24 декабря 2019 года на заседании Президиума Российской академии наук было принято решение о присуждении Премии имени А.А. Баландина доктору химических наук Когану Виктору Мироновичу (Институт органической химии им. Н.Д. Зелинского Российской академии наук), доктору химических наук Никульшину Павлу Анатольевичу (Акционерное общество «Всероссийский научно-исследовательский институт по переработке нефти») и доктору химических наук Пимерзину Андрею Алексеевичу (Самарский государственный технический университет) за цикл работ «Новые представления о катализе сульфидами переходных металлов: от моделей к промышленным катализаторам».
Премия имени А.А. Баландина присуждается Российской академией наук с 1993 года за выдающиеся работы в области катализа.
История присуждения премии:
| 2016 | В.И. Бухтияров И.В. Коптюг А.Ю. Стахеев | За серию работ «Наноструктурирование активного компонента – метод управления каталитическими свойствами нанесенных металлических катализаторов в реакциях гидрирования и окисления» |
| 2013 | Лапидус А.Л. Усачев Н.Я. Третьяков В.Ф. |
За серию работ “Теоретические основы создания каталитических процессов переработки ненефтяного сырья в углеводородные топлива и продукты для нефтехимии” |
| 2010 | Анаников В.П. Белецкая И.П. |
За работу “Катализируемые переходными металлами реакции присоединения к ацетиленовым углеводородам” |
| 2007 | Садыков В.А. | За серию работ “Роль дефектности и микроструктуры катализаторов окислительно-восстановительных реакций” |
| 2004 | Лин Г.И. Розовский А.Я. |
За цикл работ “Исследование механизма и кинетики каталитических превращений одноуглеродных молекул” |
| 2001 | Андрушкевич Т.В. Бондарева В.М. Попова Г.Я. |
За серию работ “Гетерогенно-каталитическое окисление основных органических соединений в карбоновые кислоты: механизм, кинетика, дизайн катализаторов” |
| 1998 | Грязнов В.М. | За серию работ “Исследования механизма реакций с переносом атомов водорода на металлах, создание новых катализаторов и способов проведения этих реакций” |
| 1995 | Лунин В.В. | За цикл работ “Новые гетерогенные катализаторы на основе интерметаллических соединений и их гидридов” |
| 1993 | Миначев Х.М. Слинкин А.А. Шпиро Е.С. |
За цикл работ “Структура поверхности и каталитические свойства высокодисперсных металлнанесенных систем” |
XI Международная конференция
«Механизмы каталитических реакций»
7-11 октября 2019 г., Сочи, Россия
http://conf.nsc.ru/mcr2019/en/
XI Международная конференция «Механизмы каталитических реакций» состоялась в конференц-центре отеля «Golden Tulip», Роза Хутор, г. Сочи, Краснодарский край, Россия с 7 по 11 октября 2019 г. Конференция была проведена при финансовой поддержке Российского фонда фундаментальных исследований. Финансовую поддержку конференции оказали также Партнеры конференции: фирма SPECS-TII RUS, фирма SPECS Surface Nano Analysis GmbH, Новомичуринский катализаторный завод, Компания «SocTrade», Общество с ограниченной ответственностью «Креатор Техно», LLC «Merck». В рамках конференции была организована выставка рекламных материалов компаний-партнеров.
Конференция была проведена под эгидой Европейской федерации каталитических обществ (EFCATS) и НП «Национальное каталитическое общество».
Конференция «Механизмы каталитических реакций» имеет солидную историю. Изначально она была создана в 1974 году как российская конференция, посвященная актуальным проблемам каталитической науки, но затем, в 2009 году, вышла на международный уровень, оставив английский язык в качестве единственного рабочего. География прошедших конференций включает несколько городов – это Москва (1974, 1979, 1986, 1990, 2002 гг.), Новосибирск (1982, 2009 гг.), Санкт-Петербург (2006, 2012 гг.), Светлогорск (2016 г.). С 2009 года международные МКР-конференции традиционно проходят под эгидой Европейской ассоциации каталитических обществ (EFCATS), с 2016 года – также под эгидой НП «Национальное каталитическое общество».
В конференции 2019 года приняли участие 246 ученых из 45 городов 15 стран мира, в том числе, Россия – 205, Германия – 8, Китай – 5, Великобритания, Австрия, Чехия, Польша – по 3, Мексика – 2, Израиль, Испания, Болгария, Саудовская Аравия, Швейцария – по 1.
Организаторами конференции выступили:
В ходе работы ХI Конференции участники имели возможность прослушать и обсудить 6 пленарных лекций, 4 ключевых лекции, 4 приглашенных устных доклада (20 мин), 66 устных докладов (20 мин), а также 104 стендовых презентаций. Кроме того, 9 октября в рамках Конференции была проведена Школа-конференция молодых ученых «Катализ для энергетики, переработки ископаемого и возобновляемого сырья», включавшая в себя 1 ключевую лекцию и 15 кратких устных докладов (10 мин).
Работа Конференции проходила одновременно на двух параллельных устных сессиях. Тематика включала преимущественно вопросы, связанные с фундаментальными исследованиями закономерностей каталитических реакций, дизайна новых каталитических систем, определения их физико-химических свойств; затрагивались вопросы разработки принципиально новых, а также модифицированных каталитических систем и процессов; особое внимание было уделено исследованию эволюции каталитических систем и реакционных сред в ходе проведения реакций методами in situ и operando, теории и моделированию в катализе, каталитическим системам для решения актуальных задач энергетики, наук о материалах, устойчивого развития, переработки ископаемого и возобновляемого сырья. Программа конференции включала 5 секций:
Сборник тезисов конференции был выпущен на электронном носителе. Материалы избранных устных докладов будут опубликованы в специальных выпусках журналов Topics in Catalysis (издательство Springer Nature) и Кинетика и катализ (издательство Pleiades Publishing) в 2020 году.
Пленарные лекции
Пленарные лекции затрагивали вопросы создания и использования катализаторов для решения актуальных проблем химической индустрии, установления детального механизма каталитического действия практически значимых и перспективных катализаторов и каталитических систем, а также применения и развития современных способов изучения каталитических реакций. Открывала программу Конференции Мемориальная лекция академика В.Н. Пармона (Президиум РАН) «Академик К.И. Замараев: жизнь, отданная науке и катализу», посвященная памяти академика К.И. Замараева (1939-1996 гг.). Валентин Николаевич Пармон, вице-президент РАН, ученик Кирилла Ильича, рассказал об основных вехах жизни и карьеры академика Замараева. По инициативе Кирилла Ильича в 1980-х годах 20 века в научный обиход Института катализа широко внедрялись физические методы исследования, были начаты фундаментальные разработки мирового уровня. Благодаря этому основной организатор конференции – Институт катализа им. Г.К. Борескова СО РАН – вышел на международный уровень и приобрел мировую известность.
 В лекции другой ученицы академика Замараева, д.х.н. О.А. Холдеевой (Институт катализа им. Г.К. Борескова СО РАН) «Механизмы активации пероксида водорода на центрах Ti(IV) и Nb(V)» были представлены последние достижения в области изучения механизмов активации широко используемого «зеленого» окислителя H2O2 на гетерометаллических центрах Ti(IV)- и Nb(V)-замещенных поливольфраматов. Установлено, что пероксидная форма каталитически активных центров HMO2 менее активна в отношении эпоксидирования олефинов, чем гидропероксидная форма MOOH. Показано, что для Ti-замещенных вольфраматов преобладает η1–координированная гидропероксидная форма Ti(η1-OOH), а для Nb-замещенных –
В лекции другой ученицы академика Замараева, д.х.н. О.А. Холдеевой (Институт катализа им. Г.К. Борескова СО РАН) «Механизмы активации пероксида водорода на центрах Ti(IV) и Nb(V)» были представлены последние достижения в области изучения механизмов активации широко используемого «зеленого» окислителя H2O2 на гетерометаллических центрах Ti(IV)- и Nb(V)-замещенных поливольфраматов. Установлено, что пероксидная форма каталитически активных центров HMO2 менее активна в отношении эпоксидирования олефинов, чем гидропероксидная форма MOOH. Показано, что для Ti-замещенных вольфраматов преобладает η1–координированная гидропероксидная форма Ti(η1-OOH), а для Nb-замещенных –
 В лекции профессора Юн Ли (Jun Li, Tsinghua University) были рассмотрены последние достижения в области ab initio моделирования нанокатализаторов Au20/TiO2 и Au20/CeO2 методом молекулярной динамики. Показано, что микроскопические механизмы каталитических реакций в наномасштабе включают в себя динамический «одноатомный» (single-atom) катализ (DSAC), который объясняет активность и размерный эффект золотых катализаторов. Изложены разработанные теоретические подходы к описанию явления DSAC и обобщены данные по известным явлениям данного типа.
