
12-18 мая 2014 г.
Омск, Россия
С 12 по 18 мая 2014 г. Совет молодых ученых ИППУ СО РАН совместно с Советом научной молодежи ИК СО РАН провели Всероссийскую научную молодежную школу-конференцию «Химия под знаком СИГМА: исследования, инновации, технологии – 2014».

В качестве организаторов школы-конференции традиционно выступили Институт проблем переработки углеводородов СО РАН (ИППУ СО РАН, г. Омск) и Институт катализа им. Г.К. Борескова СО РАН (ИК СО РАН, г. Новосибирск). Соорганизаторами мероприятия в этом году являлись Новосибирское отделение РХО им. Д.И. Менделеева (НСО РХО), ФГБОУ ВПО «ОмГУ им. Ф.М. Достоевского» (г. Омск), ФГБОУ ВПО «Омский государственный технический университет» (г. Омск). Круг партнеров школы-конференции с каждым разом увеличивается. В этом году информационную поддержку Всероссийской научной молодежной школе-конференции «Химия под знаком Сигма» оказали журнал «Химия в интересах устойчивого развития» (г. Новосибирск), журнал Сибирского федерального университета, серия «Химия» (г. Красноярск), журнал «Катализ в промышленности» (г. Москва). Также проведение школы-конференции поддержали фирма «РИОС-инжиниринг» (г. Омск), компания ABCR® (представительство в г. Москва), группа компаний ГАЛАХИМ (г. Москва), компания «ЭПАКСЕРВИС» (г. Омск).
История проведения научных конференций «Под знаком Сигма» насчитывает более 10 лет. Первая Всероссийская конференция «Под знаком Сигма» состоялась в 2001 г. и стала местом объединения участников различных наук: химии, физики, математики, истории, экономики. Первым и основным ее организатором на тот момент являлся ОФ ИК СО РАН (с 2004 г. – Институт проблем переработки углеводородов СО РАН). В работе конференции принимало участие большое число молодых ученых, аспирантов, студентов из различных регионов России и стран СНГ (Омск, Новосибирск, Красноярск, Томск, Владивосток, Алматы, Караганда и др.), однако наиболее многочисленной по числу участников была секция «Химия». Это позволило в дальнейшем выделить эту секцию в отдельную конференцию, которая в 2008 г. впервые была организована как Всероссийская научная молодежная школа-конференция «Химия под знаком Сигма: исследования, инновации, технологии». На конференции были представлены не только пленарные, ключевые и устные доклады, но также были проведены стендовая сессия и конкурс «УМНИК» Фонда содействия развитию малых форм предприятий в научно-технической сфере. В конкурсе приняли участие 15 молодых ученых, пятеро из которых получили гранты Фонда для реализации своих проектов.
Через два года, в мае 2010 г., состоялась вторая школа-конференция «Химия под знаком Сигма: исследования, инновации, технологии». Количество участников конференции и ее география увеличились: более 200 человек из 33 городов России, а также 20 молодых ученых из Франции приехали обсудить важнейшие аспекты исследовательской работы и поделиться опытом в развитии отдельных отраслей химической науки. Можно сказать, что данная конференция стала междисциплинарным мероприятием, своего рода площадкой для налаживания диалога между молодыми учеными из разных отраслей химической науки. Кроме того, конференция стала и местом встречи молодых ученых с учёными мирового уровня, а также ведущими специалистами промышленных предприятий. Всё это и являлось целью организаторов молодежной школы-конференции.
Третья Школа «Химия под знаком Сигма» традиционно прошла в поселке Чернолучье Омской области с 18 по 24 мая 2012 г. В её работе приняли участие 224 молодых ученых из 24 городов России. Были представлены пленарные лекции ведущих ученых-химиков, устные и стендовые доклады молодых ученых (в возрасте до 35 лет), специалистов и преподавателей по основным направлениям современных фундаментальных и прикладных исследований в области химии и химической технологии.

