22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы

Краткая информация о конференциях в области катализа:
А.Д. Гохштанд
"Методы оценки стоимости объектов интеллектуальной собственности".
Новые конкурсы ИНТАС
За рубежом

Состоявшийся в Москве 28-30 мая 2003 г. семинар был организован Институтом катализа СО РАН, Калифорнийским университетом (Беркли, США), Российским фондом фундаментальных исследований и Научным советом по катализу РАН .
Главные темы семинара:
В работе семинара приняли участие 69 ученых из академических институтов и университетов России, Казахстана и 8 - из университетов и компаний США. На семинаре были представлены 7 пленарных лекций, 30 устных и 45 стендовых докладов, практически по всем научным направлениям семинара.
Большинство пленарных лекций было посвящено применению современных физико-химических и теоретических методов для изучения механизмов каталитических реакций и определения характеристик разработанных катализаторов.
Первая пленарная лекция "Применение метода EXAFS для определения локальной структуры Fe в катализаторе Fe-ZSM-5" была прочитана проф. А.Т. Беллом (Калифорнийский Университет, США). Было показано, что Fe в Fe-ZSM-5 присутствует в виде изолированных катионов, связанных с решеткой алюминия. Локальная структура активных центров существенно не изменяется при изменении отношения Fe/Al.
В лекции В.И. Бухтиярова (ИК СО РАН, Новосибирск) "Применение физических методов исследования поверхности для in-situ изучения механизмов гетерогенных каталитических реакций" были продемонстрированы возможности различных электронных спектрометров в сочетании с методом рентгеновской фотоэлектронной спектроскопии (РФЭС) для изучения механизмов адсорбции и реакции в условиях высоких давлений, характерных для реальных каталитических процессов. В качестве примеров изученных каталитических систем были представлены адсорбция СО и разложение CH3OH на монокристаллических и напыленных образцах палладия, а также парциальное окисление метанола в формальдегид на меди.
Проф. И.И. Иванова (МГУ им. Ломоносова) в пленарной лекции "In-situ ЯМР спектроскопия в гетерогенном катализе" дала обзор применения этого метода для изучения механизмов гетерогенных каталитических реакций. Преимущества метода были продемонстрированы на примерах реакций олефинов, спиртов, эфиров, алканов, и галогенсодержащих соединений на моно- и бифункциональных цеолитных и оксидных катализаторах.
Д-р Б.Л. Троут (Массачузетский Технологический Институт, США) в лекции "Определение активности кислотных цеолитов с помощью квантово-химических методов и методов молекулярной динамики" представил сложные вычислительные алгоритмы, предназначенные для выяснения на молекулярном уровне механизмов реакций на цеолитных катализаторах и для оценки констант скоростей элементарных стадий этих механизмов. Показано, что реакция получения этанола из метанола2 MeOH ® EtOH + H2O протекает в две стадии с устойчивыми промежуточными веществами, включающими несколько стадий переноса протона и заряда.
Академик В.Б. Казанский (ИОХ РАН, Москва) в пленарном докладе "Спектральное исследование необычной локализации и необычных сверхкислотных свойств бивалентных катионов в цеолитах с высоким отношением Si/Al" сообщил о необычных каталитических свойствах цеолитов типа ZSM-5, модифицированных бивалентными катионами различных металлов. Автор объясняет эффект модифицирования двухвалентными катионами (Zn, Cu, Co) изменением специфической структуры кислотно-основных центров высококремнеземных цеолитов, проявляющих свойства сверхкислот.
Д-р В.В. Гулянц (Университет Цинциннати, США) в лекции "Смешанные Mo-V-Te оксидные катализаторы для реакции окисления пропана в акриловую кислоту" представил результаты исследования поверхностной морфологии, кристаллической структуры, объемного и поверхностного составов, и каталитической активности смешанных Mo-V-Te оксидных катализаторов для селективного окисления пропана в акриловую кислоту. Окислительная каталитическая конверсия алканов в олефины и нитрилы на многокомпонентных ванадиевых молибдатах привлекает в последнее время особое внимание исследователей, как с точки зрения ее экономичности, так и с точки зрения охраны окружающей среды.
В лекции проф. Г.И. Панова (ИК СО РАН) "Азотистые оксиды: новые окислительные реакции в органической химии" представлены недавно полученные результаты по применению закиси азота в качестве селективного окислителя. Был обнаружен эффективный способ жидкофазного, некаталитического окисления алкенов с помощью N2O в ценные карбонильные соединения с селективностью ~ 100%.
Это явление основано на свойстве закиси азота напрямую взаимодействовать с двойной С=С связью и переносить свой кислородный атом (без участия катализатора) к ненасыщенному углероду алкенов. Применение N2O в качестве окисляющего агента, как в газовой, так и в жидкой фазах, открывает новые многообещающие возможности в тонком органическом синтезе, и может привести к новым фундаментальным открытиям в области органической химии.
Представленные на устной и стендовой секциях доклады были посвящены изучению фундаментальных и прикладных аспектов катализа.
В работе семинара были представлены работы как широко известных в области катализа ученых, так и молодых коллег. Большое число работ было посвящено исследованию механизмов каталитических превращений на молекулярном уровне, выяснению природы промежуточных соединений, созданию новых каталитических композиций с уникальными каталитическими и структурными свойствами, развитию новых методов исследования и контроля параметров катализаторов. Значительная часть докладов была посвящена применению каталитических методов в целях охраны окружающей среды. Большое внимание уделялось применению теоретических методов для моделирования каталитических реакций.
Следует отметить, что значительное число работ российских ученых было выполнено при финансовой поддержке РФФИ. Проведенный семинар продемонстрировал современное состояние науки о катализе как с теоретической так и экспериментальной точек зрения. Особо следует подчеркнуть мировой уровень проведенных исследований, как с американской, так и с российской стороны. Совершенно очевидно, что для эффективного использования дорогостоящего научного оборудования необходимо проведение совместных исследований, что было показано на примере ряда совместных докладов.
Семинар выявил основные современные тенденции развития науки о катализе:
На закрытии семинара было внесено и поддержано предложение сделать семинары регулярными и проводить их поочередно в России и США один раз в три года, включая в научную программу направления катализа, наиболее интенсивно исследуемые в России и США в последнее время.
Полные тексты отобранных пленарных лекций и устных докладов российских ученых будут опубликованы в журнале Topics in Catalysis.
В. Елохин и В. Городецкий
Институт катализа
им. Г.К. Борескова СО РАН
С 29 июня по 2 июля Дом ученых в новосибирском Академгородке стал местом проведения 2-го Всеросийского семинара "Топливные элементы и энергетические установки на их основе".
В семинаре приняли участие более 90 специалистов из крупнейших российских научно-исследовательских центров, представители заинтересованных федеральных министерств, правительства г. Москвы, ведущих российских производственных компаний, а также гости из Казахстана, Украины, США и Японии. Мероприятие было организовано Институтом катализа им. Г.К. Борескова СО РАН при поддержке Министерства промышленности, науки и технологий. РФ, Минатома России, Международного научно-технического центра (МНТЦ) и Научного совета по катализу Российской академии наук.

Проблема развития технологий топливных элементов (ТЭ) как базы для новой, значительно более эффективной и экологически чистой энергетики чрезвычайно актуальна. Семинар привлек внимание ведущих ученых в данной области, представителей министерств и деловых кругов, включая такие компании, как Газпром и АвтоВАЗ, которые напрямую заинтересованы в скорейшем внедрении и коммерциализации этих технологий. Его участники представили на суд коллег более 80 докладов и стендовых презентаций, охвативших все аспекты развития и применения технологий ТЭ. Основные докладчики выступили с подробным обзором своих исследований, касающихся протон-обменных, твердооксидных и расплавно-карбонатных батарей ТЭ и топливных реформеров, преимуществ и проблем внедрения технологий ТЭ в автомобильную промышленность и другие области индустрии.
Представителями МНТЦ была проведена презентация совместной целевой инициативы МНТЦ и Минатома России в области энергоустановок на базе ТЭ, которая вызвала заинтересованный обмен мнениями участников семинара.
Участники семинара пришли к единодушному выводу, что современное состояние научных исследований в области ТЭ позволяет уже сейчас сделать следующий шаг и перейти к разработке интегральных установок на их основе.
В рамках семинара состоялся "Круглый стол", посвященный обсуждению прикладных проблем разработки и применения эне-ргоустановок на топливных элементах в нашей стране.