В лекции профессора Юн Ли (Jun Li, Tsinghua University) были рассмотрены последние достижения в области ab initio моделирования нанокатализаторов Au20/TiO2 и Au20/CeO2 методом молекулярной динамики. Показано, что микроскопические механизмы каталитических реакций в наномасштабе включают в себя динамический «одноатомный» (single-atom) катализ (DSAC), который объясняет активность и размерный эффект золотых катализаторов. Изложены разработанные теоретические подходы к описанию явления DSAC и обобщены данные по известным явлениям данного типа.
 Профессор Паоло Форнасьеро (Paolo Fornasiero, University of Trieste, Italy) в своей пленарной лекции остановился на дизайне современных катализаторов для процессов селективной конверсии биомассы, фотокаталитического получения водорода, окисления метана и восстановления углекислого газа. Особое внимание было уделено «одноатомным» (single-atom) катализаторам, не содержащим металлов органокатализаторам и так называемым «умным» («intelligent») катализаторам.
Профессор Паоло Форнасьеро (Paolo Fornasiero, University of Trieste, Italy) в своей пленарной лекции остановился на дизайне современных катализаторов для процессов селективной конверсии биомассы, фотокаталитического получения водорода, окисления метана и восстановления углекислого газа. Особое внимание было уделено «одноатомным» (single-atom) катализаторам, не содержащим металлов органокатализаторам и так называемым «умным» («intelligent») катализаторам.
 Пленарная лекция доктора Хендрика Блюма (Hendrik Bluhm, PhD, Fritz Haber Institute of the Max Planck Society, Berlin, Germany) «Гетерогенная химия на границе раздела жидкость/пар, исследованная методом фотоэлектронной спектроскопии» была посвящена анализу возможностей применения метода рентгеновской фотоэлектронной спектроскопии для in situ исследования явлений на границе твердое тело – жидкость/пар. Автор рассказал об особенностях конструирования РФЭС спектрометров, способных получать спектры при давлениях до 10 мбар и привел несколько примеров изучения адсорбции воды на металлических поверхностях.
Пленарная лекция доктора Хендрика Блюма (Hendrik Bluhm, PhD, Fritz Haber Institute of the Max Planck Society, Berlin, Germany) «Гетерогенная химия на границе раздела жидкость/пар, исследованная методом фотоэлектронной спектроскопии» была посвящена анализу возможностей применения метода рентгеновской фотоэлектронной спектроскопии для in situ исследования явлений на границе твердое тело – жидкость/пар. Автор рассказал об особенностях конструирования РФЭС спектрометров, способных получать спектры при давлениях до 10 мбар и привел несколько примеров изучения адсорбции воды на металлических поверхностях.
 В лекции проф. Грэхема Хатчингса (Graham Hutchings, Cardiff Catalysis Institute) «Катализ с использованием наноматериалов» были подведены многолетние итоги разработки катализаторов на основе наночастиц золота для ряда практически значимых каталитических процессов, таких как окисление монооксида углерода (при комнатной температуре), синтез винилхлорида гидрохлорированием ацетилена. В КНР катализаторы синтеза винилхлорида на основе наночастиц золота уже коммерциализованы. Кратко рассмотрены существующие проблемы и намечены практические перспективы развития области катализаторов на основе наночастиц золота и сплавов золота с палладием.
В лекции проф. Грэхема Хатчингса (Graham Hutchings, Cardiff Catalysis Institute) «Катализ с использованием наноматериалов» были подведены многолетние итоги разработки катализаторов на основе наночастиц золота для ряда практически значимых каталитических процессов, таких как окисление монооксида углерода (при комнатной температуре), синтез винилхлорида гидрохлорированием ацетилена. В КНР катализаторы синтеза винилхлорида на основе наночастиц золота уже коммерциализованы. Кратко рассмотрены существующие проблемы и намечены практические перспективы развития области катализаторов на основе наночастиц золота и сплавов золота с палладием.
Ключевые лекции
 Лекция доктора Джастина Харгривса (Justin Hargreaves, Uni-versity of Glasgow) была посвящена перспективам развития и
совершенствования одного из важнейших промышленных процессов XX столетия – синтеза аммиака Габера-Боша. В частности, показано, что в электро- и фотокаталитических вариантах данная реакция может быть проведена в гораздо более мягких условиях, нежели те, что используются в промышленности. Это позволит также уменьшить масштабы промышленного процесса, что даст возможность провести техническое перевооружение на промышленные установки нового поколения. Рассмотрены подходы к приготовлению металл-нитридных катализаторов синтеза аммиака, способных работать согласно механизму Марса-Ван-Кревелена.
Лекция доктора Джастина Харгривса (Justin Hargreaves, Uni-versity of Glasgow) была посвящена перспективам развития и
совершенствования одного из важнейших промышленных процессов XX столетия – синтеза аммиака Габера-Боша. В частности, показано, что в электро- и фотокаталитических вариантах данная реакция может быть проведена в гораздо более мягких условиях, нежели те, что используются в промышленности. Это позволит также уменьшить масштабы промышленного процесса, что даст возможность провести техническое перевооружение на промышленные установки нового поколения. Рассмотрены подходы к приготовлению металл-нитридных катализаторов синтеза аммиака, способных работать согласно механизму Марса-Ван-Кревелена.
 Ключевая лекция профессора К. Лимберга (Christian Limberg, Humboldt University Berlin, Germany) была посвящена исследованию молекулярных соединений оксида алюминия и железа, выступающих в качестве моделей для активных площадок. Была представлена информация по синтезу новых многоядерных силоксидных комплексов железа (II), которые отвечают за каталитическую активность железо-модифицированных цеолитов.
Ключевая лекция профессора К. Лимберга (Christian Limberg, Humboldt University Berlin, Germany) была посвящена исследованию молекулярных соединений оксида алюминия и железа, выступающих в качестве моделей для активных площадок. Была представлена информация по синтезу новых многоядерных силоксидных комплексов железа (II), которые отвечают за каталитическую активность железо-модифицированных цеолитов.
 Профессор Гюнтер Руппрехтер (Günter Rupprechter, Institute of Materials Chemistry, Technische Universität Wien, Vienna, Austria) представил ключевую лекцию «In situ поверхностная спектроскопия и микроскопия реакций на модельных катализаторах на основе диоксида циркония». Были рассмотрены результаты in situ исследований механизма окисления СО на поверхности модельных катализаторов на основе ZrO2. Показано совместное применение методов РФЭС и колебательной спектроскопии, позволяющее одновременно изучать как состояние катализатора непосредственно в условиях реакции, так и основные реакционные интермедиаты, образующиеся на его поверхности.
Профессор Гюнтер Руппрехтер (Günter Rupprechter, Institute of Materials Chemistry, Technische Universität Wien, Vienna, Austria) представил ключевую лекцию «In situ поверхностная спектроскопия и микроскопия реакций на модельных катализаторах на основе диоксида циркония». Были рассмотрены результаты in situ исследований механизма окисления СО на поверхности модельных катализаторов на основе ZrO2. Показано совместное применение методов РФЭС и колебательной спектроскопии, позволяющее одновременно изучать как состояние катализатора непосредственно в условиях реакции, так и основные реакционные интермедиаты, образующиеся на его поверхности.
 Ключевая лекция д.х.н., проф. РАН К.П. Брылякова (Институт катализа им. Г.К. Борескова СО РАН) была посвящена динамическим нелинейным эффектам (ДНЛЭ) в асимметрическом катализе –
асимметрическому автокатализу, асимметрической автоиндукции и асимметрической автоамплификации. Данные явления рассмотрены с позиций асимметрического катализа и синтетической химии. Возникновение динамической нелинейности проиллюстрировано с точки зрения химической кинетики. Данные явления могут возникать во многих стереоселективных каталитических процессах и оказывать как положительное, так и отрицательное влияние на оптический выход целевых реакций. Кроме того, показано, что ДНЛЭ могли быть причиной возникновения на Земле биологической гомохиральности, являющейся необходимым условием существования живой материи.
Ключевая лекция д.х.н., проф. РАН К.П. Брылякова (Институт катализа им. Г.К. Борескова СО РАН) была посвящена динамическим нелинейным эффектам (ДНЛЭ) в асимметрическом катализе –
асимметрическому автокатализу, асимметрической автоиндукции и асимметрической автоамплификации. Данные явления рассмотрены с позиций асимметрического катализа и синтетической химии. Возникновение динамической нелинейности проиллюстрировано с точки зрения химической кинетики. Данные явления могут возникать во многих стереоселективных каталитических процессах и оказывать как положительное, так и отрицательное влияние на оптический выход целевых реакций. Кроме того, показано, что ДНЛЭ могли быть причиной возникновения на Земле биологической гомохиральности, являющейся необходимым условием существования живой материи.