В 2014 году организаторы не стали изменять традиции проводить школу-конференцию в экологически чистой Чернолученской зоне Омской области, и в период с 12 по 18 мая была организована IV Всероссийская научная молодежная школа-конференция «Химия под знаком Сигма: исследования, инновации, технологии». Открывал конференцию чл.-корр. РАН В.А. Лихолобов (ИППУ СО РАН, Омск), который в своем приветственном слове отметил многочисленность участников школы-конференции, а также представил молодым ученым приглашенных лекторов в лице академика РАН Н.З. Ляхова (ИХТТМ СО РАН, Новосибирск), чл.-корр. РАН В.П. Ананикова (ИОХ РАН, Москва), чл.-корр. РАН В.И. Бухтиярова (ИК СО РАН, Новосибирск), чл.-корр. РАН В.П. Федина (ИНХ СО РАН, Новосибирск).
Всего на Школе было прочитано 4 лекции приглашенных лекторов, 15 пленарных лекций, 30 ключевых лекций и заслушано более 110 докладов молодых ученых и аспирантов. В бурных обсуждениях прошла стендовая сессия, состоящая преимущественно из докладов студентов из различных университетов России.
 Лекция Н.З. Ляхова (ИХТТМ СО РАН, Новосибирск) была посвящена особенностям дисперсного наномодифицирования металлов, сплавов и полимеров. В докладе было отражено влияние механохимического воздействия на формирование интерметаллических сплавов с заданными магнитострикционными свойствами. Также был рассмотрен новый способ синтеза пенополиуретановых композитов на основе литьевого полиуретана с повышенным значением предела прочности на разрыв.
Н.З. Ляхов поделился с молодыми учеными информацией о методе механохимически стимулированного низкотемпературного синтеза нитрида алюминия для получения нанокерамики с высокой теплопроводностью.
Лекция Н.З. Ляхова (ИХТТМ СО РАН, Новосибирск) была посвящена особенностям дисперсного наномодифицирования металлов, сплавов и полимеров. В докладе было отражено влияние механохимического воздействия на формирование интерметаллических сплавов с заданными магнитострикционными свойствами. Также был рассмотрен новый способ синтеза пенополиуретановых композитов на основе литьевого полиуретана с повышенным значением предела прочности на разрыв.
Н.З. Ляхов поделился с молодыми учеными информацией о методе механохимически стимулированного низкотемпературного синтеза нитрида алюминия для получения нанокерамики с высокой теплопроводностью.
В.П. Анаников (ИОХ РАН, Москва) рассказал о состоянии исследований в области адаптивного катализа комплексами и наночастицами металлов в современной органической химии. В своей лекции В.И. Бухтияров (ИК СО РАН, Новосибирск) осветил роль катализа в защите окружающей среды. В.П. Федин (ИНХ СО РАН, Новосибирск) в своем докладе рассказал о чрезвычайно интенсивно развивающейся химии пористых металл-органических координационных полимеров (МОКП), которая по-прежнему остается новой областью в междисциплинарных исследованиях, так как данный вид материалов имеет широкий спектр применения в качестве нанореакторов, катализаторов, сенсоров, материалов с магнитными свойствами.
Область исследований, связанная с созданием эффективных методов получения востребованных химических веществ, материалов и жидких биотоплив из различных видов возобновляемого сырья, была хорошо раскрыта в представленных докладах пленарных лекторов. Так, в докладе Б.Н. Кузнецова (ИХХТ СО РАН, г. Красноярск) были рассмотрены особенности каталитической переработки возобновляемой лигнинцеллюлозной биомассы в нефтехимические продукты, в частности, в биотопливо второго поколения. О современных методах и технологиях получения углеводородных биотоплив рассказал О.В. Климов (ИК СО РАН, г. Новосибирск). В.А. Яковлев (ИК СО РАН, Новосибирск) в своем докладе сообщил о разновидностях каталитических процессов получения биотоплив бензинового, керосинового и дизельного ряда из растительного сырья, а также особенностях конструирования гетерогенных каталитических систем для данных процессов.
