
Тел.: +7 (383) 330-67-71, факс: +7 (383) 330-80-56, E-mail: bic@catalysis.ru
630090, Россия, Новосибирск, пр-т Ак. Лаврентьева, 5

Тел.: +7 (383) 330-67-71, факс: +7 (383) 330-80-56, E-mail: bic@catalysis.ru
630090, Россия, Новосибирск, пр-т Ак. Лаврентьева, 5
Confined in a catalyst, this reactive intermediate can enan-tioselectively insert into a C–H bond
In the realm of reactivity, the vinyl carbocation is a beast so fleeting, so keen to combine or rearrange, that many have doubted that it can be tamed to create molecules in a stereoselective way. But by confining a vinyl carbocation within a bulky catalyst, chemists have now shown they can coax this wildly reactive intermediate to insert itself into a car-bon-hydrogen bond in an enantioselective manner.
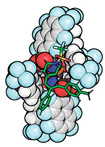 Confined to an organic catalyst (space-filling model), this vinyl carbocation substrate (stick model) is poised to react stereoselectively.
Confined to an organic catalyst (space-filling model), this vinyl carbocation substrate (stick model) is poised to react stereoselectively.
The work comes from the collaboration of researchers in Hosea M. Nelson’s group at the California Institute of Technology, Matthew S. Sigman’s group at the University of Utah, and K. N. Houk’s group at the University of California, Los Angeles.
“Historically, we’ve really struggled to make these cations catalytically and use them in synthesis,” says Sepand K. Nistanaki, a graduate student in Nelson’s lab who is the paper’s first author. “The fact that these types of carbocations can do this C–H insertion chemistry is fundamentally unique and interesting and, we think, synthetically is going to be very powerful for chemists.”
When the researchers in Nelson’s group started this project, they didn’t know if they would be able to harness the vinyl carbocation intermediates because of their extreme reactivity. After screening dozens of catalysts, the chemists found that a family of imidodiphosphorimidate organocatalysts developed by Ben List’s lab at the Max Planck Institute for Kohlenforschung were able to create and confine the vinyl cation in such a way that it would only react to form one of two possible enantiomers in a C–H insertion reaction. The chemists liken the catalyst to an enzyme in that it is able to exert stereocontrol over a highly reactive intermediate (Science 2022, DOI: 10.1126/science.ade5320).
Once his lab had observed enantioselectivity in the reaction, Nelson turned to Houk and Sigman to model the behavior computationally and verify that the reaction is indeed going through the vinyl carbocation. Now that chemists know these intermediates can be harnessed, Sigman says, they can be creative about what kind of molecules they make with this type of reaction.
“The C–H insertion of vinyl cations can be nearly barrierless,” says Matthias Brewer, a chemist at the University of Vermont who develops new reactions. “Imparting stereocontrol to this process with List’s chiral imidodi-phosphorimidate catalyst is a significant achievement,” he says in an email.
“The idea that carbocations as reactive as unstabilized vinyl cations could be engaged in highly enantioselective reactions would have seemed quite unlikely until recently,” says Harvard University’s Eric N. Jacobsen, who also develops new reactions. He says in an email that the work represents “a significant advance in asymmetric catalysis with highly reactive intermediates.”
The chemists think the area is still rich for exploration. Sigman says the information they’ve gleaned could help their team redesign the catalysts to be more practical. The chemists would also like to expand their substrates. In this work, the C–H insertion was an intramolecular transformation, in which the vinyl cation and the C–H bond were in the same molecule. Chloe G. Williams, another graduate student in Nelson’s lab, says she’d like to see the work extended to intermolecular reactions—in which the vinyl cation and the C–H bond it inserts into are on different molecules. She says the chemists would like to “understand how we can potentially use what we have learned throughout this project and apply that to other types of C–H insertion reactions to make more complex products stereoselectively.”
Recycling plastic waste using a low-cost catalyst
Hydrogenation converts polyethylene and other common polyolefins to low-mass hydrocarbons
An inexpensive catalyst can convert common commercial polymers to low molecular mass hydrocarbons under mild conditions, according to a new study. The advance may lead to cost-effective ways to produce liquid fuels and fine chemicals from plastic waste.