 Ключевую лекцию Молодежной школы-конференции «Катализ для энергетики, переработки ископаемого и возобновляемого сырья» представил известный специалист Хорхе Гаскон (Jorge Gascón, King Ab-dullah University of Science and Technology, KAUST Catalysis Center (KCC), Advanced Catalytic Materials, Thuwal, Saudi Arabia). Он рассказал о последних достижениях в области разработки новых катализаторов и каталитических процессов прямого гидрирования диоксида углерода (СО2) с целью получения таких важных химических продуктов, как метанол, легкие олефины и ароматические углеводороды. Важным аспектом данных разработок является необходимость создания катализаторов, способных работать в относительно мягких условиях, что обеспечивает максимальную селективность процесса. В качестве примеров были продемонстрированы системы на основе цеолитов, оксида индия, металлоорганических каркасов (MOF).
Ключевую лекцию Молодежной школы-конференции «Катализ для энергетики, переработки ископаемого и возобновляемого сырья» представил известный специалист Хорхе Гаскон (Jorge Gascón, King Ab-dullah University of Science and Technology, KAUST Catalysis Center (KCC), Advanced Catalytic Materials, Thuwal, Saudi Arabia). Он рассказал о последних достижениях в области разработки новых катализаторов и каталитических процессов прямого гидрирования диоксида углерода (СО2) с целью получения таких важных химических продуктов, как метанол, легкие олефины и ароматические углеводороды. Важным аспектом данных разработок является необходимость создания катализаторов, способных работать в относительно мягких условиях, что обеспечивает максимальную селективность процесса. В качестве примеров были продемонстрированы системы на основе цеолитов, оксида индия, металлоорганических каркасов (MOF).
Устные доклады
 I секция. В приглашенном устном докладе к.х.н. К.В. Ковтунова (K. I. Kovtunov, Международный томографический центр СО РАН, Новосибирск, Россия) «In situ исследование механизмов гетерогенного гидрирования параводородом» представлен новый подход к исследованию механизма процессов гидрирования непредельных углеводородов с помощью спектроскопии 1Н ЯМР с использованием явления индуцированной параводородом поляризации ядер. Ранее подобные подходы применялись лишь для исследования механизмов гомогенно-каталитических процессов гидрирования. С помощью данного метода получен ряд новых результатов: так, показано, что гидрирование пропилена на гетерогенных катализаторах частично протекает парным образом, что формально противоречит существующим представлениям, подразумевающим диссоциативный механизм адсорбции молекулы водорода. Предложены гипотезы для объяснения наблюдаемых особенностей.
I секция. В приглашенном устном докладе к.х.н. К.В. Ковтунова (K. I. Kovtunov, Международный томографический центр СО РАН, Новосибирск, Россия) «In situ исследование механизмов гетерогенного гидрирования параводородом» представлен новый подход к исследованию механизма процессов гидрирования непредельных углеводородов с помощью спектроскопии 1Н ЯМР с использованием явления индуцированной параводородом поляризации ядер. Ранее подобные подходы применялись лишь для исследования механизмов гомогенно-каталитических процессов гидрирования. С помощью данного метода получен ряд новых результатов: так, показано, что гидрирование пропилена на гетерогенных катализаторах частично протекает парным образом, что формально противоречит существующим представлениям, подразумевающим диссоциативный механизм адсорбции молекулы водорода. Предложены гипотезы для объяснения наблюдаемых особенностей.
Д.х.н. М.Ю. Синев (Институт химической физики РАН, Москва, Россия) представил доклад «Соотношения между наблюдаемой кинетикой и механизмом окислительно-восстановительных процессов: активация кислорода и маршруты окисления легких алканов». В докладе проанализированы кинетические особенности ряда каталитических процессов окисления легких алканов, не содержащих благородных и переходных металлов. Обрисованы подходы к решению обратной кинетической задачи для сложных многостадийных процессов окисления, для которых известен механизм и проведены кинетические измерения.
 Профессор Вэй Сун (Wei Sun, Lanzhou Institute of Chemical
Physics, Lanzhou, China) в докладе «Исследование механизма процессов энантиоселективного окисления в присутствии хиральных комплексов марганца» изложил ряд новых результатов, позволяющих составить представление о природе каталитически активных центров процессов энантиоселективного эпоксидирования олефинов и окислительной десимметризации спироциклических соединений пероксидом водорода в присутствии комплексов марганца с хиральными N4-донорными лигандами. Показано, что данные «биомиметические» катализаторы в некоторых отношениях ведут себя подобно природным металлоферментам, активируя С-Н связи по рекомбинационному механизму, характерному для ферментов семейства цитохромов P450.
Профессор Вэй Сун (Wei Sun, Lanzhou Institute of Chemical
Physics, Lanzhou, China) в докладе «Исследование механизма процессов энантиоселективного окисления в присутствии хиральных комплексов марганца» изложил ряд новых результатов, позволяющих составить представление о природе каталитически активных центров процессов энантиоселективного эпоксидирования олефинов и окислительной десимметризации спироциклических соединений пероксидом водорода в присутствии комплексов марганца с хиральными N4-донорными лигандами. Показано, что данные «биомиметические» катализаторы в некоторых отношениях ведут себя подобно природным металлоферментам, активируя С-Н связи по рекомбинационному механизму, характерному для ферментов семейства цитохромов P450.
К.х.н. Е.А. Лашина (Институт катализа им. Г.К. Борескова СО РАН) в докладе «Автоколебания в ходе окисления CH4, C2H6 и C3H8 на металлических катализаторах: математическое моделирование с использованием квазистационарного приближения» представила математическую модель изотермических автоколебаний в реакциях окисления легких алканов (C1-C3) на поверхности никеля. Автоколебания в реакции окисления никеля удовлетворительно описываются системой трех дифференциальных уравнений. Показано, что для окисления пропана применение метода квазистационарных концентраций также позволяет существенно сократить число дифференциальных уравнений и получить адекватную математическую модель наблюдаемых явлений.
II cекция. Секция II была посвящена современным методам изучения механизмов каталитических реакций. В докладах были представлены новые способы исследования механизмов с использованием современных физико-химических методов, включая методы in situ и operando, а также новые способы обработки данных. К.х.н. О. А. Булавченко (Институт катализа им. Г.К. Борескова СО РАН) в докладе «In situ РД и РФЭС исследование восстановления смешанных Mn-Zr и Mn-Co оксидных катализаторов окисления CO» рассказала об исследовании окисления СО на оксидных катализаторах на основе циркония, кобальта и марганца методами рентгеновской дифракции и РФЭС. К.х.н. А.А. Сараев (Институт катализа им. Г.К. Борескова СО РАН) представил доклад «CuFeAl нанокомпозитные катализаторы окисления СО: Operando XAS исследования», в котором рассказал о исследовании катализаторов методами EXAFS и XANES. К.х.н. А.В. Бухтияров (Институт катализа им. Г.К. Борескова СО РАН) представил результаты РФЭС исследования окисления СО на модельных биметаллических катализаторах Pd-Au/HOPG методом РФЭС.
 Приглашенный устный доклад профессора Е. Кондратенко (E. Kondratenko, Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Rostock, Germany) «Потенциал время-разрешенной operando UV-Vis спектроскопии для изучения образования и удаления кокса в дегидрировании пропана» был посвящен operando исследованиям дегидрирования пропана на катализаторах на основе оксида циркония. Данное направление очень актуально в мире вследствие добычи природного газа, обогащенного пропанам, на новых шельфовых месторождениях. С российской стороны тему исследований продолжил к.х.н. В.В. Каичев (Институт катализа им. Г.К. Борескова СО РАН) докладом «Дегидрирование пропана на ванадий-титановых катализаторах: активные центры и механизм реакций». Автор представил результаты in situ исследований механизмов окислительного и неокислительного дегидрирования пропана на монослойных катализаторах V2O5/TiO2. Исследования проводились методами РФЭС и ИК-спектроскопии, что позволило определить состояние катализатора и основные интермедиаты реакции.