Участники семинара отмечают отсутствие водородной энергетики в Перечне критических технологий Российской федерации и крайне ограниченную государственную финансовую поддержку работ в области топливных элементов, что отрицательно сказывается на сроках создания отечественных энергоустановок на основе топливных элементов.
В целях ускорения создания отечественных энергоустановок на основе топливных элементов участники семинара "Топливные элементы и энергоустановки на их основе" рекомендуют:

Продолжаем публикацию наиболее важных материалов прошедшего в Новосибирске (25-28 февраля с.г.) семинара "Современное состояние и перспективы промышленной реализации результатов научных исследований". Предлагаем вниманию читателей лекцию А.Д. Гохштанда "Методы оценки стоимости объектов интеллектуальной собственности"
А.Д. Гохштанд
ЗАО "Экспертиза и оценка", Москва
тел.: (095) 7918916, Е-mail: agoh@rambler.ru
Обычно результаты интеллектуальной деятельности, предназначенные к реализации, и приравненные к ним средства индивидуализации называются объектами интеллектуальной собственности (ОИС).
К ОИС относятся три группы объектов с различным правовым режимом:
Интеллектуальная собственность - товар особого рода. Его отличия от материальных объектов (движимого и недвижимого имущества) заключаются в следующем: этот товар
В правовом отношении различаются следующие виды прав на ОИС:
Имущественные права на товары (Т), работы (Р) и услуги (У), в которых использованы объекты интеллектуальной (И) собственности (С) - ТРУИС, не являются интеллектуальной собственностью. Переход права собственности на товары, работы и услуги сам по себе не влечет передачи имущественных прав на результаты интеллектуальной деятельности, выраженные в них.
Интеллектуальная собственность, оцениваемая и учитываемая в хозяйственном обороте, это имущественные права, т.е. права коммерческого использования ОИС, как исключительные, так и неисключительные (п.п. 3, 4, 5).
Личные неимущественные права не участвуют в хозяйственном обороте, это моральные права, которые не могут быть проданы и поэтому не являются интеллектуальной собственностью.
К числу имущественных прав относятся:
Имущественные права возникают в силу:
Обладание имущественными правами является необходимым, но не достаточным условием освоения и реализации новых технологий на основе ОИС. Эту возможность еще необходимо превратить в действительность, добиться более высокой по сравнению с имеющимися технологиями доходности от ОИС.
Освоение новых ОИС и, соответственно, новых технологий чрезвычайно сложный и, как правило, длительный процесс, связанный с высоким риском. Не всякая ОИС доходит до стадии превращения в новую технологию, обеспечивающую получение чистого дохода с нужным уровнем доходности.
Результативность новых технологий проявляется, в конечном счете, в экономических характеристиках ТРУИС, в реализации ТРУИС и получении за счет этого дополнительной прибыли (дохода).
Рассмотрим этапы экономического оборота интеллектуальной собственности.
Первый этап - это создание результата интеллектуальной деятельности, т.е. объекта интеллектуальной собственности.
Второй этап заключается в получении исключительных имущественных прав на ОИС в соответствии с патентным и авторским правом или в обеспечении конфиденциальности.
Третий возможный этап - распоряжение имущественными правами путем выдачи лицензий или передачи прав в качестве вклада в уставный капитал предприятия или другими способами.
Четвертый по последовательности этап экономического оборота - работы по доведению ОИС до состояния, в котором он может быть использован в запланированных целях.
Пятый этап - организация производства ТРУИС.
Шестой этап - реализация ТРУИС.
И, наконец, седьмой этап - использование ТРУИС конечными потребителями.
Центральное место при создании ОИС занимает владелец исключительных имущественных прав на ОИС, охватывающих все возможные способы и территории использования, весь срок действия прав по закону, т.е. правообладатель.
Правообладателем может стать или автор, или организация-разработчик, или заказчик, финансировавший создание ОИС, или лицо, которому полностью уступлены исключительные имущественные права.
Правообладателем может быть и государство через свои органы, если оно финансирует создание ОИС из своих бюджетных и внебюджетных средств, а также в некоторых других случаях, предусмотренных законодательством.
Через реализацию ТРУИС компенсируются все затраты на создание ОИС и подготовку их к коммерческому использованию и поступают доходы от использования ОИС. От соотношения общих затрат на создание ОИС и подготовку производства ТРУИС и доходов от реализации ТРУИС зависит их экономическая эффективность.
Правообладатель определяет стратегию использования своей интеллектуальной собственности, при этом он обычно исходит из всего потенциала дохода, который может быть получен, и должен учитывать всю совокупность предстоящих затрат.
В большинстве случаев правообладатель самостоятельно не может реализовать весь проект с использованием ОИС: на это нужны инвестиции, производственная база и ряд других условий, которыми он может не располагать. Поэтому правообладатель передает за определенное вознаграждение часть или все имущественные права на ОИС другим юридическим или физическим лицам, обладающим необходимыми ресурсами. При этом распределяется не только рынок ТРУИС и, следовательно, будущие доходы, но и затраты на подготовку ОИС к практическому использованию.
На этой стадии - передачи правообладателем части прав другому лицу правополучателю - возникает важная проблема множественности оценок интеллектуальной собственности.
Цена на товар-вещь обычно зависит только от коммерческих условий реализации. Цена товара "интеллектуальная собственность", кроме этого, зависит от 4 групп специфических факторов.
1-я группа факторов определяет объем передаваемых или оцениваемых имущественных прав (см.п.п. 3, 4, 5 перечня прав).
2-я группа факторов определяет степень готовности ОИС к практическому использованию.
3-я группа определяет степень риска, вызываемого неопределенностью экономических параметров и условий реализации ТРУИС в результате внедрения нововведений.
4-я группа определяет степень риска, вызываемого недобросовестной конкуренцией в области интеллектуальной собственности.
Оценка интеллектуальной собственности зависит, прежде всего, от объема передаваемых прав, т.е., как ранее отмечалось, от величины передаваемого потенциального рынка ТРУИС и, соответственно, доходов от него. Далее, оценка интеллектуальной собственности зависит от уровня готовности ОИС к практическому использованию. Естественно, что на ОИС на уровне поисковой работы затрачивается меньше средств, чем на ОИС, прошедшем практическую обкатку и готовом к применению. При этом затраты на создание ОИС по уровню готовности к применению могут различаться в разы и даже на порядки. Из этого следует, что соотношение собственно интеллектуальных и прочих затрат по созданию ОИС и затрат по приведению ОИС к готовности для практического использования и по подготовке и освоению производства и реализации ТРУИС может колебаться в очень широких пределах.
ОИС реализуется в новых ТРУИС или в новых технологиях и, как у всякого новшества, его будущие экономические характеристики имеют высокую степень неопределенности. Это относится и к объему рынка применения новшества и к себестоимости и цене на ТРУИС, к их потребительским и эксплуатационным свойствам.
Оценка интеллектуальной собственности во многом зависит и от рисков недобросовестной конкуренции и пиратства и возрастает в меру производимых затрат по защите своих прав. Объекты авторского и патентного права зачастую легко тиражируемы, вероятность неправомерного их использования может быть достаточно велика и это снижает стоимость интеллектуальной собственности.
Стоимостная оценка интеллектуальной собственности состоит в конечном счете в определении чистой текущей стоимости, т.е. в определении разницы между дисконтированными потоками будущих доходов и расходов, ожидаемых от реализации инновационного проекта.
При использовании исключительных прав на ОИС или распоряжении ими возможны различные виды соглашений:
Лицензирование - это предоставление владельцем интеллектуальной собственности другому лицу (юридическому или физическому) разрешения на использование ОИС в коммерческих целях.
Стоимостная оценка прав на ОИС в большинстве случаев определяется по дополнительной прибыли, которую покупатель лицензии ожидает получить от использования ОИС на своем предприятии. Конечная цель покупателя лицензии состоит в получении конкурентных преимуществ на рынке от использования ОИС. Для партнеров по лицензионной сделке главное - это получение такого размера доходов, который покроет их расходы на разработку ОИС, покупку- продажу имущественных прав на реализацию предмета лицензии, а также обеспечит получение ими дополнительной прибыли в течение срока действия договора.
В качестве источников получения доходов от использования интеллектуальной собственности в зависимости от типа ОИС могут учитываться:
При определении цены лицензии обычно учитываются следующие факторы:
При определении цены лицензии используются традиционные для стоимостной оценки подходы: затратный, сравнительный (рыночный) и доходный.
