22 января 2026
Главный научный сотрудник ЦКП "СКИФ" Ян Зубавичус: "Мы готовимся изо всех сил, чтобы с первого фотона начать активную работу"
15 января 2026
Российские и китайские ученые развивают низкотемпературное получение водорода из биомассы

З.Р. Исмагилов
(К 60-летию со дня рождения)
III Международная конференция
«Катализ: теория и практика»
Российско-немецкий семинар
"Связь между реальным и модельным катализом"
За рубежом
Приглашения на конференции
НОВОСТИ НАУКИ
MULTITASKING CATALYSTS
BIOSYNTHESIS: Mix-and-match enzymes can catalyze all four isoprenoid coupling reactions
ENZYMES MADE OF BITS and pieces of two other enzymes can catalyze all four reactions used to build the skeletons of isoprenoid natural products, chemists have discovered. The multitasking catalysts may yield clues about how nature evolved the ability to make these compounds.
Isoprenoids are the most chemically diverse family of natural products. More than 55,000 naturally occurring isoprenoids or terpenoids are known, with examples from all over the world. This broad family includes compounds that play important roles in metabolism and cell structure. Remarkably, this vast array of compounds is generated from simple precursors by a set of just four coupling reactions: chain elongation, cyclopropanation, branching, and cyclobutanation.
MULTIPLE CHOICE isoprenoid skeletons are made from two simple precursors via four coupling reactions
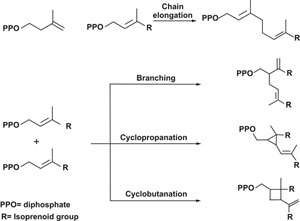
In nature, each reaction is usually carried out by a separate enzyme. In the course of studying these enzymes, chemistry professor C. Dale Poulter and coworkers at the University of Utah have created chimeras that can perform all four reactions (Science 2007,316,73). Starting with the chain elongation and cyclopropanation enzymes from a sagebrush plant, they replaced segments of the active site of the chain elongation enzyme with corresponding bits from the cyclopropanation enzyme.
"We're not changing the size of the protein, and we're not introducing extra active sites," Poulter says. "All we're doing is changing the amino acids used to construct the active site."
The unexpected discovery that some of the chimeras can perform all four of the coupling reactions could provide insight into how the natural enzymes that catalyze these reactions could have evolved from a single precursor. "Once you have the ability to do all four reactions, then making one pathway selective over another is basically what the job of evolution is about," Poulter says. The efficiency of the multitasking enzymes for the various reactions was dramatically different, he notes.
In an accompanying commentary, David W. Christianson, a chemistry professor at the University of Pennsylvania, writes that the "chimeras exhibit remarkable trends in biosynthetic versatility." In addition, the work provides "compelling evidence" that the enzymes "that catalyze these fundamental coupling reactions diverged from a common ancestor early in the evolution of terpenoid biosynthesis," Christiansor writes. - CELIA ARNAUD
www.cen-online.org
APRIL 9.2007
ACID CATALYSIS IN BASIC SOLUTION
By confining a reactant inside electrostatically tuned molecular cages, chemists have performed acid catalysis in basic solution (Science 2007,316, 85). Michael D. Pluth, Robert G. Bergman, and Kenneth N. Raymond of the University of California, Berkeley, show that a water-soluble, tetrahedral metal-ligand assembly thermodynamicaily drives the protonation of a guest molecule (space-filling model) trapped inside its highly charged cavity. The researchers exploit this host-induced shift in the guest's ability to accept a proton to do acid catalysis in basic solution. In the presence of catalytic amounts of the host, orthoformates [HC(OR)3, R = alkyl] in-basiс solution are trapped inside and protonated by water, resulting in rapid hydrolysis of the otherwise stable-to-base orthoformate. A similar strategy could be used to hydrolyze other acid-sensitive molecules in basic environments, the researchers note. The host's ability to select appropriately sized substrates is of particular interest, they add, because size selectivity is "often used by nature but rarely incorporated into standard homogeneous or heterogeneous catalysis."
www.cen-online.org
APRIL 9.2007
ПРЕМИИ И НАГРАДЫ
E. V. MURPHREE AWARD
iN INDUSTRIAL & ENGINEERING CHEMISTRY
snonsored by ExxonMobil Reseatch & Engineering Co. and ExxonMobil Chemical Co.