Приглашенный устный доклад профессора Е. Кондратенко (E. Kondratenko, Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Rostock, Germany) «Потенциал время-разрешенной operando UV-Vis спектроскопии для изучения образования и удаления кокса в дегидрировании пропана» был посвящен operando исследованиям дегидрирования пропана на катализаторах на основе оксида циркония. Данное направление очень актуально в мире вследствие добычи природного газа, обогащенного пропанам, на новых шельфовых месторождениях. С российской стороны тему исследований продолжил к.х.н. В.В. Каичев (Институт катализа им. Г.К. Борескова СО РАН) докладом «Дегидрирование пропана на ванадий-титановых катализаторах: активные центры и механизм реакций». Автор представил результаты in situ исследований механизмов окислительного и неокислительного дегидрирования пропана на монослойных катализаторах V2O5/TiO2. Исследования проводились методами РФЭС и ИК-спектроскопии, что позволило определить состояние катализатора и основные интермедиаты реакции.
 В. Мурзин (V. Murzin, Deutsches Elektronen-Synchrotron DESY, Hamburg, Germany) представил доклад «Исследования катализаторов методом спектроскопии рентгеновского поглощения на станции P64/P65 в Международном центре синхротронного излучения DESY», в котором продемонстрировал возможности станций и привел ряд примеров использования синхротронного излучения в исследованиях катализаторов. Александр Клюшин (A.Yu. Klyushin, Fritz Haber Institute of the Max Planck Society, Berlin, Germany) в своем докладе «Активация золота через эффект сильного взаимодействия метал-носитель в реакции окисления СО» рассмотрел результаты, полученные в Международном центре синхротронного излучения BESSY. Проведенное им in situ исследование методом РФЭС и масс-спектроскопии позволило выявить причины активации золота, нанесенного на оксид титана.
В. Мурзин (V. Murzin, Deutsches Elektronen-Synchrotron DESY, Hamburg, Germany) представил доклад «Исследования катализаторов методом спектроскопии рентгеновского поглощения на станции P64/P65 в Международном центре синхротронного излучения DESY», в котором продемонстрировал возможности станций и привел ряд примеров использования синхротронного излучения в исследованиях катализаторов. Александр Клюшин (A.Yu. Klyushin, Fritz Haber Institute of the Max Planck Society, Berlin, Germany) в своем докладе «Активация золота через эффект сильного взаимодействия метал-носитель в реакции окисления СО» рассмотрел результаты, полученные в Международном центре синхротронного излучения BESSY. Проведенное им in situ исследование методом РФЭС и масс-спектроскопии позволило выявить причины активации золота, нанесенного на оксид титана.
 III cекция. В устном приглашенном докладе С. Пеннера
(S. Penner, Institute of Physical Chemistry, University of Innsbruck,
Innsbruck, Austria) «Определенная in situ структурная динамика как ключевой параметр механизма в реакционной способности катализаторов сухого риформинга метана на основе LaNiO3» рассмотрены закономерности динамики восстановительной трансформации катализатора углекислотной конверсии метана на основе никелата лантана LaNiO3 под воздействием реакционной среды, изученные с помощью рентгеноструктурных исследований in situ на синхротронном излучении. Показана сложность таких процессов, протекающих через промежуточное образование слоистого никелата лантана со структурой Раддлсдена–Поппера, что определяет как размеры наночастиц металлического никеля, так и их сильное взаимодействие с оксидом/гидроксокарбонатом лантана в стационарном состоянии, влияющее на каталитическую активность и устойчивость к зауглероживанию.
III cекция. В устном приглашенном докладе С. Пеннера
(S. Penner, Institute of Physical Chemistry, University of Innsbruck,
Innsbruck, Austria) «Определенная in situ структурная динамика как ключевой параметр механизма в реакционной способности катализаторов сухого риформинга метана на основе LaNiO3» рассмотрены закономерности динамики восстановительной трансформации катализатора углекислотной конверсии метана на основе никелата лантана LaNiO3 под воздействием реакционной среды, изученные с помощью рентгеноструктурных исследований in situ на синхротронном излучении. Показана сложность таких процессов, протекающих через промежуточное образование слоистого никелата лантана со структурой Раддлсдена–Поппера, что определяет как размеры наночастиц металлического никеля, так и их сильное взаимодействие с оксидом/гидроксокарбонатом лантана в стационарном состоянии, влияющее на каталитическую активность и устойчивость к зауглероживанию.
В устном докладе д.х.н. В.А. Садыкова (Институт катализа им. Г.К. Борескова СО РАН) «Детальный механизм трансформации этанола в сингаз на нанокомпозитных катализаторах» представлены результаты изучения детального механизма трансформации этанола в синтез-газ (селективное окисление, паровая конверсия, парокислородная конверсия и др.) на мезопористых нанокомпозитных катализаторах (нанесенные на алюминат магния сложные оксиды со структурой шпинели, перовскита и флюорита, обладающие высокой подвижностью и реакционной способностью кислорода, промотированные никелем и рутением) с использованием комбинации релаксационных методов (18O SSITKA, in situ ИК-спектроскопия в проточных ячейках) и импульсной микрокалориметрии. Квантовохимический метод DFT был использован для анализа энергетических барьеров стадий трансформации реагентов и интермедиатов. Оценка констант базовых стадий механизма показала, что лимитирующим является разрыв С-С связи в этоксикомплексе или ацетальдегиде. Энергетический барьер этой стадии снижается при промотировании катализаторов рутением за счет реализации нового маршрута внедрения терминального кислорода Ru-O по С-С связи интермедиатов, адсорбированных на соседних катионах переходных/редкоземельных элементов.
 В докладе А.А. Габриенко (Институт катализа СО РАН им. Г.К. Борескова) «Активация метана на Cu/H-ZSM-5 цеолитах: спектроскопическое изучение взаимодействия метана с различными Cu центрами» рассмотрены маршруты активации
метана на цеолите Cu/H-ZSM-5, определяющие его каталитические свойства в реакции трансформации метана в метанол. Применение комбинации спектроскопических методов (DRIFTS, UV-Vis DRS, 13C CP/MAS NMR) и H-D обмена показало, что активация метана с образованием метокси-групп протекает с участием как катионов Cu2+, так и бренстедовских кислотных центров. Использование 13C CP/MAS NMR спектроскопии позволило изучить закономерности взаимодействия метокси-групп с СО, Н2О, бензолом и др., определяющими селективности побочных процессов.
В докладе А.А. Габриенко (Институт катализа СО РАН им. Г.К. Борескова) «Активация метана на Cu/H-ZSM-5 цеолитах: спектроскопическое изучение взаимодействия метана с различными Cu центрами» рассмотрены маршруты активации
метана на цеолите Cu/H-ZSM-5, определяющие его каталитические свойства в реакции трансформации метана в метанол. Применение комбинации спектроскопических методов (DRIFTS, UV-Vis DRS, 13C CP/MAS NMR) и H-D обмена показало, что активация метана с образованием метокси-групп протекает с участием как катионов Cu2+, так и бренстедовских кислотных центров. Использование 13C CP/MAS NMR спектроскопии позволило изучить закономерности взаимодействия метокси-групп с СО, Н2О, бензолом и др., определяющими селективности побочных процессов.
В докладе М.Л. Грингольц (Институт нефтехимического синтеза им. Топчиева, Москва) «Новые макромолекулярные реакции кросс-метатезиса в смесях полинорборнена с полидиенами на катализаторах Граббса: кинетическое исследование» рассмотрены проблемы кинетики и механизма сложных реакций кросс-метатезиса различных полимеров, содержащих двойные С=С связи, катализируемых карбеном рутения (катализатор Граббса), что позволяет разработать научные подходы к синтезу многоблочных сополимеров, обладающих улучшенными механическими свойствами и повышенной стабильностью. Кинетика процесса взаимодействия промышленных полимеров (полинорборнена) и полидиенов, а именно, полибутадиена и цис-/транс-полиизопрена, была исследована с помощью in situ 1H NMR для контроля трансформации Ru-карбеновых комплексов как активных центров и ex situ 13C NMR для контроля характеристик полимеров. Несмотря на крайнюю сложность протекающих процессов, авторы смогли показать адекватность разработанной ранее кинетической схемы и оценить константы элементарных стадий процесса. Установлено, что самой быстрой стадией является взаимодействие карбенов рутений-полиноpборнены с двойными связями в цепях полибутадиена.
IV cекция. В качестве приглашенного устного докладчика на секции IV выступила профессор Е.В. Скорб (Университет ИТМО, Санкт-Петербург, Россия) с лекцией «Дизайн слоистых материалов с фотокаталитически активной поверхностью для бионических приборов и инфохимии». Основная часть доклада была посвящена фотогенерации и использованию градиента локализованной концентрации ионов.
Чл.-корр. РАН А.А. Ремпель (Институт металлургии УрО РАН, Екатеринбург, Россия) представил устный доклад «Окисление органических молекул на гибридных фотокатализаторах диоксид титана – сульфид кадмия, активных под видимым светом», в котором обсуждались методы синтеза фотокатализаторов, активных в процессах окисления под действием видимого света, и их каталитические свойства.