Затратный подход заключается в определении стоимости оцениваемых имущественных прав на использование ОИС величиной затрат на создание, воссоздание или замещение этой ОИС в текущих ценах. Затратный подход реализуется при оценке с помощью метода стоимости создания (воссоздания, замещения) ОИС
Метод стоимости создания ОИС
Стоимость определяется суммированием всех затрат, произведенных при создании (воссоздании, замещении) ОИС, оцененных в текущих ценах и увеличенных на принятый коэффициент рентабельности (ставку дохода на инвестиции). При необходимости из этой величины вычитается накопленная амортизация ОИС.
При оценке по этому методу производятся следующие работы:
Этот метод применяется или в качестве одного из возможных методов оценки или в виде теста для проверки значений стоимости ОИС. Этот метод используется для оценки НИОКР, проектно-изыскательских и прочего вида работ.
Сравнительный подход основан на принципах эффективно работающего рынка, на котором покупаются и продаются аналогичного вида активы, а субъекты права принимают независимые индивидуальные решения, он реализуется с помощью метода сравнительного анализа продаж.
Метод сравнительного анализа продаж
При оценке по этому методу проводятся следующие работы:
Этот метод применяется для определения стоимости франшиз и товарных знаков (знаков обслуживания).
Доходный подход основан на расчете экономических выгод, ожидаемых от использования прав на оцениваемые ОИС. Он заключается в определении стоимости оцениваемой лицензии - текущей стоимостью чистого дохода, который может быть получен от использования ОИС за экономически обоснованный срок его службы.
Этот подход базируется на следующих операциях:
У этого подхода имеются две разновидности:
В доходном подходе используются следующие методы
Метод дополнительной прибыли
Стоимость имущественных прав на использование ОИС в этом методе определяется капитализацией дополнительной прибыли (чистого дохода), получаемой от продажи ТРУИС, имеющих преимущества в конструкции, составе, дизайне, потребительских характеристиках и т. п., за счет использования ОИС: изобретений, промышленных образцов, товарных знаков, фирменных наименований и т.п. У этого метода есть две разновидности. Дополнительная прибыль может получаться за счет надбавок (повышенной рентабельности) в цене ТРУИС или за счет расширения рынка продаж при сохранении цены изделия на уровне цен-конкурентов.
Метод экономии себестоимости (выигрыша в себестоимости)
Стоимость имущественных прав на использование ОИС в этом методе определяется капитализацией прибыли (чистого дохода), получаемой от экономии себестоимости при использовании ОИС технологического, производственного или управленческого назначения: изобретений на способ производства, ноу-хау и других ОИС. Применение таких ОИС позволяет экономить трудозатраты, затраты на материалы, энергию и накладные расходы.
Метод избыточных прибылей
При этом методе предполагается, что использование имущественных прав на ОИС позволяет предприятию получать прибыли, превышающие нормативный или средний уровень, характерный для данного производства.
Основные этапы расчета стоимости имущественных прав на использование ОИС методом избыточных прибылей:
При оценке интеллектуальной собственности используется еще один метод, который носит название метод освобождения от роялти (иногда он называется методом стандартных роялти). Этот метод является сочетанием сравнительного и доходного подходов, при этом рыночным путем определяется объем использования ОИС и ставка роялти. Как уже отмечалось, роялти - это вознаграждение лицензиару в виде фиксированных сумм или ставок от стоимости реализуемой лицензиатом продукции, изготовленной по лицензии. В ставках роялти содержится доля лицензиара в дополнительной прибыли лицензиата, определяемая не расчетным путем, а эмпирически, путем применения усредняемых на основе мирового опыта размеров роялти для различных отраслей промышленности, обычно называемых стандартными ставками роялти.
Виды платежей за лицензии. В лицензионной торговле применяются три вида выплаты вознаграждения по лицензии:
При определении лицензионного вознаграждения в форме роялти важным является выбор базы расчета, в качестве которой может быть цена продукции, выпускаемой по лицензии, объем производства продукции по лицензии, прибыль или другая выгода, себестоимость. При составлении лицензионного договора чаще всего в качестве базы для определения роялти используется цена продукции, выпускаемой по лицензии. Фактические цены и объемы реализуемой продукции позволяют реально контролировать использование лицензии. Между партнерами по лицензионной сделке в этом случае реже всего возникают конфликты по выплатам вознаграждения.
Для стимулирования лицензиата в увеличении объемов производства и реализации продукции нередко используются "скользящие", т.е. дифференцированные ставки роялти, уменьшающиеся по продукции, выпускаемой сверх оговоренного сторонами объема производства.
Роялти как форма платежа выгодна в большей степени для лицензиата, чем для лицензиара. Лицензиат выплачивает лицензионное вознаграждение после получения доходов от выпуска продукции по лицензии, лицензиар же вынужден тратить свои средства на подготовку документации, доработку технологии, инженерные услуги без получения какой либо компенсации до начала производства продукции по лицензии. Лицензиар не получает гарантий компенсации своих расходов при недостаточном объеме реализации продукции или досрочном расторжении лицензионного договора. Чтобы защитить свои интересы лицензиар обычно настаивает на включении в договор требования о выплате лицензиатом ежегодных минимально-гарантированных платежей, которые обычно составляют до 50 % от суммы ежегодного платежа по роялти.
Паушальные платежи представляют собой сумму, которая выплачивается либо в виде единовременного платежа, либо по частям: при вступлении договора в силу, в момент передачи технической документации и после начала выпуска продукции по лицензии.
Цена лицензии при паушальном платеже определяется по той же методике, что при платежах в форме роялти, с учетом разницы в сроках получения вознаграждения, т.е. с применением коэффициента дисконтирования.
В применении паушальной формы платежа заинтересован лицензиар, так как он быстро и в полном объеме получает лицензионное вознаграждение, но при этом он теряет возможность получения дополнительных сумм при превышении расчетных объемов производства продукции по лицензии.
Комбинированные платежи - это сочетание единовременных платежей с периодическими на базе роялти. Первоначальный платеж на начальном этапе реализации договора предусматривает оплату расходов лицензиара на подготовку и передачу технической документации и других расходов, понесенных им на стадиях предшествующих заключению договора. Единовременный первоначальный платеж обычно составляет от 10 до 30 % от цены лицензии, рассчитанной на базе роялти. Комбинированная форма платежа за лицензию наиболее часто используется в международных лицензионных сделках, так как позволяет наиболее полно учесть интересы сторон.
При передаче имущественных прав на ОИС в уставный капитал предприятия в отличие от лицензионного договора не предусматривается прямого вознаграждения. Величина стоимости интеллектуальной собственности, вносимой в уставный капитал предприятия, это номинальная оценка, определяющая долю, пакет акций в уставном капитале, на которые согласился обладатель интеллектуальной собственности при создании предприятия для будущего распределения предполагаемых доходов от использования новшества.
Далеко не всегда номинальная стоимость интеллектуальной собственности, и, соответственно, ее доля в уставном капитале предприятия совпадает с реальной оценочной, но последняя должна превышать первую.
Общая схема стоимостной оценки вклада интеллектуальной собственности в уставный капитал предприятия обычно выглядит следующим образом.
Для организации предприятия необходимо создать имущественный комплекс в составе:
Для каждой составляющей имущественного комплекса, кроме интеллектуальной собственности, вносимой в уставный капитал предприятия, устанавливаются рыночные стоимость и норматив доходности, для определения рыночных, нормализованных доходов от использования материальных и денежных ресурсов.
Затем определяется предполагаемая доходность от деятельности имущественного комплекса или проекта с использованием интеллектуальной собственности по годам за весь расчетный срок действия проекта.
Из общего расчетного дохода вычитаются нормализованные доходы, полученную разность обычно называют избыточной прибылью. Избыточные прибыли, ассоциирующиеся с интеллектуальной собственностью, дисконтируются по годам, а сумма, полученная за весь расчетный срок действия проекта, принимается за оценочную стоимость интеллектуальной собственности. Этот метод, как уже отмечалось, называется методом избыточных прибылей.
Описанная выше схема расчета предполагает, что рентабельность инновационного проекта или предприятия будет существенно выше, чем обычные коммерческие и обычные инвестиционные проекты, в противном случае повышенные инновационные риски будут неоправданными.
Стоимость интеллектуальной собственности, вносимая в уставный капитал предприятия, - это инвестиционная стоимость, которая может быть определена при помощи методов доходного, сравнительного или затратного подходов.