As a boy, Wolfgang F. Hölderich, 60, had interest in all things explosive and stinky. His first experiments were performed in his grandmother's basement, and her garden was used as "a place for launching self-constructed rockets." It was this freewheeling spirit that led Hölderich to pursue a career in chemistry along a path that led him to academia, to industry, and back again to academia.
Hölderich has spent his career straddling - and uniting - the worlds of industry and academia. He received a master's degree in chemistry in 1972 from the University of Karlsruhe, in Germany, and a Ph.D. in 1975 from the university's Institute of Inorganic Chemistry. Hölderich then became a postdoc at Massachusetts Institute of Technology on a National Science Foundation/North Atlantic Treaty Organization Fellowship in 1976.
In 1978, he started a 14-year career with BASF, in Ludwigshafen, Germany, where he held several leadership positions in the area of heterogeneous catalysis research. Hölderich transitioned back into academia in 1992 and is currently university professor and director of the department of chemical technology and heterogeneous catalysis at RWTH Aachen University of Technology, in Germany.
"It is difficult to succeed both as a prolific industrial inventor and as a noted academic in one life span," says L. Louis Hegedus, recently retired seniorvice president of R&D for Arkema and a C&EN adviser. But Hölderich has done just that, racking up a number of impressive accomplishments along the way.
Hölderich completed undergraduate work and received his master's degree in only four years. That swiftness contrast s with the average stint of six to seven years at that time in Germany. While working toward his Ph.D., he was able to revisit the interests of his youth, working with "stinky and explosive compounds such as phosphines, silylphosphanes, boranes, and phosphinoboranes," he says.
Hölderich first gained interest in heterogeneous catalysis when he moved into industry at BASF. While there, he was the lead inventor for many BASF patents and developed aggressive, successful patenting strategies. Hölderich says his success in zeolite chemistry and the industrialization of new processes increased his interest in the field and his knowledge of chemical engineering. "It is still fascinating and fulfilling to see howprocesses can be commercialized and how lab-scale experiments can be transformed to large-scale units," he adds.
Hölderich then took his interest and knowlege of heterogeneous catalysis back to academia, starting up a world-class catalysis research institute at Aachen. In this continuing role, he has presentedhundreds of lectures at international catalysis conferences. He has authored more than 250 papers, holds over 200 patents, and has been an author or editor of four books in the field of heterogeneous catalysis.
He has also acted as a conduit between industry and academia by serving as a consultant for chemical, petrochemical, and pharmaceutical companies throughout Europe, Japan, and the U.S. In collaboration with Tokyo-based Sumitomo Chemical, he was heavily involved in the development of a process for the production of caprolactam, the monomer that polymerizes into nylon 6. The process, for which Hölderich was granted the basic patent, unfolds via the Beckmann rearrangement of cyclo-hexanone oxime catalyzed by an extremely weakly acidic pentasil zeolite.The transformation is carried out in a novel fluidized-bed reactor with continuous catalyst regeneration and was commercialized in Japan by Sumitomo.
It is perhaps Hölderich's own words that best reveal the reason for his success as an innovator: "You haveto observe carefully and be persistent in following ideas even when first experiments fail. If you get an unexpected result, follow the new direction rather than throwing your data away."