Далее выступила д.х.н., проф. И.И. Михайленко (РУДН, Москва, Россия) с докладом «Дегидратация изобутанола на катализаторах Ag-ZP, полученных золь-гель методом». Ее работа посвящена изучению реакции дегидратации изобутанола для получения желаемого продукта превращения – изобутена.
Доклад д.х.н. Е.А. Козловой (Институт катализа им. Г.К. Борескова СО РАН) «Фотокаталитическое восстановление CO2 на катализаторах на основе Cd1-xZnxS: влияние фазового состава на скорость реакции и распределение продуктов» был посвящен изучению фотокатализаторов на основе твердых растворов кадмия и цинка для актуального процесса восстановления углекислого газа под действием видимого света. Работа к.х.н. Ю.С. Демидовой (Институт катализа им. Г.К. Борескова СО РАН) «Гидрирование монотерпеноидов в присутствии катализаторов на основе золота и платины» была посвящена гидрированию монотерпеноидов с использованием катализаторов с золотом и платиной. В докладе проф. Е.С. Локтевой (Московский государственный университет, Москва, Россия) «Эффект природы темплата и состава двойных и тройных оксидных катализаторов на основе CeO2 в окислении СО» было рассмотрено влияние природы темплата и состава оксидных катализаторов на их активность в процессе окисления угарного газа.
Школа-конференция молодых ученых
«Катализ для производства энергии, топлив, возобновляемых ресурсов»
На конференции были широко представлены работы молодых ученых из России и зарубежных стран, причем уровень многих докладов был достаточно высоким. Выступления докладчиков показали, что во многих далеких от столицы исследовательских центрах имеется современное научное оборудование для физико-химических исследований и квантовохимических расчетов, позволяющее проводить детальное изучение каталитических реакций и их механизмов и публиковать данные в международных научных журналах с высокими индексами цитируемости. Можно отметить следующие доклады, продемонстрировавшие высокий уровень фундаментальных достижений и практическую значимость.
 Большинство устных докладов молодых ученых было посвящено фундаментальным исследованиям свойств катализаторов различной природы.
Р. Нугид (R. Nuguid, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland) в своем докладе «Изучение механизма селективного каталитического восстановления NO методом рамановской спектроскопии с модулированным возбуждением» представил новый подход к характеризации активных центров катализатора V2O5/TiO2, используемого для реакции селективного восстановления NO в NH3. В основе подхода лежит метод КР-спектроскопии с модулируемым возбуждением. Это выступление было признано оргкомитетом лучшим докладом молодого ученого, а Р. Нугид был награжден специальным дипломом.
Большинство устных докладов молодых ученых было посвящено фундаментальным исследованиям свойств катализаторов различной природы.
Р. Нугид (R. Nuguid, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland) в своем докладе «Изучение механизма селективного каталитического восстановления NO методом рамановской спектроскопии с модулированным возбуждением» представил новый подход к характеризации активных центров катализатора V2O5/TiO2, используемого для реакции селективного восстановления NO в NH3. В основе подхода лежит метод КР-спектроскопии с модулируемым возбуждением. Это выступление было признано оргкомитетом лучшим докладом молодого ученого, а Р. Нугид был награжден специальным дипломом.
Доклад М. Закржевского (M. Zakrzewski, PhD, Lodz University of Technology, Lodz, Poland) «Электрохимический синтез нового Me/TiO2-CNT катализатора и его каталитические свойства в фотокаталитическом восстановлении диоксида углерода» был посвящен изучению углеродистых отложений, образующихся на катализаторах риформинга метана. С применением различных спектроскопических методов было исследовано несколько видов отработанных катализаторов на основе Ni/Al2O3, допированных добавками металлического рутения или оксида церия, что позволило установить природу, состав и структуру углеродистых отложений. Практически важные результаты были представлены в докладе аспирантки Института катализа им. Г.К. Борескова СО РАН Е.А. Столяровой «Влияние добавления силиказоля на каталитическую активность CoMoP катализаторов гидропереработки». В ее работе было проведено комплексное исследование нового катализатора NiMoP/Al2O3 переработки прямогонных дизельных топлив и вторичных легких фракций, продуктов парового крекинга вакуумного газойля. Данный катализатор продемонстрировал перспективность для применения в процессах получения компонентов моторных топлив, удовлетворяющих современным техническим требованиям. Другой доклад из области прикладного катализа «Влияние добавления цинка на Pd/Сибунит катализатор селективного гидрирования ацетилена» был представлен аспиранткой Д.В. Глыздовой (Центр новых химических технологий, г. Омск). Было проведено изучение свойств Pd-Zn системы, нанесенной на углеродный носитель Сибунит (Sib), применительно к реакции селективного гидрирования ацетилена. С помощью рентгеновских методов (XRD и EXAFS) было изучено формирование активного компонента, его состав и структура, а также каталитические свойства системы Pd-Zn/Sib.
М.А. Салаев (Томский государственный университет, г. Томск) и А.А. Скорынина (Южный федеральный университет, г. Ростов-на-Дону) представили работы, выполненные с применением расчетных методов квантовой химии. В первой работе «Теоретическое изучение влияния промоторов серебряных катализаторов эпоксидирования этилена» было исследовано влияние добавок щелочных металлов и оксида рения на каталитические свойства системы Ag/Al2O3 в реакции эпоксидирования этилена. Во второй работе «Теоретическое исследование активных металлических центров Pt- и Pd-функционализированных металл-органических каркасов» были представлены результаты по изучению формирования активных частиц Pd и Pt, нанесенных на металлорганический каркас UiO-67. Также были представлены фундаментальные работы, выполненные в Институте катализа им. Г.К. Борескова СО РАН. Доклад И.А. Четырина «In situ РФЭС и МС изучение окисления метана на биметаллических Pt-Pd/Al2O3 катализаторах» был посвящен изучению электронных и каталитических свойств системы Pd-Pt/Al2O3 c применением методов рентгенофлуоресцентной спектроскопии и масс-спектрометрии в режиме in situ. А.В. Селиванова в докладе «In situ изучение адсорбции метанола на Pt(111) и Pd(111) при низких температурах методом спектроскопии ИК-модуляции отражения-поглощения» рассказала о применении современного метода ИК-спектроскопии, спектроскопии ИК-модуляции отражения-поглощения (PM-IRRAS), для исследования особенностей адсорбции метанола на Pt(111) and Pd(111) при низких температурах в режиме in situ. А.Ю. Куренкова в своем докладе «Фотокаталитическое выделение водорода из водных растворов глюкозы при облучении видимым светом» продемонстрировала интересные результаты по изучению свойств фотокатализаторов на основе систем Cd0.3Zn0.7S в реакции разложения глюкозы с получением водорода из водных растворов.
Стендовая секция конференции прошла в атмосфере творческих дискуссий и позволила всем участникам пообщаться и обменяться знаниями и подходами, а молодым ученым – получить консультации и советы более опытных коллег. Дипломами оргкомитета были награждены молодые авторы двух лучших стендовых докладов: Полина Березкина (Уральский Федеральный Университет) и Олег Усольцев (Южный Федеральный Университет).

XI Международная конференция «Механизмы каталитических реакций» подтвердила свой уникальный статус проводящегося на территории России международного научного мероприятия, полностью посвященного фундаментальным проблемам катализа. Созданные Конференцией возможности по обмену научными знаниями между российскими и зарубежными учеными, несомненно, будут иметь долгосрочное положительное воздействие на развитие отечественной каталитической науки и технологий, формирование новых и эволюцию существующих научных направлений.
Заметим, что по сравнению с предыдущими конференциями, данное мероприятие имело несколько более «прикладной» характер: организаторы постарались выделить ряд стоящих перед обществом вызовов, в нахождении ответов на которые роль катализа могла бы быть (и, по всей видимости, в перспективе будет) определяющей. В частности, речь идет о проблемах развития энергетики, переработки ископаемого и возобновляемого сырья, утилизации диоксида углерода, синтеза новых материалов и, в целом, устойчивого развития и повышения качества жизни людей. Широко были представлены современные экспериментальные методы исследования механизмов каталитических реакций in situ и operando, а также различные квантовохимические методы анализа возможных интермедиатов, маршрутов их трансформации и структуры активных центров. Практически на всех, а не только на специализированных секциях, преобладали работы, выполненные с использованием этих подходов. По сравнению с предыдущими конференциями данной серии был заметен значительный рост технических возможностей исследователей, занимающихся характеризацией новых материалов и исследованиями реакций на поверхности твердых тел. Программа XI Конференции продемонстрировала беспрецедентное разнообразие тематик, что, в том числе, позволяет сделать заключение о расширении сотрудничества между научными организациями РФ и промышленностью.