Researchers worldwide are developing methods for recycling plastics by chemically decomposing polymers into smaller molecular units and reusing them. Pyrolysis, for example, an approach backed by major chemical companies, converts plastics to pyrolysis oil, which can be used as a fuel or a feedstock to make other compounds. But this process is energy intensive—it often runs at 500 °C, and it produces a large mixture of products and carbonaceous waste.
Catalytic depolymerization can be more selective. And recent studies have shown that catalytic hydrogenolysis, which uses hydrogen to decompose plastics, is effective in breaking down polyethylene, polypropylene, and other common polyolefins, which tend to be fairly inert because of their strong carbon-carbon bonds. But hydrogenolysis generally uses platinum or other expensive catalysts, and it requires high temperatures and long reaction times.
To get around those shortcomings, a team of researchers based at Northwestern University looked for a highly active earth-abundant catalyst that quickly drives hydrogenolysis of polyolefins under mild conditions. Led by Michael J. Bedzyk, Yosi Kratish, and Tobin J. Marks, the team reports that they came up with an organozirconium compound supported on sulfated alumina that does the job well (Nat. Commun. 2022, DOI: 10.1038/s41467-022-34707-6).
The team studied decomposition of several polymer samples, including polypropylene, polyethylene food packaging, polyethylene copolymers, and other types of polyethylene. In one test, they found that treating 1.5 g of polyethylene with 202 kPa (2 atm) of hydrogen at 200 °C in the presence of a small amount of the catalyst completely converted the polymer to liquid and gas products in just 45 minutes. Other samples also quickly turned into low-weight hydrocarbon products. The team’s experimental and computational investigations indicate that decomposition occurs via a C–C bond scission step rather than by way of σ-bond metathesis, which is common for other catalysts.
Colorado State University’s Eugene Chen says that one of the most intriguing aspects of this “exciting and insightful” work is that the proposed catalytic species responsible for this fast polyolefin deconstruction also represents the form of a polymerization catalyst that converts α-olefins to polyolefins. “It’s conceivable that this catalyst could be used for both polyolefin production and deconstruction simply by switching hydrogen on and off and adjusting the reaction temperature,” he says.
Susannah L. Scott of the University of California, Santa Barbara, says it’s remarkable that the active form of the catalyst appears to be air stable. This is important, she explains, because real plastic waste will have many different contaminants that the catalyst must tolerate. She notes, however, that the quantity of hydrogen used in this study may represent a challenge for a large-scale process. To convert all the world’s polyole-fin waste in this way would require a significant fraction of current world hydrogen production, she says.
This catalyst is a golden egg
Eggshells impregnated with gold nanoparticles make an easy, cheap, and reusable ‘megacatalyst’
By infusing eggshells with gold nanoparticles, researchers have made a low-cost, sustainable catalyst that can be reused and eventually recycled (ACS Appl. Mater. Interfaces 2022, DOI: 10.1021/acsami.2c13564). They used the robust “megacatalyst” to detoxify dye waste and run other organic reactions by dropping the eggshell catalyst into reaction solutions, and they could simply remove it by hand when done.
Typically, to make nanoparticle catalysts easy to reuse, chemists anchor them to porous materials such as hydrogels and metal-organic frameworks. These impregnated materials are easy to separate from a reaction mixture, but making them is laborious and expensive. Also, recovering the metal from the support material usually requires time- or energy-intensive methods like magnetic separation and centrifugation.
Anil K. Suresh of SRM University, Andhra Pradesh, and colleagues chose eggshells as supports because of their high porosity and large surface area with a protein layer rich in amino acids. The team dipped waste eggshells collected from restaurants in a suspension containing gold chloride for 6 h, and amino acids on the shells reduced the gold ions to form crystalline gold nanoparticles. Suresh claims this is the simplest synthesis of a supported catalyst ever reported.
The researchers tested the recyclability of the eggshell catalyst by using it to detoxify dye waste from local textile operations. They reused the eggshell 14 times to break down a total of 2.8 L of waste. They also used a gold-infused eggshell to make several grams of a precursor to aceta-minophen by hydrogenating 4-nitrophenol, with a conversion efficiency of 99%.
Dipping the eggshell in nitric acid and hydrochloric acid dissolved the protein layer on its surface, releasing the nanoparticles and allowing the team to recoup about 77% of the gold. Suresh says he and his colleagues have also have grafted catalytic silver and copper oxide on eggshells and are now working on methods to do this for platinum and palladium.
Chemical & Engineering News