.9 | 3.4 | 4.2 |
| Catalysis Letters | 1.917 | 2.546 | 2.252 | 2.171 | 2.051 | 3.3 | 3.5 | 3.9 | 4.4 | 4.6 |
| Zeolites | 1.989 | 1.918 | 2.255 | 1.771 | 1.992 | 6.0 | 6.3 | 6.7 | 7.2 | 8.4 |
| Journal of Molecular Catalysis - A Chemical | 1.178 | 1.478 | 1.657 | 1.744 | 1.4 | 2.0 | 2.4 | 3.0 | ||
| Catalysis Today | 1.760 | 1.791 | 1.537 | 1.860 | 1.723 | 3.9 | 4.2 | 4.6 | 4.5 | 4.5 |
| Applied Catalysis A General | 1.669 | 1.774 | 2.020 | 1.553 | 1.557 | 2.5 | 3.0 | 3.3 | 3.6 | 4.0 |
| AlChE Journal | 1.431 | 1.736 | 1.338 | 1.420 | 1.537 | >10 | >10 | >10 | >10 | >10 |
| Industrial Engineering Chemistry Research | 1.159 | 1.181 | 1.211 | 1.229 | 1.290 | 4.5 | 4.8 | 5.0 | 5.3 | 5.5 |
| Chemical Engineering Science | 1.033 | 1.405 | 1.083 | 0.998 | 1.218 | 9.6 | 9.7 | 9.5 | >10 | 9.6 |
| Journal ot Molecular Catalysis- B Enzymatic | 1.132 | 1.386 | 1.126 | 2.1 | 2.3 | |||||
| Studies in Surface Science and Catalysis | 0.565 | 0.905 | 0.883 | 0.698 | 0.655 | 4.7 | 5.2 | 4.3 | 4.6 | 5.1 |
| Reaction Kinetics and Catalysis Letters | 0.408 | 0.429 | 0.379 | 0.404 | 0.514 | 8.3 | 9.0 | 8.9 | 9.0 | 8.1 |
Источник:Institute for Scientific Information (ISI) Philadelphia, PA, USA
Импакт-факторы и полупериод цитирования (J. of Catalysis)
| за четыре года (1996-1999г.) | за 2000 год | |||
|---|---|---|---|---|
| Impact Factor | Half-Life (yr) | Impact Factor | Half-Life (yr) | |
| Catalysis Reviews | 7.3 | >10 | 6.6 | >10 |
| Journal of Physical Chemistry | 3.6 | 5.8 | 2.8/3.4 A/B | 2.5/2.6a |
| Journal of Catalysis | 3.3 | 8.7 | 3.0 | 8.8 |
| Chemical Communications | 3.3 | 6.3 | - | - |
| Applied Catalysis B: Environ | 3.0 | 3.4 | 3.0 | 4.0 |
| Langmuir | - | - | 3.0 | 4.4 |
| Topics in Catalysis | 2.6 | 2.5b | 3.2 | 2.8b |
| Microporous Materials | 2.3 | 3.3 | 1.8 | 2.0 |
| Catalysis Letters | 2.3 | 4.1 | 1.8 | 5.1 |
| Surface Science | - | - | 2.2 | 7.5 |
| Zeolites | 2.0 | 5.7 | - | - |
| Journal of Mol.Catal. A: General | 1.5 | 2.2 | 1.7 | 3.4 |
| Catalysis Today | 1.7 | 4.5 | 1.9 | 4.5 |
| Applied Catalysis A: General | 1.7 | 3.5 | 1.6 | 4.2 |
| AIChE Journal | 1.5 | >10 | - | - |
| Chemical Engineering Science | 1.2 | 9.7 | - | - |
| Journal Mol. Catal. B: Enzymatic | 1.2 | 2.2 | 1.4 | 2.5 |
| Studies in Surface Sci. Catal. | 0.8 | 4.8 | - | - |
Сроки публикаций (в месяцах) в журнале "Journal of Catalysis" за период с 1995 по 2001 г.г.
| Получены для рассмотрения | Одобрение на публикацию | Получены для опубликования | Количество представленных статей | |
|---|---|---|---|---|
| 1995 | 7.1 | 3.6 | 10.8 | - |
| 1996 | 7.3 | 4.5 | 11.9 | - |
| 1997 | 6.2 | 4.0 | 10.2 | - |
| 1998 | 4.4 | 3.9 | 8.8 | 560 |
| 1999 | 4.2 | 3.8 | 8.0 | 584 |
| 2000 | 3.9 | 3.7 | 7.6 | 588 |
| 2001 | 4.1 | 3.8 | 7.9 | 611 |