Вопреки распространенному в общественных дискуссиях тезису о невысоком уровне российской науки, XI Конференция «Механизмы каталитических реакций» продемонстрировала, что в области катализа алармистская риторика преждевременна. Ситуация далека от критической, уровень большинства докладов, представленных российскими участниками – сотрудниками основных научных центров страны (Москва и Московская область, Новосибирск, Санкт-Петербург), соответствует мировому уровню. Увеличивается количество докладов из тех регионов, которые в общественном сознании не увязаны с научной деятельностью (Ростов, Иркутск, Тверь и т.д.).
По сравнению с предыдущей конференцией (Светлогорск, 2016 г.) значительно (в 1,5 раза) выросло число участников, расширилась география участия (45 городов против 39; 15 стран против 14). Необходимо отметить полное отсутствие ученых из стран бывшего СССР (Беларуси, Украины, Азербайджана и др.), что может свидетельствовать об усугублении негативных тенденций в научной сфере указанных стран, а также о дальнейшей деградации научных связей РФ с этими странами. В то же время, несмотря на географическую удаленность, в конференции 2019 года приняли участие сразу пять представителей китайских университетов и научно-исследовательских институтов (в то время как в 2016 году не было ни одного китайского участника). Это является следствием объективных тенденций расширения сотрудничества между нашими странами, в том числе – между научными организациями России и КНР.
В неформальных дискуссиях и на церемонии закрытия был отмечен высокий уровень организации конференции, творческая, деловая и доброжелательная атмосфера, созданная организаторами и участниками. Вместе с тем, было указано на целесообразность расширения присутствия иностранных участников на Конференции. В качестве одной из возможностей было названо проведение следующей, XII Международной конференции «Механизмы каталитических реакций» в 2022 году за пределами Российской Федерации.
Материал подготовили:
В.В. Каичев, Е.А. Козлова, В.А. Садыков,
А.А. Габриенко, М.А. Клюса,
В.И. Бухтияров, К.П. Брыляков
(Институт катализа СО РАН, Новосибирск)
Mechanical chirality gives new shape to catalysis
Mechanically chiral rotaxane creates a catalytic pocket a little like an enzyme
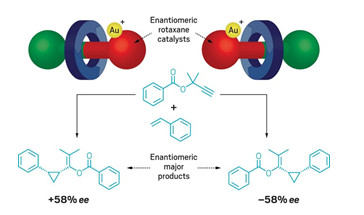
Researchers in the UK have used a mechanically planar chiral rotaxane as the basis for an enantioselective gold catalyst (Chem 2020, DOI: 10.1016/j.chempr.2020.02.006). Stephen Goldup and Andrew Heard at the University of Southampton say their work is a proof of concept that mechanical bonds can be used to build “pockets” for selective synthesis akin to those found in enzymes. Heard started by building and separating two mechanically chiral rotaxanes. Rotaxanes are mechanically inter-locked molecules in which a macrocycle is threaded onto a dumbbell-shaped compound or axle. Heard’s chiral rotaxanes differ from one an-other by the direction that the macrocyles loop around the central axles. By adding gold to a phosphine ligand at one end of the axle, Heard creat-ed chiral catalysts for a cyclopropanation reaction (shown). Each catalyst gave a different enantiomeric product and worked about as well as current catalysts. However, Goldup doesn’t expect that synthetic chemists will be using his new catalyst just yet—not least because these chiral rotaxanes are hard to make and isolate. The team now hopes to show that chiral ro-taxanes can help build enantioselective catalysts that are currently impos-sible to create otherwise—opening up new vistas of synthetic chemistry would make it well worth dealing with the fussy rotaxanes.
Photocatalyst converts fatty acids to diesel and jet-fuel molecules selectively
Method provides petroleum-free way to turn industrial biowaste into valuable commodity
Industrial waste containing bioderived long-chain fatty acids could serve as sustainable feedstocks for diesel and jet fuels, which today are pro-duced by refining petroleum sources. But several of the methods for re-moving oxygen from fatty acids to convert them to long-chain alkanes, the principal components of these fuels, require temperatures above 250 °C and high-pressure hydrogen, making them expensive and energy intensive. Decarboxylation methods, which convert fatty acids to alkanes by stripping carbon dioxide groups, run under milder conditions. But these methods tend to suffer from low selectivity: they generate a distr-bution of desirable and undesirable products. Now, Zhipeng Huang, Zhitong Zhao, and Feng Wang of the Dalian Institute of Chemical Physics and coworkers report that under mild conditions (30 °C and 0.2 MPa hy-drogen) and in the presence of ultraviolet light, a Pt-TiO2 catalyst decar-boxylates fatty acids selectively (Nat. Catal. 2020, DOI: 10.1038/s41929-020-0423-3). For example, the method converted pure stearic and linoleic acids to n-heptadecane in greater than 90% yield. In tests of crude soybean and tall-oil fatty acids, which are inedible by-products of soybean processing and the pulp industry, respectively, the method produced mixtures of long-chain alkanes at up to roughly 90% yield.
Straightforward path to linear alkylbenzenes identified
Nickel catalyst provides a route to the cleaning product chemicals with high yields and selectivity
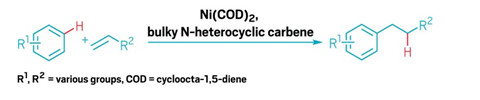
The world used about 3.5 million metric tons of linear alkylbenzenes, worth about $4.8 billion, in 2019, mostly to make detergents and cleaning products. However, the industrial synthesis of these compounds tends to make mixtures containing branched alkylbenzenes, which don’t biodegrade easily and can pollute rivers, lakes, and oceans. Anti-Markovnikov transition metalcatalyzed hydroarylation, in which the aryl compound bonds to the less substituted carbon of an alkene, could make linear alkylbenzenes, but these reactions have been plagued by poor yields and selectivity.
Now John Hartwig of the University of California, Berkeley; Yoshiaki Nakao of Kyoto University; and coworkers have coupled select arenes with an unconjugated terminal alkene to form linear alkylbenzenes in one step with 85–96% yields and high selectivity (Nat. Chem. 2020, DOI: 10.1038/s41557-019-0409-4).
The current acylation and reduction methods to produce these compounds is through a Friedel-Crafts acylation, which requires multiple steps. The new reaction is the first to be done in one step, with approximately 50:1 linear to branched selectivity, Hartwig says. In addition, the catalyst, a nickel cycloocta-1,5-diene compound, could turn over 280 times and still give an 85% yield. This turnover is more than 10-fold higher than reac-tions involving catalytic hydroarylation of benzene, Hartwig says.
Normally in these reactions, the alkenes isomerize between internal and terminal alkenes, Hartwig says. If the reaction is not selective for terminal alkenes, it will either give a mixture of linear and branched alkylbenzenes, or more or less stop. This new reaction only occurs with the termial alkene, which gets around the isomerization problem. The researchers didn’t intentionally design this selectivity into their system, but it’s a pathway that in principle could be generalized to similar types of X–H bonds adding to alkenes, Hartwig says.
Hartwig and coworkers found that the reaction goes through an alkyl nickel–aryl intermediate and involves an unexpected hydrogen transfer between catalyst ligands followed by reductive elimination. Further analysis of the mechanism revealed that the bulk of the N-heterocyclic carbene ligands did not affect the activity. The reactivity has more to do with noncovalent attractive interactions between ligands, instead of repulsive ones, Hartwig says.
These high-selectivity results from Hartwig and coworkers achieve a long-standing goal, says T. Brent Gunnoe, an organometallic chemist at the University of Virginia. “The use of large N-heterocyclic carbene ligands and noncovalent interactions to enhance the rate of catalysis is an unexpected result and a clever idea that could have broader impact in the design of other catalysts,” he says.
If the catalyst activity were high enough, this reaction would be easy to scale up to the industrial level, Hartwig says, because it’s a simple addition reaction, and there are no side products. However, it’s not perfect. “The biggest drawback of this system, in addition to catalyst lifetime, is its low tolerance for a lot of functional groups,” such as esters and nitriles, he says.
Nickel catalyst fends off air attack
Air-stable complex offers a more convenient approach to coupling reactions
Nickel catalysts have shot to synthetic stardom over the past decade or so, proving particularly useful in coupling reactions that form carbon-carbon or carbon-nitrogen bonds. The most popular catalyst for these reactions is bis(1,5-cyclooctadiene)nickel (Ni(COD)2), and although it’s very effective, the complex decomposes rapidly in air, making it troublesome to use.