Актуальная на сегодняшний день тематика получения экологически чистых моторных топлив нашла своё отражение в докладах пленарных лекторов А.С. Белого, А.В. Лавренова, В.П. Доронина, А.А. Ламберова. Так, в докладе А.С. Белого (ИППУ СО РАН, Омск) были обобщены результаты исследований состояния платины в алюмоплатиновых катализаторах риформинга, выполненных в ИППУ СО РАН за последние 20 лет, представлены результаты промышленного использования этих катализаторов в процессах совместной переработки легких и С5+-алканов в типичные компоненты высокооктановых моторных топлив. Лекция А.В. Лавренова (ИППУ СО РАН, Омск) была посвящена созданию современных интегрированных технологий получения экологически чистых топлив, в частности, гидроизомеризации бензолсодержащих бензиновых фракций. Особенностью предлагаемых для данного процесса бифункциональных каталитических систем является использование в качестве кислотных носителей смешанных оксидных систем на основе анион-модифицированного оксида алюминия. В.П. Доронин (ИППУ СО РАН, Омск) представил доклад о цеолитсодержащих катализаторах крекинга, разработанных совместно ИППУ СО РАН и ОАО «Газпромнефть-ОНПЗ, которые в настоящее время используются на ряде нефтеперерабатывающих предприятий России. В докладе было отмечено, что в связи с ухудшением качества исходного сырья и повышением требований к качеству получаемых бензинов возникла необходимость в разработке и внедрении современного катализатора крекинга вакуумного газойля, обеспечивающего наивысшие показатели процесса. Опытом промышленного внедрения разработанных каталитических систем на примере опыта сотрудничества с ОАО «Нижнекамскнефтехим» поделился с участниками школы-конференции А.А. Ламберов (КФУ, Казань). С.В. Черепановой (ИК СО РАН, г. Новосибирск) в рамках школы-конференции был сделан пленарный доклад, посвященный исследованию структуры катализаторов с использованием метода рентгеновской дифракции. Катализ, нефтепереработка и физико-химические методы исследования тесно связаны с аналитической химией. Своим опытом применения структурированного подхода к описанию полученных результатов исследований для упрощения анализа объектов поделился В.И. Вершинин (ОмГУ, Омск).
Среди ключевых докладов, прочитанных в секции «Углеродные и неорганические материалы», можно отметить доклад Л.Г. Пьяновой (ИППУ СО РАН, г. Омск), посвященный разработке модифицированных сорбентов с биоспецифическими свойствами, а также доклад И.В. Мишакова (ИК СО РАН, г. Новосибирск) о новых возможностях получения катализаторов и синтеза углеродных наноматериалов с помощью явления управляемой углеродной эрозии. Из доклада М.М. Симунина (НИУ МИЭТ, г. Москва) молодые ученые узнали о применяемых в настоящее время перспективных технологиях синтеза углеродных нанотрубок.

В секции «Аналитическая химия и физико-химические методы исследования» заметный интерес вызвал доклад
На фоне интенсивного развития промышленности во всем мире остро стоит вопрос разработки эффективных методов очистки промышленных сточных вод. В докладах О.П. Таран (ИК СО РАН, г. Новосибирск) и Bryony Tolhurst (университет Брайтона, Великобритания) были предложены возможные методы решения данной проблемы.
Лучшие устные и стендовые доклады молодых ученых были отмечены дипломами НСО РХО (г. Новосибирск), а также ценными призами партнеров школы-конференции.
Победителем стендовой сессии был признан доклад «Исследование влияния модифицирующих добавок на активность и физико-химические свойства никелевых катализаторов гидрооблагораживания бионефти», представленный

В рамках программы конференции был организован круглый стол, посвященный вопросам интеграции науки и химического образования в Омской области, в котором приняли участие представители научной сферы – А.В. Лавренов (ИППУ СО РАН, г. Омск), О.О. Мироненко (ИППУ СО РАН, г. Омск), ВУЗов – Г.П. Сагитуллина (ОмГУ, г. Омск), И.В. Власова (ОмГУ, г. Омск), В.Ф. Фефелов (ОмГТУ, г. Омск) и представитель бизнес-инкубатора – возможной площадки для реализации научных проектов – О.В. Метель (г. Омск).