По существу для стоимостной оценки интеллектуальной собственности, вносимой в уставный капитал предприятия, необходимо разработать бизнес-план инновационного проекта: провести анализ рынков сбыта, составить производственные планы, планы маркетинга, финансовые планы и т.д.
В конечном счете, следует определить эффективность инновационного проекта и на его основе стоимость интеллектуальной собственности, вносимой в качестве вклада в уставный капитал предприятия.
При определении стоимости интеллектуальной собственности большое значение имеет учет рисков при использовании ОИС.
Риски специфичные для инноваций:
Стоимостная оценка инновационных рисков может определяться затратами на защиту прав и секретов, а также стоимостью убытков от несанкционированного использования ОИС.
Следует различать риски инвестора и риски владельца интеллектуальной собственности. Первый рискует капиталом и доходом на него, второй - только доходом от использования ОИС. Это необходимо учитывать при распределении долей в уставном капитале предприятия.
На практике используются различные методы анализа и учета рисков при оценке интеллектуальной собственности. К наиболее распространенным относятся: метод использования нормы дисконта и метод коэффициентов достоверности.
Метод использования нормы дисконта является самым простым и поэтому широко применяемым на практике. В этом методе к минимально приемлемой ставке дисконта прибавляется премия за риск, при этом, чем выше риск, ассоциированный с интеллектуальной собственностью, тем выше величина премии, которая может определяться экспертным путем или по формальным процедурам.
Типичная норма дисконта (ставка доходности) КД может рассматриваться как сумма трех слагаемых, а именно свободная от риска учетная ставка - У, поправка на ожидаемую инфляцию - И, и поправка на риск - Р, а их связь может быть представлена уравнением <1+КД=(1+У)*(1+И)*(1+Р)
Например, свободная от рисков ставка составляет-10 %, ожидаемая инфляция -10 %, а поправка на риск 8 %, то <1+КД= (1+0,1)*(1+0,1)*(1+0,8)=1,3,
т.е. необходимая ставка доходности составляет 30 %.
Метод корректировки нормы дисконта предполагает постоянство риска во времени, хотя для многих проектов с использованием ОИС характерно наличие высоких рисков в начальные периоды и их постепенное снижение к концу реализации проекта. При существенно отличающихся рисках на разных стадиях освоения новшества использование единой ставки доходности может привести к серьезным ошибкам при определении оценочной стоимости.
Более правильным было бы разделить поправки на время и риск, при этом денежные потоки в конкретном году корректировались бы принятой для этого года поправкой на риск. При этом методе корректируются обычно не нормы дисконта, а ожидаемые денежные потоки путем применения специальных понижающих коэффициентов (коэффициентов достоверности)
Таким образом осуществляется приведение ожидаемых денежных поступлений к достоверному уровню, который не вызывает сомнений или который определяется более или менее точно. На практике коэффициенты достоверности часто определяют экспертным путем.
Введением в действие положений Налогового Кодекса (НК) определены основные положения по бухгалтерскому учету в целях налогообложения. В них дана единая терминология, технология и правила бухгалтерского учета вообще и налогового учета в частности. В них определены и основы бухгалтерского учета интеллектуальной собственности.
Налоговым Кодексом определены понятия объектов налогообложения и, прежде всего, такие как имущество, товар, работа и услуга (п. 2, 3, 4, и 5 ст.38 НК).
Под имуществом понимаются виды объектов гражданских прав, относящихся к имуществу. В соответствии со ст. 128 ГК к объектам гражданских прав относятся вещи, включая деньги и ценные бумаги, иное имущество, в том числе имущественные права; работы и услуги; информация; результаты интеллектуальной деятельности, в том числе исключительные права на них (интеллектуальная собственность); нематериальные блага.
Товаром признается любое имущество, реализуемое либо предназначенное для реализации.
Работой признается деятельность, результаты которой имеют материальное выражение и могут быть реализованы для удовлетворения потребностей организации и (или) физических лиц. К этому виду относятся научно-технические работы.
Услугой признается деятельность, результаты которой не имеют материального выражения, реализуются и потребляются в процессе осуществления этой деятельности.
Налогом облагается имущество организации, добавленная стоимость и прибыль, получаемая при продаже товаров, работ, услуг и имущественных прав. Кроме того, организация платит единый социальный налог. Действует также налог на доходы физических лиц.
Результаты интеллектуальной деятельности, включая интеллектуальную собственность, при использовании организацией в своей деятельности, могут быть отнесены к амортизируемому имуществу.
Амортизируемым имуществом признаются имущество, результаты интеллектуальной деятельности и иные объекты интеллектуальной собственности, которые находятся у налогоплательщика на праве собственности (если иное не предусмотрено настоящей главой), используются им для извлечения дохода, и стоимость которых погашается путем начисления амортизации. Амортизируемым имуществом признается имущество со сроком полезного использования более 12 месяцев и первоначальной стоимостью более 10 000 рублей. Нематериальными активами признаются приобретенные и (или) созданные налогоплательщиком результаты интеллектуальной деятельности и иные объекты интеллектуальной собственности (исключительные права на них), используемые в производстве продукции (выполнении работ, оказании услуг) или для управленческих нужд организации в течение длительного времени.
Для признания нематериального актива необходимо наличие способности приносить налогоплательщику экономические выгоды (доход), а также наличие надлежаще оформленных документов, подтверждающих существование самого нематериального актива и (или) исключительного права у налогоплательщика на результаты интеллектуальной деятельности (в том числе патенты, свидетельства, другие охранные документы, договор уступки (приобретения) патента, товарного знака). К нематериальным активам, в частности, относятся:
1) исключительное право патентообладателя на изобретение, промышленный образец, полезную модель;
2) исключительное право автора и иного правообладателя на использование программы для ЭВМ, базы данных;
3) исключительное право автора или иного правообладателя на использование топологии интегральных микросхем;
4) исключительное право на товарный знак, знак обслуживания, наименование места происхождения товаров и фирменное наименование;
5) исключительное право патентообладателя на селекционные достижения;
6) владение "ноу-хау", секретной формулой или процессом, информацией в отношении промышленного, коммерческого или научного опыта.
Первоначальная стоимость амортизируемых нематериальных активов определяется как сумма расходов на их приобретение (создание) и доведение их до состояния, в котором они пригодны для использования, за исключением сумм налогов, учитываемых в составе расходов. Стоимость нематериальных активов, созданных самой организацией, определяется как сумма фактических расходов на их создание, изготовление (в том числе материальных расходов, расходов на оплату труда, расходов на услуги сторонних организаций, патентные пошлины, связанные с получением патентов, свидетельств), за исключением сумм налогов, учитываемых в составе расходов.
К нематериальным активам могут быть отнесены и имущественные права, полученные по договорам на использование интеллектуальной собственности (лицензионный и авторский договоры). Об этом свидетельствуют следующие положения гл.25 НК:
Ст.256. "...Не подлежат амортизации следующие виды амортизируемого имущества:
8) приобретенные права на результаты интеллектуальной деятельности и иные объекты интеллектуальной собственности, если по договору на приобретение указанных прав оплата должна производиться периодическими платежами в течение срока действия указанного договора."
"В качестве основного условия отнесения имущества к амортизируемому является принадлежность этого имущества налогоплательщику на праве собственности, приобретенном в установленном законом порядке.
Исключение составляет лизинговое имущество, числящееся на балансе лизингополучателя." (Методические указания МНС к ст.256 НК).
В состав внереализационных расходов (ст.265 НК), не связанных с производством и реализацией, относятся, в частности: расходы на содержание переданного по договору аренды (лизинга) имущества (включая амортизацию по этому имуществу).
Для организаций, предоставляющих на систематической основе за плату во временное пользование и (или) временное владение и пользование свое имущество и (или) исключительные права, возникающие из патентов на изобретения, промышленные образцы и другие виды интеллектуальной собственности, расходами, связанными с производством и реализацией, считаются расходы, связанные с этой деятельностью"
Из этих положений следует, что приобретенные права на результаты интеллектуальной деятельности и иные объекты интеллектуальной собственности, если по договору о приобретении указанных прав оплата производится фиксированными (единовременными платежами), относятся, во-первых, к амортизируемому имуществу и, во-вторых, это имущество амортизируется. Договоры на передачу во временное пользование и \ или во временное владение и пользование исключительных прав, возникающих из патентов на изобретения, промышленные образцы и другие виды интеллектуальной собственности приравниваются к договорам аренды (лизинга), и что эти договора относятся к числу исключений при отнесении этих имущественных прав к амортизируемому имуществу, одним из условий принадлежности к которому является владение им по праву собственности.