Researchers at the Max Planck Institute for Coal Research have now developed an air-stable alternative to Ni(COD)2 that could make nickel ca-talysis much more accessible to users (Nat. Catal. 2019, DOI: 10.1038/s41929-019-0392-6). “Air stability has been a huge challenge,” says Josep Cornellà, who led the research, “and a lot of people don’t have access to glove boxes.”
Nickel is increasingly used in reactions traditionally dominated by palladium catalysts. Not only is nickel cheaper, Cornellà says, it can also catalyze a wider range of reactions than palladium. But the nickel catalysts used in these reactions are often extremely reactive, so they must be freshly generated within the reaction mixture. Chemists do this by mixing a precatalyst like Ni(COD)2 with other ligands, such as phosphines or bi-pyridines, which take the place of cyclooctadiene to form the active catalyst.
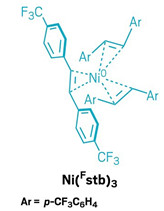 The new precatalyst is based on a nickel atom surrounded by three stil-bene ligands that bear trifluoromethyl groups (Ni(Fstb)3). These ligands enclose the nickel and protect it from oxygen. Cornellà’s team could make the red solid in 20 g batches by mixing a cheap precursor—nickel acetylacetonate—with the stilbene ligand and a reducing agent called triethylaluminum. The complex is stable for months in a freezer and only starts to decompose after several days at room temperature.
The new precatalyst is based on a nickel atom surrounded by three stil-bene ligands that bear trifluoromethyl groups (Ni(Fstb)3). These ligands enclose the nickel and protect it from oxygen. Cornellà’s team could make the red solid in 20 g batches by mixing a cheap precursor—nickel acetylacetonate—with the stilbene ligand and a reducing agent called triethylaluminum. The complex is stable for months in a freezer and only starts to decompose after several days at room temperature.
“People will use this immediately because it’s a drop-in replacement,” for Ni(COD)2, says Nilay Hazari of Yale University, who develops transition metal catalysts. “In my mind, it’s a very significant step forward.”
The Max Planck researchers used Ni(Fstb)3 to generate active catalysts in more than a dozen different reactions, including classics such as a Suzuki-Miyaura coupling, Buchwald-Hartwig carbon-nitrogen bond formations, and a Heck reaction. It generally performed as well as Ni(COD)2, giving the intended products in high yields.
Teamed with a carbene ligand, the new complex also catalyzed a coupling reaction between pentafluorobenzene and an alkyne, a feat that Ni(COD)2 cannot manage. Some reactions were less successful, though—a coupling reaction that involved butadiene or isoprene failed because these compounds could not displace the stilbene ligands from the complex.
Hazari says that Ni(Fstb)3 could be particularly helpful in catalyst development studies. For example, it could easily be loaded onto a 96-well plate with a different ligand in each well to assess which of the resulting active catalysts were most effective for a particular reaction. “For screening ligands, this is fantastic,” Hazari says.
However, he thinks there is still room for improvement. Triethylaluminum can ignite spontaneously in air, so it would be better to use a safer reducing agent to prepare the complex. It would also be helpful to improve the complex’s thermal stability, he adds. Cornellà and his colleagues are now testing a library of other stilbene ligands to fine-tune the reactivity of their complex.
Chemical & Engineering News
|
18-20
мая 2020 г. VI Всероссийская научная молодежная школа-конференция «Химия под знаком СИГМА: исследования, инновации, технологии» (видео-конференция на платформе Zoom) Омск, Россия |
http://conf.nsc.ru/sigma-6/ru |
|
May
25-27, 2020 School of Catalysis –Experimental Techniques in Catalysis Liblice Castle, Czech Republic |
https://school.katalyza.cz/ |
|
June
14-19, 2020 17th International Congress on Catalysis (ICC 2020) San Diego, California, USA |
https://2020icc.com/ |
|
June
22-25, 2020 Workshop on of Zeolites Liblice Castle, Czech Republic |
https://workshop2020.katalyza.cz/ |
|
July
12-16, 2020 84th Prague Meeting on Macromolecules - Frontiers of Polymer Colloids Prague, Czech Republic |
https://www.imc.cas.cz/sympo/84pmm/ |
|
July
12-15, 2020 8th Conference of the Federation of European Zeolite Associations (FEZA 2020) Brighton, UK |
http://www.fezaconference.org |
|
August
24-26, 2020 19th Nordic Symposium on Catalysis (NSC 2020) Espoo, Finland |
http://nsc2020.fi/ |
|
August
30 – September 2, 2020 4th International Symposium on Multiscale Multiphase Process Engineering (MMPE) Berlin, Germany |
https://dechema.de/en/mmpe2020.html |
|
August
30 – September 4, 2020 XXIV International Conference on Chemical Reactors CHEMREACTOR-24 Milan, Italy |
http://conf.nsc.ru/CR-24 |
|
August
31 – September 4, 2020 11th European Conference on Solar Chemistry and Photocatalysis: Environmental Applications (SPEA11) Turin, Italy |
http://www.spea11.unito.it/home |
|
31
августа – 4 сентября 2020 г. ХI Всероссийская научная конференция и школа «Аналитика Сибири и Дальнего Востока», посвященная 100-летию со дня рождения И.Г. Юделевича Новосибирск, Россия |
http://conf.nsc.ru/asfe-11 |
|
1-4
сентября 2020 г. 3-я Всероссийская научная конференция «Методы исследования состава и структуры функциональных материалов» (МИССФМ 2020) Новосибирск, Россия |
http://conf.nsc.ru/missfm-3/ru |
|
September
5-10, 2020 VI International Virtual (distant learning) School for Young Scientists: Magnetic Resonance and Magnetic Phenomena in Chemical and Biological Physics Roshchino, St. Petersburg (Leningrad) region, Russia |
http://roshchino2020.tomo.nsc.ru/ |
|
September
6-9, 2020 11th International Conference on Environmental Catalysis (ICEC 2020) Manchester, UK |
http://www.confercare.manchester.ac.uk/events/icec2020/ |
|
September
6-9, 2020 6th European Symposium on Photopolymer Science İstanbul, Turkey |
http://www.esps2020.org/ |
|
7-11
сентября 2020 г. XIV Конференция "Металлургия цветных, редких и благородных металлов» в рамках XII Международного конгресса и выставки «Цветные металлы и минералы" Красноярск, Россия |
https://nfmsib.ru/ |
|
September
8-11, 2020 Workshop on Low Dimensional Materials Liblice Castle, Czech Republic |
https://ldm.katalyza.cz/ |
|
September
15-19, 2020 2020 Summer School of the European Federation of Catalysis Societies (EFCATS): “Engineering Materials for Catalysis” Portorož-Portorose, Slovenia |
https://skd2020.chem-soc.si/en/2020-efcats-summer-school/ |
|
19-28
сентября 2020 г. XXXII Симпозиум «Современная химическая физика» Туапсе, Россия |
http://www.chemicalphysics.ru |
|
September
20-23, 2020 7th International Conference on Metal-Organic Frameworks and Open Framework Compounds Dresden, Germany |
https://dechema.de/en/MOF2020.html |
|
September
21-25, 2020 IV Scientific-Technological Symposium “Catalytic Hydroprocessing in Oil Refining” (STS HydroCat-2020) Thessaloniki, Greece |
http://conf.nsc.ru/STS_4/en |
|
21-25
сентября 2020 г. XVIII Российская конференция с международным участием «Физическая химия и электрохимия расплавленных и твердых электролитов» г. Нальчик, пос. Эльбрус, Кабардино-Балкарская республика, Россия |
https://kbsu.ru/events/pcee2020 |
|
21-25
сентября 2020 г. XIII Международная Конференция «Кремний-2020» и XII Школа молодых ученых и специалистов по актуальным проблемам физики, материаловедения, технологии и диагностики кремния, нанометровых структур и приборов на его основе г. Ялта (Гурзуф), Республика Крым, Россия |
http://www.si2020.niime.ru/ |
|
28
сентября – 2 октября 2020 г. XI Международная конференция «Химия нефти и газа» Томск, Россия |
http://petroleum-chemistry.ru/ |
|
28
сентября – 2 октября 2020 г. 74-я Международная молодежная научная конференция «Нефть и газ – 2020» Москва, Россия |
http://neftegaz.gubkin.ru/konferentciia/ |
|
1
октября 2020 г. XIII Всероссийская научно-техническая конференция «Актуальные проблемы развития нефтегазового комплекса России» Москва, Россия |
http://ap.gubkin.ru/ |
|
4-9
октября 2020 г. VI Семинар памяти профессора Ю.И. Ермакова «Гомогенные и закрепленные металлокомплексные катализаторы для процессов полимеризации и нефтехимии» Сочи, Россия |