Уже не в первый раз на школе-конференции «Химия под знаком СИГМА» проходил тренинг-семинар «Интеграция», целью которого было активировать и объединить знания и навыки, приобретенные участниками семинара в различных областях науки, обсудить возможности и перспективы научного сотрудничества, получить опыт работы в команде. Опыт, полученный в подобных тренингах, в будущем поможет молодым ученым реализовать собственные интеграционные проекты, что является неоспоримым плюсом в развитии их карьеры.
Помимо научной программы, молодые ученые приняли активное участие в спортивных соревнованиях «Сигма-2014» по футболу, волейболу, настольному теннису и шахматам. Победители были награждены почетными призами и дипломами. Игра-квест «Веревочный курс», проведенный компанией ИП «Увлечён И Я», позволил сплотить участников из разных уголков России в неформальной обстановке, что способствовало расширению и закреплению их научных связей.
Организаторам школы-конференции удалось обеспечить высокий уровень проведения мероприятия, актуальность представленных тематик и привлечь к участию российских ученых с мировым именем. Школа-конференция получила хорошие отзывы гостей и участников. Молодые ученые, принявшие участие в конференции, получили возможность узнать о последних достижениях во многих областях современной химии, а также представить и обсудить результаты своих исследований с коллегами, работающими в различных областях химической науки.
Организаторы выражают свою признательность гостям школы-конференции за активное участие в ее работе, а также выражают благодарность спонсорам и партнерам за помощь в проведении данного мероприятия и приглашают принять участие в следующей конференции «Химия под знаком СИГМА», которую планируется провести в 2016 году.
Л.Ф. Сайфулина, Н.Н. Леонтьева, Д.А. Шляпин
(Институт проблем переработки углеводородов СО РАН, Омск)
29 мая 2014 г.
Новосибирск, Россия
29 мая 2014 года в Институте катализа им. Г.К. Борескова СО РАН состоялось заседание секции Научно-технического совета ОАО «Газпром» «Комплексная переработка газа и газового конденсата». Основными направлениями докладов, представленных на заседании, стали вопросы разработки катализаторов, адсорбентов и технологий их использования в переработке природного газа, а также проблемы и перспективы развития данной отрасли. Организаторами заседания выступили Институт катализа им. Г.К. Борескова СО РАН и ОАО «Газпром».

Заседание секции НТС ОАО «Газпром» собрало 70 участников из 15 городов России. В мероприятии приняли участие представители как промышленных организаций, так и академических институтов.
В ходе заседания ведущими специалистами в области каталитических методов переработки газа было представлено 18 устных сообщений и 8 стендовых докладов с предшествующими им флэш-презентациями.
В своем выступлении академик В.Н. Пармон представил разработки Института катализа, в частности, новые высокоактивные катализаторы для реакции окислительного дегидрирования этана. Данные катализаторы позволяют обеспечить выход этилена до 75%. Также было отмечено, что разработки ИК СО РАН успешно применяются на ведущих газоперерабатывающих заводах России.
Генеральный директор ОАО «ВНИИУС» А.М. Мазгаров сделал сообщение о разработке и опыте применения фталоцианиновых катализаторов окислительной очистки углеводородного сырья и сточных вод от сернистых соединений. В Казани организовано производство катализатора ИВКАЗ [PcCo(SO3)2Cl2,(OH)2] мощностью 10 тонн/год. Проф. Мазгаров отметил, что в настоящее время в России и за рубежом эксплуатируется более 40 установок ДМС, ДМД и Серокс с использованием катализатора ИВКАЗ.
В выступлении чл.-корр. РАН, профессора З.Р. Исмагилова особое внимание было уделено каталитическим технологиям подготовки сернистых природных и попутных нефтяных газов для дальнейшего энергетического и химического использования. По мнению проф. Исмагилова, наиболее перспективным является прямое окисление сероводорода, что делает возможным проводить очистку сернистых газов в компактных установках непосредственно в местах добычи.