К нематериальным активам не относятся:
1) не давшие положительного результата научно-исследовательские, опытно-конструкторские и технологические работы;
2) интеллектуальные и деловые качества работников организации, их квалификация и способность к труду.
Амортизация по объектам амортизируемого имущества начисляется и учитывается в целях налогообложения в уменьшение налоговой базы до полного списания стоимости объекта или до выбытия объекта из состава амортизируемого имущества по любым основаниям (продажа, ликвидация и т.п.).
Амортизируемое имущество облагается налогом на имущество по действующей в настоящее время ставке 2 %.
Налог на добавленную стоимость (гл.21 НК) берется со всех операций с результатами интеллектуальной деятельности, включая интеллектуальную собственность, за исключением:
К учреждениям культуры и искусства относятся театры, кинотеатры, концертные организации и коллективы, театральные и концертные кассы, цирки, библиотеки, музеи, выставки, дома и дворцы культуры, клубы, дома (в частности, кино, литератора, композитора), планетарии, парки культуры и отдыха, лектории и народные университеты, экскурсионные бюро (за исключением туристических экскурсионных бюро), заповедники, ботанические сады и зоопарки, национальные парки, природные парки и ландшафтные парки (НК, Статья 149. п.2 п.п.20 и п.п.21).
Не подлежат налогообложению (НДС) операции:
Налогообложению подлежит прибыль, получаемая от продаж продукции, работ, услуг, полученных с использованием результатов интеллектуальной деятельности, и имущественных прав на интеллектуальную собственность.
Прибылью признаются полученные доходы, уменьшенные на величину произведенных расходов.
К доходам относятся:
1) доходы от реализации товаров (работ, услуг) и имущественных прав,
2) внереализационные доходы.
При определении доходов из них исключаются суммы налогов, предъявленные покупателю (приобретателю) товаров (работ, услуг, имущественных прав). К подобным налогам относятся налог на добавленную стоимость, налог с продаж, акциз.
Согласно статье 41 НК РФ доходом признается экономическая выгода в денежной или натуральной форме, учитываемая в случае возможности ее оценки и в той мере, в которой такую выгоду можно оценить. Величина доходов определяется исходя из цен сделки. В случае получения доходов в натуральной форме, доходы определяются исходя из рыночных цен.
Пунктом 4 статьи 454 Гражданского Кодекса (ГК) РФ предусмотрено, что к продаже имущественных прав применяются общие положения о купле-продаже, если иное не вытекает из содержания или характера этих прав. В зависимости от содержания или характера имущественного права следует руководствоваться положениями ГК РФ, Закона СССР N 2213-1, Закона СССР N 2328-1, Закона РФ N 3517-1.
Доходом от реализации признаются выручка от реализации товаров (работ, услуг) как собственного производства, так и ранее приобретенных, выручка от реализации имущественных прав.
Выручка от реализации определяется исходя из всех поступлений, связанных с расчетами за реализованные товары (работы, услуги) или имущественные права, выраженные в денежной и (или) натуральной формах.
Внереализационными доходами признаются доходы, не указанные в выручке от реализации.
Внереализационными доходами налогоплательщика признаются, в частности, доходы:
Если организация операции по передаче имущества в аренду (субаренду) осуществляет на постоянной (систематической) основе, то доходы от таких операций учитываются в доходах от реализации, а если операции по передаче имущества в аренду носят разовый характер, то доходы от таких операций учитываются в составе внереализационных доходов. Понятие систематичности используется, если такие операции производятся два раза и более в течение календарного года.
3) в виде безвозмездно полученного имущества (работ, услуг) или имущественных прав.
При получении имущества (работ, услуг) безвозмездно оценка доходов осуществляется исходя из рыночных цен, но не ниже остаточной стоимости - по амортизируемому имуществу и не ниже затрат на производство (приобретение) - по иному имуществу (выполненным работам, оказанным услугам). Информация о ценах должна быть подтверждена налогоплательщиком - получателем имущества (работ, услуг) документально или путем проведения независимой оценки.
При определении налоговой базы не учитываются следующие доходы:
1) в виде имущества, имущественных прав, работ или услуг, которые получены от других лиц в порядке предварительной оплаты товаров (работ, услуг) налогоплательщиками, определяющими доходы и расходы по методу начисления;
2) в виде имущества, имущественных прав, которые получены в форме залога или задатка в качестве обеспечения обязательств;
3) в виде имущества, имущественных прав или неимущественных прав, имеющих денежную оценку, которые получены в виде взносов (вкладов) в уставный (складочный) капитал (фонд) организации (включая доход в виде превышения цены размещения акций (долей) над их номинальной стоимостью (первоначальным размером);
4) в виде имущества, имущественных прав, которые получены в пределах первоначального взноса участником хозяйственного общества или товарищества (его правопреемником или наследником) при выходе (выбытии) из хозяйственного общества или товарищества либо при распределении имущества ликвидируемого хозяйственного общества или товарищества между его участниками;
5) в виде имущества, имущественных прав и (или) неимущественных прав, имеющих денежную оценку, которые получены в пределах первоначального взноса участником договора простого товарищества (договора о совместной деятельности) или его правопреемником в случае выделения его доли из имущества, находящегося в общей собственности участников договора, или раздела такого имущества.
Расходами признаются обоснованные и документально подтвержденные затраты осуществленные (понесенные) налогоплательщиком. Под обоснованными расходами понимаются экономически оправданные затраты, оценка которых выражена в денежной форме. Под документально подтвержденными расходами понимаются затраты, подтвержденные документами, оформленными в соответствии с законодательством Российской Федерации. Расходами признаются любые затраты при условии, что они произведены для осуществления деятельности, направленной на получение дохода. Расходы в зависимости от их характера, а также условий осуществления и направлений деятельности налогоплательщика подразделяются на расходы, связанные с производством и реализацией, и внереализационные расходы. Если некоторые затраты с равными основаниями могут быть отнесены одновременно к нескольким группам расходов, налогоплательщик вправе самостоятельно определить, к какой именно группе он отнесет такие расходы.
Расходы, связанные с производством и реализацией, включают в себя:
1) расходы, связанные с изготовлением (производством), хранением и доставкой товаров, выполнением работ, оказанием услуг, приобретением и (или) реализацией товаров (работ, услуг, имущественных прав);
2) расходы на содержание и эксплуатацию, ремонт и техническое обслуживание основных средств и иного имущества, а также на поддержание их в исправном (актуальном) состоянии;
3) расходы на освоение природных ресурсов;
4) расходы на научные исследования и опытно-конструкторские разработки;
5) расходы на обязательное и добровольное страхование;
6) прочие расходы, связанные с производством и (или) реализацией.
Расходы, связанные с производством и (или) реализацией, подразделяются на следующие элементы:
1) материальные расходы;
2) расходы на оплату труда;
3) суммы начисленной амортизации;
4) прочие расходы.
Расходами на научные исследования и (или) опытно-конструкторские разработки признаются расходы, относящиеся к созданию новой или усовершенствованию производимой продукции (товаров, работ, услуг), в частности расходы на изобретательство, а также расходы на формирование Российского фонда технологического развития и иных отраслевых и межотраслевых фондов финансирования научно-исследовательских и опытно-конструкторских работ по перечню, утверждаемому Правительством Российской Федерации в соответствии с Федеральным законом "О науке и государственной научно-технической политике".
Расходы налогоплательщика на научные исследования и (или) опытно-конструкторские разработки, относящиеся к созданию новой или усовершенствованию производимой продукции (товаров, работ, услуг), в частности расходы на изобретательство, осуществленные им самостоятельно или совместно с другими организациями (в размере, соответствующем его доле расходов), равно как на основании договоров, по которым он выступает в качестве заказчика таких исследований или разработок, признаются для целей налогообложения после завершения этих исследований или разработок (завершения отдельных этапов работ) и подписания сторонами акта сдачи-приемки в порядке, предусмотренном настоящей статьей.
Расходы налогоплательщика на научные исследования и (или) опытно-конструкторские разработки, осуществленные в целях создания новых или совершенствования применяемых технологий, создания новых видов сырья или материалов, которые не дали положительного результата, также подлежат включению в состав прочих расходов равномерно в течение трех лет в размере, не превышающем 70 процентов фактически осуществленных расходов, в порядке, предусмотренном настоящим пунктом.