The award address will be presented before the Division of Industrial & Engineering Chemistry. -KIMBERLY DUNHAM
www.cen-online.org
January 29.2007
George a. olah award in hydrocarbon or petroleum chemistry
Sponsored by the George A. Olah Endowment
Bruce E. Koel "has had a major impact on hydrocarbon chemistry," says John L. Falconer, a professor of chemical and biological engineering at the University of Colorado. And his work "has had a major impact on others" as indicated by his large number of invited talks and a citation rate of approximately
24 citations per paper.
Koel's career began with a solid background in surface science. His papers are considered important contributions to the surface science literature.
Next, he began integrating surface science techniques into the study of fundamental surface chemical problems relevant to many important technologies including catalysis and electrochemistry. A principal contribution was his investigation of the chemistry and catalysis on well-defined bimetallic alloy surfaces.
Chemisorption and surface reactions of dozens of small molecules, hydrocarbons, and intermediates have now been studied under ultra-high-vacuum (UHV) conditions, where the powerful spectroscopic tools of modern surface science can be utilized. In addition, these studies are being correlated with measurements, in his lab, of the high-pressure catalytic hydrogenation of crotonaldehyde and cyclohexanone over these some serfaces.
Koel's work to discover, characterize, and utilize ordered surface alloys (inter-metallic compounds) grown on single-crystal substrates has greatly improved the understanding of fundamental principles that underpin alloy chemistry and that can be used to interpret or predict their catalytic properties.
"His work comprises the most detailed and complete exploration of the chemistry on platinum-tin alloy surfaces," notes David W. Goodman, a professor of chemistry at Texas A&M University. "Other than a few studies on oxidation of PtSn alloys, there was only the early work of Sachtler's on H2 and ethylene adsorption on polycrystalline material when Bruce began. His most important contributions have derived from chemisorption studies on these surface alloys, correlating surface structure with chemisorption properties."
In other studies tackling fuel-cell electrode chemistry, Koel has shown that PtSn alloys are remarkably weakly interacting with organic compounds given the oxophilicity of tin. Methanol, ethanol, water, and even nitromethane are reversibly adsorbed under UHV conditions.
His chemisorption studies have led to the discovery of novel catalytic processes occurring in UHV. The two most significant aspects of these studies are the utilization of ordered surface alloys to provide a clear probe of site requirements for adsorption and reactions, showing clearly the importance of the threefold hollow site to hydrocarbon reactions, and the discovery of the importance of a modifier precursor in controlling adsorption kinetics on chemically modified or bimetallic surfaces.
Koel, 51, received abachelor's degree in chemistry in 1976 from Emporia State University, in Kansas, and a Ph.D. in chemistry from the University of Texas, Austin. He is the recipient of numerous awards and has trained 43 graduate students and
23 postdoctoral fellows.
The award address will be presented before the Division of Colloid & Surface Chemistry.-WILLIAM SCHULZ
www.cen-online.org
January 8.2007
ARthur w. adamson award for distinguished service in the advancement of surface chemistry
Sponsored by Occidental Petroleum Corp.
Charles T. Campbell was captivated more than 30 years ago by the potential of surface chemistry to reveal molecular secrets about heterogeneous catalysis. "I was fascinated by the energy issues: the possibility of using catalysts to help the chemical industry run more efficiently," Campbell says. Three decades later, he's still hooked, and it's for the same reasons.
As an undergraduate student at the University of Texas, Austin, Campbell was introduced to surface science by chemistry professor John M. (Mike) White. "Mike was so inspirational in the way he taught," Campbell remarks, that "I just fell in love with the science." According to Campbell, the Texas professor had a knack for encouraging students and "always had positive things to say, no matter how badly you did." The latter quality served Campbell especially well one afternoon when he "goofed" while trying to repair a leaking glass vacuum system. As Campbell recalls, after White had finished extensive glass-blowing work to assemble the equipment, Campbell checked the apparatus for leaks and found one. Deciding he could fix it without assistance, Campbell went to work with the glass-blowing torch. Unfortunately, "the whole thing just shattered," Campbell recalls. Always patient, White simply encouraged his student to keep working, Campbell says.