Большое внимание аудитории вызвал доклад с.н.с. ИК СО РАН, к.х.н. О.Н. Коваленко, целью работы которой являлось повышение степени очистки газов от серосодержащих соединений за счет повышения эффективности реакции Клауса и реакций гидролиза COS и CS2. В Институте катализа разработан новый алюмооксидный катализатор процесса Клауса сферической формы с объемом мезопор (d = 3-10 нм) не менее 0,12 см3/г и соотношением объема мезопор (d = 3-10 нм) к объему ультрамакропор (d > 1000 нм) не менее 5. Катализатор имеет высокую прочность (> 6 МПа) и низкий насыпной вес – 0,63-0,66 г/см3. Показано, что катализатор за счет оптимизированной пористой структуры превосходит известные аналоги по выходу серы в реакции Клауса на промышленных гранулах.
Продолжил тему извлечения серы из серосодержащих газов директор по развитию ОАО «СКТБ «Катализатор»
Доклад заведующего лабораторией ОАО «ВНИИ НП», к.т.н. В.И. Юзефовича был посвящен разработке и организации производства универсального широкопористого алюмосиликатного адсорбента АС-230ш. Этот адсорбент был получен на ОАО «Химический завод им. Л.Я. Карпова» (г. Менделеевск, Республика Татарстан). Опытно-промышленная партия нового адсорбента уже прошла испытания, давшие положительные результаты, на многих предприятиях топливно-энергетического комплекса.
Опытом сотрудничества ООО «СкатЗ» и ОАО «Газпром» в области адсорбционных и каталитических процессов поделился коммерческий директор ООО «Салаватский катализаторный завод» Д.А. Медведев. Силикагелевые адсорбенты, производимые ООО «СкатЗ», успешно применяются для осушки воздуха, в том числе с использованием метода КЦА. На основании многолетнего опыта производства и применения силикагелей и результатах научно-исследовательских работ освоено производство микропористого силикагеля АСМ для осушки и «отбензинивания» газа при подготовке к транспортировке. Опытный пробег силикагелевого адсорбента на КС «Краснодарская» ОАО «Газпром» показал полное соответствие заявленным характеристикам на уровне импортных аналогов.
Генеральный директор ООО «Ишимбайский специализированный химический завод катализаторов» А.Б. Бодрый представил доклад «Производство адсорбентов и катализаторов для процессов газопереработки и нефтегазохимии» с информацией о катализаторах, разработанных в ОАО «НИПИГАЗПЕРЕРАБОТКА», а также о промышленном опыте их применения.

В своем докладе «Основные направления исследований и переработки матричной нефти» генеральный директор ОАО «ВНИПИнефть», д.т.н., академик РАЕН, профессор В.М. Капустин отметил, что матричная нефть является углеводородным сырьем, произведенным карбонатной нефтегазоматеринской системой газоконденсатных месторождений. Высокомолекулярные компоненты матричной нефти (асфальтены, смолы) содержат большие концентрации микроэлементов, редких и редкоземельных металлов, причем концентрация в них целого ряда благородных, редких и редкоземельных металлов характеризуется аномально высокими значениями. Однако при всех достоинствах матричной нефти, традиционные технологии для ее добычи неприменимы. В настоящее время наиболее приемлемый метод извлечения ВМС – технология «промыва» продуктивного пласта растворителем от нагнетательной скважины к добывающим с дополнительной прокачкой газообразного агента для ее вытеснения. Вместе с тем, часть углеводородов матричной нефти растворяется в углеводородах газоконденсата и извлекается в виде затемненной газоконденсатной смеси утяжеленного состава.