Расходы налогоплательщика на научные исследования и (или) опытно-конструкторские разработки, осуществленные в форме отчислений на формирование Российского фонда технологического развития и иных отраслевых и межотраслевых фондов финансирования научно-исследовательских и опытно-конструкторских работ по перечню, утверждаемому Правительством Российской Федерации в соответствии с Федеральным законом "О науке и государственной научно-технической политике", признаются для целей налогообложения в пределах 0,5 процента доходов (валовой выручки) налогоплательщика.
Эти положения не распространяются на расходы на научные исследования и (или) опытно-конструкторские разработки в организациях, выполняющих научные исследования и (или) опытно-конструкторские разработки в качестве исполнителя (подрядчика или субподрядчика). Указанные расходы рассматриваются как расходы на осуществление деятельности этими организациями, направленной на получение доходов.
В случае, если в результате произведенных расходов на научные исследования и (или) опытно-конструкторские разработки организация-налогоплательщик получает исключительные права на результаты интеллектуальной деятельности, данные права признаются нематериальными активами, которые подлежат амортизации.
К прочим расходам, связанным с производством и реализацией, относятся в частности следующие расходы налогоплательщика:
В состав расходов включаются все налоги и сборы, начисляемые организацией в соответствии с законодательством о налогах и сборах (единый социальный налог, налог на имущество и т.д.), по которым она является налогоплательщиком, за исключением налога на добавленную стоимость, акцизов, налога с продаж, предъявленных налогоплательщиком покупателю (приобретателю) товаров (работ, услуг, имущественных прав), а также сумм налога на прибыль и платежей за сверхнормативные выбросы загрязняющих веществ в окружающую среду, которые не учитываются в целях налогообложения
Суммы начисленной амортизации по основным средствам, используемым, в частности, для управления организацией, и по нематериальным активам учитываются в составе косвенных расходов. Следует учесть, что в состав прямых расходов не включаются расходы по добровольному страхованию всех работников организации (независимо от их участия в производственном процессе ).
В состав косвенных расходов включаются также расходы, осуществленные налогоплательщиком в течение предыдущих отчетных (налоговых) периодов, и сформировавшие определенные группы расходов, часть которых включается в состав расходов текущего периода (в частности, расходы, связанные с освоением природных ресурсов, расходы на осуществление НИОКР), через алгоритм расходов будущих периодов.
Особенности ведения налогового учета операций с амортизируемым имуществом:
Налог на доходы физических лиц
К доходам от источников в Российской Федерации относятся:
Не подлежат налогообложению (освобождаются от налогообложения) следующие виды доходов физических лиц:
При исчислении налоговой базы право на получение профессиональных налоговых вычетов имеют следующие категории налогоплательщиков:
Если эти расходы не могут быть подтверждены документально, они принимаются к вычету в следующих размерах:
| Нормативы затрат (в процентах к сумме начисленного дохода) | |
| Создание литературных произведений, в том числе для театра, кино, эстрады и цирка | 20 |
| Создание художественно-графических произведений, фоторабот для печати, произведений архитектуры и дизайна | 30 |
| Создание произведений скульптуры, монументально-декоративной живописи, декоративно-прикладного и оформительского искусства, станковой живописи, театрально и кинодекорационного искусства и графики, выполненных в различной технике | 40 |
| Создание аудиовизуальных произведений (видео-, теле- и кинофильмов) | 30 |
| Создание музыкальных произведений: музыкально-сценических произведений (опер, балетов, музыкальных комедий), симфонических, хоровых, камерных произведений, произведений для духового оркестра, оригинальной музыки для кино-, теле- и видеофильмов и театральных постановок | 40 |
| других музыкальных произведений, в том числе подготовленных к опубликованию | 25 |
| Исполнение произведений литературы и искусства | 20 |
| Создание научных трудов и разработок | 20 |
| Открытия, изобретения и создание промышленных образцов (к сумме дохода, полученного за первые два года использования) | 30 |
При определении налоговой базы расходы, подтвержденные документально, не могут учитываться одновременно с расходами в пределах установленного норматива.
Единый социальный налог
В налоговую базу (в части суммы налога, подлежащей уплате в Фонд социального страхования Российской Федерации) не включаются любые вознаграждения, выплачиваемые физическим лицам по договорам гражданско-правового характера, авторским и лицензионным договорам.
От уплаты налога освобождаются:
российские фонды поддержки образования и науки - с сумм выплат гражданам Российской Федерации в виде грантов (безвозмездной помощи), предоставляемых учителям, преподавателям, школьникам, студентам и (или) аспирантам государственных и (или) муниципальных образовательных учреждений.
В сентябре 2003 г. были объявлены новые конкурсы ИНТАС по программе "Сопутствующие мероприятия на 2003-2006 гг.", направленные на поддержку совместной научной деятельности ученых Новых независимых государств (ННГ) и стран-членов ИНТАС во всех областях точных и естественных наук, а также гуманитарных и социальных науках и экономике.
Информация подготовлена Сибирским информационно-консультационным центром для удобства пользователей из ННГ. Для подготовки заявок необходимо обратиться к оригинальному объявлению о конкурсах и использовать информационный пакет на английском языке, размещенные на сайте ИНТАС http://www.intas.be/mainfs.htm или копии на сайте СИКЦ (http://www-sbras.nsc.ru/sicc/intas_2003_app.htm)
Международная конференция по высокоорганизованным каталитическим системам
(Highly Organized Catalytic Systems, HOCS2004)
14 - 17 июня 2004 года
Химический факультет МГУ им. М.В. Ломоносова, Москва
Организаторы конференции:
Химический факультет МГУ им. М.В. Ломоносова, Москва, Россия
Министерство промышленности, науки и технологий РФ, Москва
Академия Наук РФ, Москва
Российский фонд фундаментальных исследований, Москва
Фирма Haldor TopsØe A/S , Дания
Фирма "Нейрок-катализаторы"
Организационный комитет:
академик Лунин В.В. (Россия) - председатель,
академик Шилов А.Е. (Россия) - сопредседатель,
проф. Смирнов В.В. - зам. председателя
к.х.н. Л.А.Тюрина - ученый секретарь
Научные направления конференции:
В программе конференции - пленарные лекции (45 мин.), устные доклады (15-30 мин.) и стендовые сессии.
Ключевые даты:
15 февраля 2004 г. - предварительная регистрация
15 апреля 2004 г. - срок представления тезисов докладов
Информация о конференции:
Л.А. ТЮРИНА
е-mail:hocs@kinet.chem.msu.ru
Международный симпозиум
"Каталитическая полимеризация олефинов"
22-25 июня 2004 года
Институт химической физики им. Н.Н. Семенова, Москва
Организаторы симпозиума :
Институт химической физики им. Н.Н. Семенова РАН, Москва
Институт катализа им. Г.К. Борескова СО РАН, Новосибирск
Научный совет по катализу ОХНМ РАН, Москва
Тематика симпозиума:
Информация о симпозиуме:
|
Ковалева Наталья
Институт химической физики им. Н.Н. Семенова ул. Косыгина, 4б 119991 Москва Тел.: 095 939 73 73 Факс: (095) 137 82 84 E-mail:mospol@center.chph.ras.ru Web site: http://mospol.chph.ras.ru/ |
Замулина Татьяна Владимировна
Институт катализа им. Г.К. Борескова СО РАН просп. Акад. Лаврентьева, 5 630090 Новосибирск тел.\факс: (3832) 34-12-97 E-mail: zam@catalysis.nsk.su |
International symposium on Carbon for catalysis CarboCat-2004
18-20 July, 2004, Lausanne, Switzerland
Swiss Federal Institute of Technology
The Symposium is organazed by the Swiss Federal Institute of Technology in Lausanne (EPFL) under the auspices of the European Federation of Catalysis Societies (EFCATS) and of the European Federation of Chemical Engineering (EFCE) with the participation of the DACHEMA Subject Division Catalysis, the Swiss National Science Foundation and the Boreskov Institute of Catalysis (BIC, Novosibirsk, Russia).