Eventually, Campbell gained recognition for developing surface methods and applying them to catalytic reactions, such as methanol synthesis on Cu/ZnO catalysts and dehydrogenation of hydrocarbons on platinum. He's also recognized for innovations in single-crystal adsorption microcalorimetry and for refining methods for quantitative surface analysis of high-surface-area catalysts.
As Texas A&M chemistry professor D. Wayne Goodman notes, Campbell has "melded" surface analysis and catalysis "into a single discipline" through his work in identifying reaction intermediates and pathways and in measuring absolute reaction rates. In addition, Goodman notes, Campbell "is one of few scientists who have succeeded in using this approach to obtain insightful results accessible to the catalysis community."
Campbell, 53, graduated in 1975 from the University of Texas, Austin, with a bachelor's degree in chemical engineering and completed his Ph.D. education there in 1979. From Texas, he went to the University of Munich, in Germany, where he conducted postdoctoral research with noted surface scientist Gerhard Ertl.
From 1981 to 1986, Campbell served as a staff scientist at Los Alamos National Laboratory, in New Mexico, and then began his academic career at Indiana University, Bloomington. He has been a professor of chemistry at the University of Washington, Seattle, since 1989.
Campbell has served on the editorial boards of the Journal of Catalysis, the Journal of Chemical Physics, and other journals, and he has held the position of editor-in-chief of Surface Science since 2002. He has been honored with several awards, including the ACS Award in Colloid or Surface Chemistry in 2001 and the Alexander von Humboldt Research Award in 2003.
The award address will be presented to the Division of Physical Chemistry and the Division of Colloid & Surface Chemistry. - MITCH JACOBY
www.cen-online.org
January 22.2007
GABOR A. SOMORJAI AWARD FOR CREATIVE RESEARCH IN CATALYSIS
Sponsored by the GaborA. & JudithK. Somoorjai EndowmentFund
Chemical systems that are too complex to be probed in a straightforward way often need to be substituted with simplified stand-ins in order to reveal pertinent molecular secrets. But designing model test subjects and suitable experimental conditions to uncover those secrets is challenging. According to leading researchers, Hans-Joachim (Hajo) Freund, a professor and director of the Fritz Haber Institute of the Max Planck Society, in Berlin, excels in designing model catalysts and probing them with novel surface-sensitive techniques.
Freund's "innovative methods" for preparing single-crystal model oxide catalysts and his development of new surface-analysis techniques have led to "major contributions" in the evolution of catalysis, says Gabor A. Somorjai, a professor of chemistry at the University of California, Berkeley. Somorjai adds that Freund's 25 years of "outstanding and creative research" have elevated him to the level of "unquestioned leader in Europe of the surface science approach to heterogeneous catalysis."
Among Freund's numerous scientific accomplishments, various seminal studies are singled out by experts for their broad impact. Examples include development of synthetic methods for preparing model catalysts based on the oxides of nickel, aluminum, silicon, niobium, magnesium, and other materials. The Berlin scientist is also recognized for his advances in electron spin resonance techniques and single-particle luminescence methods and for his application of sum frequency generation and other vibrational spectroscopy procedures to probe model catalysts.
The impact of Freund's work continues to grow. As Texas A&M University chemistry professor D. Wayne Goodman notes, Freund initiated much of the oxide-based catalyst research that is underway in laboratories around the world. Goodman adds that Freund and his coworkers have played a major role in bridging the gap between the traditional surface science and catalysis communities and have served as a springboard for "an entirely new approach to fundamental catalytic research."
Freund, 55, earned bachelor's and master's degrees in chemistry and physics at the University of Cologne, in Germany, in 1972 and 1975, respectively, and completed his Ph.D. education there in 1978. Following postdoctoral positions at the University of Pennsylvania and Xerox, he began his academic career at Erlangen University, Nuremberg, Germany, where he remained until 1987. He then moved to Ruhr-University Bochum, also in Germany, where he served as a chemistry professor from 1987 to 1996. In 1996, he was appointed director of the Fritz Haber Institute.