http://conf.ict.nsc.ru/ermak-VI/ |
|
October
11-14, 2020 5th International Conference on Bioinspired and Biobased Chemistry & Materials Nice, France |
http://www.nice-conference.com/ |
|
11-15
октября 2020 г. IX Международный российско-казахстанский симпозиум «Углехимия и экология Кузбасса» Кемерово, Россия |
http://www.iccms.sbras.ru/ccsymp-2020 |
|
14-17
октября 2020 г. Международная научно-техническая конференция «Катализ: переработка углеводородного сырья и экология Ташкент, Узбекистан |
http://conf.nsc.ru/tashkent-2020/ru |
|
15-16
октября 2020 г. II Конференция «Фундаментальные и прикладные вопросы электрохимического и химико-каталитического осаждения и защиты металлов и сплавов», посвященная памяти чл.-корр. Ю.М. Полукарова Москва, Россия |
http://www.polukarov.lsps.ru/index.php?option=com_content&view=article&id=4&Itemid=7 |
|
October
18-22, 2020 9th IUPAC International Conference on Green Chemistry (ICGC-9) Athens, Greece |
http://www.greeniupac2020.or |
|
October
19-23, 2020 11th International Frumkin Symposium on Electrochemistry Moscow, Russia |
http://www.ihte.uran.ru/?p=13522 |
|
November
3-6, 2020 Workshop on Water in Zeolites Liblice Castle, Czech Republic |
https://water2020.katalyza.cz/ |
|
January
26-30, 2021 26th IUPAC International Conference on Chemistry Education (ICCE 2020) Cape Town, South Africa |
https://www.icce2020.org.za/ |
|
21-24 March, 2021 11th International Symposium on Catalysis in Multiphase Reactors & 10th International Symposium on Multifunctional Reactors (CAMURE 11 & ISMR 10) Milano, Italy |
|
|
May
16-20, 2021 48th World Polymer Congress (MACRO2020) Jeju Island, Korea |
http://www.macro2020.org |
|
May
17-19, 2021 New Trends in Polymer Science (Polymers 2020) Turin, Italy |
https://polymers2020.sciforum.net/ |
|
May
19-22, 2021 2nd International Conference on Reaction Kinetics, Mechanisms and Catalysis (RKMC 2021) Budapest, Hungary |
https://www.akcongress.com/ https://rkmc.akcongress.com/ |
|
Spring
2021 First International Symposium on High-Throughput Catalysts Design (HTCD 2020) Villeneuve D'Ascq, France |
https://htcd2020.sciencesconf.org/ |
|
May-June,
2021 10th International Symposium “Molecular Order and Mobility in Polymer Systems” (MOMPS-X) Saint Petersburg, Russia |
http://momps2020.macro.ru/ |
|
June
27 – July 2, 2021 44th International Conference on Coordination Chemistry Rimini, Italy |
https://www.iccc2020.com/ |
|
June
– July, 2021 International BioEPR School-conference Novosibirsk, Russia |
http://www.bioepr2020.ru |
|
July
25-30, 2021 12th Triennial Congress of the World Association of Theoretical and Computational Chemists (WATOC 2020) Vancouver, Canada |
http://watoc2020.ca |
|
August
29 – September 3, 2021 European Congress on Catalysis (EuropaCat 2021) Prague, Czech Republic |
www.europacat2021.eu |
|
September
12-15, 2021 4th European Conference on Metal Organic Frameworks and Porous Polymers (EuroMOF 2021) Krakow, Poland |
https://dechema.de/en/EuroMOF2021.html |
|
September
19-23, 2021 13th European Congress of Chemical Engineering and 6th European Congress of Applied Biotechnology (ECCE 13 & ECAB 6) Berlin, Germany |
http://ecce-ecab2021.eu/ |

9 марта 2020 года ушел из жизни выдающийся ученый, педагог и организатор науки, президент химического факультета Московского государственного университета имени Ломоносова, лауреат Государственной премии РФ, академик РАН Валерий Васильевич Лунин – всемирно признанный специалист в области гетерогенного катализа и физической химии поверхности.
Валерий Васильевич Лунин родился в 1940 году в деревне Богдановка Орловской области. В 1957 году поступил на Химический факультет МГУ. В 1972 году успешно защитил кандидатскую работу, а через 10 лет – докторскую диссертацию. За время работы на химическом факультете В.В. Лунин прошел путь от младшего научного сотрудника до профессора, заведующего одной из крупнейших кафедр мира – кафедры физической химии. В 1992 году был избран деканом химического факультета МГУ, в 2018 году стал первым Президентом факультета. С 1991 года он – член-корреспондент РАН, с 2000 года – академик.
Академик В.В. Лунин – автор более 1000 научных работ, в том числе 5 монографий, более 20 учебных пособий, 100 авторских свидетельств и патентов. Основатель и руководитель ведущей научной школы России «Фундаментальные представления о закономерностях формирования новых каталитически активных систем на базе интерметаллических соединений и их гидридов», насчитывающей сотни учеников и последователей. Им подготовлено более 80 кандидатов и 7 докторов химических наук.
В.В. Луниным открыто и изучено явление ускорения структурных и фазовых превращений в полиметаллических системах под влиянием водорода гидридных фаз, созданы принципиально новые катализаторы для важнейших процессов промышленного органического синтеза, нефтехимии и нефтепереработки, в том числе углубленной переработки тяжелых нефтяных остатков, синтеза метанола и высших спиртов из СО и Н2, процессов экологической защиты водной и воздушной среды, разложения и утилизации хлорсодержащих органических отходов. В 1995 г. за цикл работ «Новые гетерогенные катализаторы на основе интерметаллических соединений и их гидридов» В.В. Лунину присуждена премия имени А.А. Баландина Президиума РАН. В 1999 г. коллектив авторов во главе с В.В. Луниным удостоен Премии Правительства РФ за разработку и промышленную реализацию технологии двухступенчатого окисления аммиака в производстве азотной кислоты на основе сотового оксидного катализатора. В 2003 г. Валерий Васильевич в составе творческого коллектива получил Государственную Премию РФ в области науки и техники за цикл работ «Полиядерные соединения: молекулярные магнетики и катализ».
В.В. Лунин руководил научными исследованиями по использованию сверхкритических флюидов для создания новых технологий и материалов, внес крупный вклад в химию озона, в том числе по его применению в процессах водоочистки, гетерогенно-каталитических процессах, делигнификации лигнинсодержащих материалов.
В 2006 г. В.В. Лунин с коллективом авторов получил Премию Правительства РФ в области науки и техники за работу «Исследование физико-химических основ синтеза озона, разработка и широкое внедрение принципиально новых лечебных технологий с использованием озона». В 2008 г. Валерий Васильевич награжден Юбилейной медалью к 175-летию со дня рождения Дмитрия Ивановича Менделеева «За активную работу по изучению и пропаганде наследия великого русского ученого Д.И.Менделеева», а в 2009 г. его работы отмечены золотой медалью салона изобретений EURECA и премией имени В.Н. Ипатьева Президиума РАН. В 2010 году В.В. Лунин с сотрудниками удостоен Ломоносовской Премии Ученого Совета МГУ за работу «Эффекты синергизма в промышленных процессах гидрирования и гидродехлорирования». В 2017 году серия работ «Разработка новых катализаторов процессов экологического катализа» отмечена премией имени В.А. Коптюга Президиума РАН. В этом же году В.В. Лунин в составе авторского коллектива получил премию Правительства РФ в области науки и техники за «разработку, промышленное производство и масштабное внедрение высокоэффективных катализаторов двойного назначения для синтеза химических продуктов, получения технологических и очистки выбросных газов на предприятиях химической, нефтехимической, металлургической, машиностроительной, пищевой, атомной, оборонной, медицинской и других отраслей промышленности, а также в сфере жилищно-коммунального хозяйства». Фонд содействия отечественной науке высоко оценил вклад Валерия Васильевича; в 2005-2006 гг. он стал лауреатом в номинации «Выдающиеся ученые России. Химия».
В.В. Лунин был главным редактором журналов «Вестник Московского университета. Серия химия», «Журнал физической химии», «Сверхкритические флюиды. Теория и практика», членом редколлегий ряда научных журналов, председателем Научного совета РАН по химии ископаемого твердого топлива, заместителем председателя Научного совета РАН «Катализ и его промышленное использование» и Научного совета по катализу ОХНМ РАН.
Валерий Васильевич Лунин был человеком высоких моральных принципов и большого личного обаяния. Добрая и благодарная память о нем останется навсегда в сердцах его учеников и коллег.