Symposium topics:
Executive organizing committee
| Swiss Federal Institute of Technology (EPFL), Lausanne/CH
Dr. Lioubov Kiwi-Minsker, Chair Ingrid Margot, Secretary Paule Anken, Secretary carbocat@epfl.ch http://isp.epfl.ch/Carbocat Tel.:+41-21-693 3175, +41-21-693 3692 Fax: +41-21-693 3190, +41-21-693 5690 Members of the LGRC-EPFL |
BIC, Novosibirsk, Russia
(for Russian participants) Liudmila Startseva, Secretary Boreskov Institute of Catalysis Prosp. Akad. Lavrentieva, 5 Novosibirsk, 630090, Russia Phone/Fax: +7(3832) 34-12-97 E-mail: star@catalysis.nsk.su |
Key Dates
Abstract submission - 15 November 2003 - 31 January 2004
e-mail: carbocat@epfl.ch
Registration/ Accommodation - from 15 November 2003
http://isp.epfl.ch/Carbocat
Notification of abstract acceptance - 31 March 2004
Opening ceremony and welcome - 18 July 2004
V Российская конференция с участием стран СНГ
"Научные основы приготовления и технологии катализаторов"
IV Российская конференция с участием стран СНГ
"Проблемы дезактивации катализаторов"
13 -16 сентября 2004 г.
г. Омск
Адрес для переписки:
Старцевой Людмиле Яковлевне
Институт катализа
им. Г.К. Борескова СО РАН
просп. Акад. Лаврентьева, 5
Новосибирск, 630090
тел.\факс: (3832) 34-12-97
E-mail: star@catalysis.nsk.su
Simple, direct indole synthesis
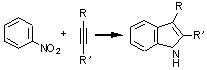
A remarkable metal-catalyzed reaction of nitroaromatic compounds with alkynes (shown) goes through N-centered reduction, C-H activation, and the formation of both C-N and C-C bonds to give indoles in moderate yields [Chem. Commun, 2002, 484]. Kenneth M. Nicholas, professor of chemistry and biochemistry, postdoc Andrea Penoni, and undergraduate Jerome Volkmann at the University of Oklahoma, Norman, find that nitrosoaromatics also yield indoles when reacted with alkynes, suggesting that the nitroso compounds are intermediates in this catalytic reductive annulation [Org. Lett., 4,699 (2002)]. The reactions are regioselective, selectively forming 3-substituted indoles with terminal alkynes. The researchers currently are exploring the scope and mechanism of the reactions and plan to use them in the synthesis of some of the many bioactive indoles.
C & EN / MARCH 11, 2002
Nornicotine catalyzes aldol reactions in H2O
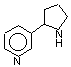
A metabolite of nicotine can catalyze aldol reactions under physiological
conditions, according to chemists at Scripps Research Institute [J. Am. Chem. Soc., 124, 3220 (2002)]. The intriguing finding has potentially large implications for understanding the physiology of cigarette addiction and for nicotine replacement therapy. Chemistry professor Kim D. Janda and graduate student Tobin J. Dickerson find that nornicotine (shown) catalyzes the reaction of acetone with 4-nitrobenzaldehyde in aqueous phosphate buffer, giving the aldol addition product in 81% yield. Nornicotine also catalyzes aldol reactions
of other compounds, including pyruvate, an important player in metabolism. Such aldol reactions usually proceed only in organic solvents; in water, they require enzymes or Lewis acid catalysts.
A constituent of tobacco and a minor metabolite of nicotine, nornicotine has a longer half-life than its parent compound, and heavy smokers can have fairly constant levels in their blood. The Scripps researchers are investigating the physiological relevance of their findings.
C & EN / APRIL 1, 2002
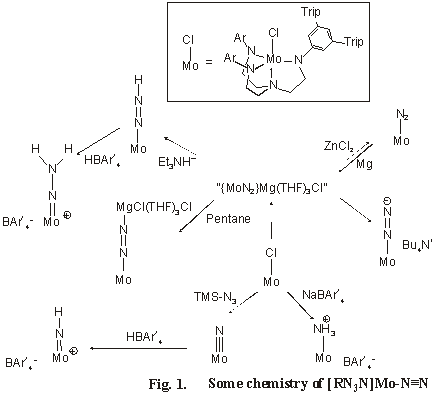
Reduction of ligated N2 to ammonia
Yandulov and Schrock have been exploring the chemistry of triamidoamine ([(RNCH2CH2)3N]3-=[RN3N]3-) molybdenum complexes with the aim of understanding how catalytic reduction of dinitrogen might be achieved on a single-site homogeneous catalyst. These workers now have made the study of the process more transparent, with the development of a complex having a highly protected molybdenum reaction site (D Yandulov & R Schrock, J Am Chem Soc 2002. 124. 6252).
When R is an aryl group substituted in the 3 and 5 positions, it is possible to isolate complexes (see Figure 1) in which dinitrogen binds to molybdenum to form a monomeric species (avoiding formation of relatively stable [RN3N]Mo-Nº N-Mo[RN3N] complexes). The figure outlines some of the substitution, protonation and redox reactions that surround the synthesis and reactivity of [RN3N]Mo-Nº N complexes, but the reduction of dinitrogen to ammonia reported by these authors still requires reduction by CoCp2. Nevertheless, this ligand system allows the isolation of several intermediates in the reduction process - and knowledge that may be invaluable in the search for the ultimate objective: catalytic reduction of dinitrogen to ammonia.
Timothy Hughbanks Texas A&M University
arbon-free precursor to BN-nanotubes
The synthesis of carbon nanotubes led quickly to the investigation of corresponding boron nitride analogues. However, methods of synthesising carbon nanotubes have not been adaptable to the reliable synthesis of BN nanotubes. Thus, there is no way to obtain BN nanotubes in reasonable quantity or, especially, purity. Tang's group (C Tang Y Bando, T Sato & K Kurashima, Chem Commun 2002. 1290) has exploited the fact that boron reacts with MgO at elevated temperatures (11000C) to yield gaseous magnesium and B2O2. The latter reacts with ammonia that is flowed into the chamber, to produce the boron nitride product:
B (s) + MgO (s) ® B2O2(g) + Mg (g)
B2O2 (g) + NH3 (g) ®
2BN(s) + 2H2O (g) + H2 (g)
Catalysed additions of triphenylphosphine
In Chemistry Letters (2002. 272) Arisawa, Momozuka & Yamaguchi describe a rhodium-mediated process where triphenylphosphine adds to dienes (see Reaction 1). The regioselectivities observed are usually highly in favour of addition of the phosphine to the diene terminus. Where the regioselectivity of the addition is imperfect, then many products can be recrystallised to purity.
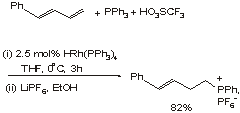
Z-Alkenes are less reactive than the E-isomers, hence the addition process can be used to remove the latter from mixtures of diene starting materials.
Kevin Burgess Texas A&M University
Palladium-catalysed arylation of thiophenesM
Continuing their work on arylation of electron-rich aromatic compounds. Miura and coworkers have investigated palladium-catalysed reactions of thiophenes with aryl halides. They find that 2-thiophene carboxamides undergo triarylatlon with loss ofthe amide functionality, 3-substituted thiophenes can be triarylated (especially if the 3-substituent is an electron withdrawing group), and the reaction conditions may be controlled such that diarylation of them is possible (reactions 3-5, respectively).
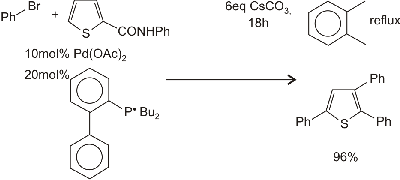 reaction 3 |
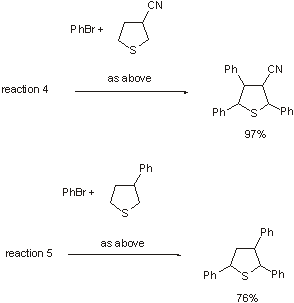 reactions 4 and 5 |
Hydrogenation of hindered alkenes
There are not many homogeneous catalysts that mediate hydrogenations of tri-and tetra-substituted, unfunctionalised alkenes. The gold standard in this field is Crabtree's catalyst, [Ir(PCy3)(py)(COD)]+PF6¯ which can achieve this at ambient temperature and pressure. In Organometallic. Weller and coworkers have reported a rhodium-based complex that has hydrogenation activities marginally inferior to Crabtree's catalyst (2002, in press). They report good evidence that the rhodium complex 1, under hydrogen, is transformed into 2 in which the [closo-CB11H6Br6]¯ anion complexes the metal as shown. The stated rationale behind investigation of this complex was that, '... the weakly coordinating carborane anion could ... move to one side to allow olefin and dihydrogen to coordinate the rhodium, while remaining available in order to stabilise reactive cationic species.' This hypothesis seems to be born out by the results. Under 1 atm of hydrogen at room temperature, complex 1 catalysed the hydrogenation of 1-methylcyclohexane and of 2,3-dimethylbut-2-ene, with turnover frequencies that are not significantly less than those measured for Crabtree's catalyst.