Freund has received numerous honorary professorships, including those at the Free University, Technical University, and Humboldt University, all in Berlin. He has been honored with various lectureships around the globe and has been a fellow of the American Physical Society since 2001. He has served on several international science panels and committees and has been named to the editorial boards of numerous chemistry and physics journals.
In two-and-a-half decades of service to the surface science and catalysis communities, Freund has educated nearly 80 Ph.D. students and more than 80 postdoctoral researchers and has published more than 450 scientific papers.
The award address will be presented before the Division of Colloid & Surface Chemistry - MITCH JACOBY.
www.cen-online.org
January 22.2007
L.J. Cooßen Receives Duisberg Prize
Lukas J.Gooßen (Universitat Kaisers-lautern) has been awarded the Carl Duisberg Memorial Prize by the GDCh for the development of new catalytic reactions for organic synthesis. He is particularly interested in carbonic acids as substrates for catalytic transformations, for catalytic additions, and in the mechanisms of palladium-catalyzed reactions. He recently reported in Angewandte Chetnie on the ruthenium-catalyzed anti-Markovnikov addition of amides and alkenes and its application to the regio- and stereoselective synthesis of enamides.
Gooßen studied at the Universities of Bielefeld (Germany) and Michigan (Ann Arbor. USA), and he completed his PhD in 1997 under the guidance of W. A. Herrmann at the Technische Universität München on the topic of functionalized imidazolin-2-ylidene metal complexes in catalysis. He then joined K. B. Sharpless at the Scripps Resears Institute in La Jolla (CA, USA) for a year as a postdoctoral researcher. In 1999/2000, he was a head of laboratory with Bayer. In 2004 he completed his habilitation at the Max-Planck-Institut für Kohlenforschung in Mülheim with M. Reetz on the subject of new transition-metal-catalyzed reactions for organic synthesis. In September 2004 he joined the faculty at the RWTH Aachen as a Heisenberg fellow, and in 2005 he accepted a professorship at the Universität Kaiserslautern.
ADUC Awards for Young Chemists
The Association of German University Professors of Chemistry (Arbeitsgemeinschaft Deutscher Universitätprofessoren für Chemie; ADUC) prizes for 2007 will be presented to the following:
Congratulations to Nobel Laureate Gerhard Ertl
The Editors of The Journal of Physical Chemistry congratulate the winner of the 2007 Nobel Prize in Chemistry, Gerhard Ertl, a frequent contributor to the Journal. In 2004, JPC published the Gerhard Ertl Festschrift. This special issue, as well as numerous other articles listed below, are available now to read free of charge. Mostafa El-Sayed, Editor-in-Chief of JPC when this Special Issue was published, said at the time, "This guy is going to get the Nobel Prize some day." We are pleased to see he did.
George C. Schatz,Editor-in-Chief
Donna Minton,Managing Editor
Gerhard Ertl Festschrift -The Journal of Physical Chemistry B, Vol. 108, Iss. 38
Free access to all 81 articles published in this special issue, including:
Surface Femtochemistry: Associative Desorption of Hydrogen from Ru(001) Induced by Electronic Excitations
Denzler, D.N., Frischkorn, C., Wolf, M., and Ertl, G., J. Phys. Chem. B.; (Article); 2004; 108(38); 14503-14510. DOI:10.1021/jp049199i
Other articles by Gerhard Ertl published in ACS journals:
Other Special Issues on Nobel Laureates from The Journal of Physical Chemistry:
Related Links:
ACS Publications' Gerhard Ertl page
Nobel Laureates in Chemistry published in ACS journals
C&EN article: A Nobel Surprise Birthday
American Chemical Society comment on award of 2007 Nobel Prize in Chemistry