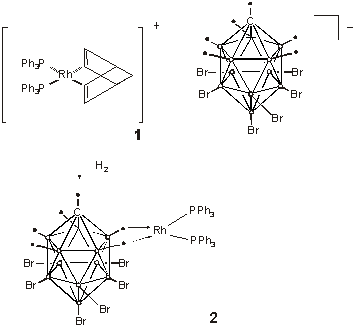
Complex 1 and 2
Chemistry & Industry - 15 July 2002
Calalytic epoxidation of alkenes by NO2
Nitrous oxide, often overlooked as an oxidant, has the obvious advantage of generating only a very innocuous by product on reduction. It now appears that it can be used to epoxidise alkenes in a process catalysed by the polyoxometalate [n-Oct3MeN]10[MnIII2ZnW(ZnW9O34)2]. Unfortunatuly, the process requires temperatures of 150oC and only proceeds at a rate of about one turnover/hour. However, the reaction is 100% stereoselective in that cis-stilbene only gives cis-stilbene oxide. It is also interesting from a mechanistic standpoint as, surprisingly appreciable quantities of Mn11 are formed reversibly in the reaction (EPR).
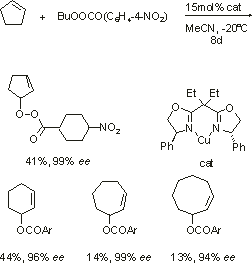
Fig. Allylis oxidation - typical reaction, catalyst and product
Chemistry & Industry - 16 September 2002
1-D zeolites trap hydrocarbons
One-dimensional zeolites, a class of porous solids with relatively low interconnectivity, may be useful as hydrocarbon traps in automotive catalysis, according to a study conducted at Northwestem University. At engine start-up catalytic converters are cold and ineffective in catalyzing reactions of hydrocarbons and other exhaust stream components - resulting in a couple of minutes of high-level tailpipe emissions. A proposed solution to the "cold start" problem involves trapping the hydrocarbons up-stream of the converter and releasing them after the converter is warm enough to cause the gases to react. Success with that approach, however, has been limited by a lack of suitable adsorbent materials, particularly for lightweight hydrocarbons. Now, chemical engineering associate professor Randall Q. Snurr, graduate student Kenneth F. Czaplewski, and coworkers have found that when test mixtures of propane and toluene are adsorbed in EUO and mordenite-1-D zeolites characterized by nonintersecting narrow channels-propane's desorption temperature is raised by 35 to 100oC compared to the desorption temperature of pure propane [Microporous Mesoporous Mater., 56, 55 (2002)]. The group explains that low concentrations of tightly adsorbed toluene are sufficient to clog the narrow pores at low temperatures.
C&EN / OCTOBER 28 2002/ 33
Combinatorial materials finding catalysts faster
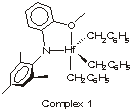
Symyx-Dow collaboration yields new class of polyolefin catalysts
A four-year collaboration between Symyx Technologies and Dow Chemical has borne fruit in the discovery of a new class of single-site catalysts for olefin polymerization. The new amide-ether-based hafnium catalysts were found using a fully integrated high-throughput screening methodology [J. Am. Chem. Soc., 125, 306 (2003)].
"The results are stunning," says one of the coauthors, Dow chemist James C. Stevens, because the new screening methodology enabled them to do in days or weeks what used to take many months or years.
The Dow-Symyx researchers set out to discover new catalysts with potential for the production of linear low-density polyethylene, which is a copolymer of ethyiene and an α-olefin, such as 1-octene. They first synthesized a library of hafnium and zirconium complexes containing 23 different bidentate and tridentate ligands. To screen these complexes under different activation conditions, they carried out 384 polymerization experiments in just a few hours.
The work uncovered many catalytically active metal-ligand combinations. One hafnium catalyst (complex 1) was found capable, under the right conditions, of polymerizing 1-octene with 100% conversion. Further experiments demonstrated that this complex can produce high-molecular-weight ethylene-1-octene copolymers.
In the course of one day, the researchers then studied the activity at 130oC of 96 other hafnium complexes containing related amine-ether ligands. They found that many of these catalysts are even better at copolymerizing ethylene and 1-octene than complex 1.
In larger scale batch reactor experiments, complex 1 and a closely related hafnium complex were found to perform as well as or better than Dow's workhorse metallocene polyolefin catalyst system.
Dow isn't necessarily "marching to commercialization" with any of the catalysts reported in the JACS paper, Stevens tells C&EN. "But we will be talking soon, I think, about another catalyst that's come out of this procedure that is going to be commercialized. "-RON DAGANI.
C&EN / APRIL 7, 2003
Co catalysts, CO insertion, and bioplastics
Poly[(R)]-β-hydroxybutyrate] (PHB) is a biodegradable polyester that is made by bacteria and has properties similar to polypropylene. Its environmentally friendly nature is attractive, but high fermentation production costs make it impractical for many commercial applications. Chemistry professor Geoffrey W. Coates and his group at Cornell University have now come up with a new class of catalysts, [Lewis acid]+[Co(CO)4]-, which they believe could be part of a potentially economical chemical route to PHB. The researchers have shown that the Co catalysts readily insert CO into epoxides and aziridines to make β-lactones and β-lactams, respectively. For example, they have made multigram quantities of (R)-β -butyrolactone from (R)- propylene oxide with 95% yield and complete retention of configuration. The R isomer is critical to make PHB, Coates notes, since a mix of isomers leads to polymers with poor properties. Coates's group also has developed di-iminate zinc alkoxide catalysts for ring-opening polymerization of the β-lactones to PHB and related poly (β-hydroxy-alkanoates) [J. Am. Chem. Soc., 124, 15239 (2002)]. Other aspects of the work include using the catalysts to make substituted lactones, lactams, and other heterocycles for pharmaceutical building blocks.
C&EN / APRIL 14, 2003
First catalytic, asymmetric Passerini reaction devised
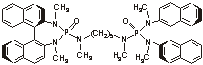
Chemists have found a way to carry out the Passerini reaction both catalytically and enantioselectively [J. Am. Chem. Soc., 125, 7825 (2003)]. The Passerini reaction is a carbon-carbon bond-forming process that's widely used to synthesize natural products and diverse libraries of drug candidates. It involves the formation, in one step, of an α-acyloxy carboxamide from three components: an isocyanide, a ketone or an aldehyde, and a carboxylic acid. Chemistry professor Scott E. Denmark and graduate student Yu Fan at the University of Illinois, Urbana-Champaign, show that, in the presence of silicon tetrachloride, a chiral bisphosphor-amide (shown) catalyzes the Passerini reaction enantioselectively. The catalyst, a Lewis base, promotes the ionization of SiCl4, a weak Lewis acid. The resulting catalyst-SiCl3+ adduct affords highly enandomerically enriched α-hydroxy amides and esters from a wide range of aldehyde and isocyanide starting materials.
Atmospheric' direct route to propylene oxide
A significant research problem in industrial chemistry has been to find a direct oxidation process to make propylene oxide, a versatile chemical intermediate. In theory, combining O2 with propylene should work, but the conversion is low. So instead, a pair of two-step processes currently are used to manufacture propylene oxide - the chlorohydrin process and the peroxidation process - but both have major coproducts. Now, Torsten Berndt of the Institute for Tropospheric Research in Leipzig, Germany, and coworkers have uncovered a potentially viable direct route to propylene oxide by reacting propylene with nitrogen(V) oxides such as nitrate radicals in the gas phase - chemistry the researchers observed while conducting atmospheric studies [Ind. Eng. Chem. Res., 42, 2870 (2003)]. The researchers combine NO2 with O3 and add the mixture to propylene in a flow-through gas reactor. The nitrogen (V) oxides formed act as an oxygen-transfer agent to convert propylene to propylene oxide with yields and selectivity comparable with the current industrial routes, Berndt notes. This noncatalytic nitrate oxidation route would be a lucrative alternative if it can be successfully scaled up, the researchers believe.
C&EN / June 23, 2003