О новых направлениях переработки природных и попутных газов рассказал заведующий лабораторией Института химической физики РАН, д.х.н., профессор В.С. Арутюнов. Он отметил, что прогресс в технологиях добычи нетрадиционных ресурсов природного газа привел к резкому росту их добычи и снижению цены газа по отношению к нефти. Ожидается, что к 2040 г. нетрадиционные источники обеспечат до 30% мировой добычи газа. Однако современные газохимические технологии слишком сложны и капиталоёмки для практического применения в специфических условиях российских газодобывающих регионов. Главной проблемой существующих «Gas To Liquid» (GTL) технологий являются большие затраты на получение синтез-газа. Малотоннажная переработка газа в жидкие продукты диктует необходимость создания принципиально новых технологий получения синтез-газа либо альтернативных технологий, не требующих предварительной конверсии природного газа в синтез-газ.
В своем докладе проф. Арутюнов представил работы ИПХФ РАН и ИХФ РАН по указанным направлениям, включая конверсию природных и попутных газов в синтез-газ на основе объемных матричных конверторов, а также ряд альтернативных процессов, таких как прямое окисление метана в метанол и процессы на его основе, окислительный крекинг жирных и попутных газов и др.
Тему поиска оптимальных GTL технологий – процессов получения газового конденсата и высокооктанового бензина – продолжил заместитель директора Института нефтехимического синтеза им. А.В. Топчиева РАН, д.х.н. А.Л. Максимов. Им была представлена проточно-циркуляционная система, обладающая определёнными преимуществами, к которым относятся возможность использования простого аппаратурного оформления на стадии получения оксигенатов – многоступенчатого адиабатического реактора с охлаждением контактного газа путем впрыска холодного потока – квенча; использование одноступенчатого адиабатического реактора на стадии получения бензина; снижение коксообразования; снижение выхода ароматических соединений с сильноразветвленными боковыми цепочками; увеличение срока пробега катализатора синтеза бензина до регенерации. Подобная пилотная установка получения бензина из синтез-газа установлена в г. Электрогорск (ОАО «ЭлИНП»).
Внимание аудитории привлек доклад заведующей сектором «Каталитического синтеза на основе оксидов углерода и углеводородов им. А.Н. Башкирова» Института нефтехимического синтеза РАН, к.х.н. М.В. Куликовой «Высокоэффективный отечественный процесс и катализатор производства по Фишеру-Тропшу в сларри-реакторе синтетической нефти из природного и попутного газов». В докладе была представлена разработанная в ИНХС РАН технология получения синтетической нефти по Фишеру-Тропшу в трехфазном сларри-реакторе с наноразмерным катализатором. Катализатор представляет собой суспензию активных наноразмерных Ме-содержащих частиц, диспергированных в углеводородной среде сларри-реактора. Данная технология предполагает достижение следующих показателей: мощность по жидким синтетическим углеводородам – не ниже 1,0-2,0 л/сутки при селективности по жидким углеводородам – не ниже 85%.
В докладе генерального директора ООО «БИ АЙ Технолоджи» Ю.В. Аристовича речь шла о процессе мягкого парового риформинга. Основная цель разработанного процесса – одностадийная конверсия попутного нефтяного газа и жирных видов природного газа в нормализованный газ, годный к непосредственной транспортировке и/или использованию в качестве энергоносителя для квалифицированного сжигания с получением энергии или генерации других видов энергии (электрическая, механическая). Кроме того, оказалось, что мягкий паровой риформинг как нельзя лучше подходит сразу для нескольких вариантов производства метано-водородных смесей, которые получают всё более заметное распространение в мировой практике. Применение таких смесей рассматривается ОАО «Газпром» как одно из перспективных направлений развития отрасли.
Успехами в разработке катализатора процесса олигомеризации для установки ОАО «Газпромнефть – МНПЗ» поделился главный технолог завода С.Е. Кузнецов. Для ОАО «Газпромнефть – МНПЗ» процесс олигомеризации бутан-бутиленовой фракции является одним из основных процессов переработки непредельных газов вторичных процессов для получения высокооктанового компонента моторного топлива в силу невозможности строительства установок сернокислотного или фтористоводородного алкилирования. В ходе проведения научных исследований разработан цеолитный модифицированный катализатор Ga-ZSM-5/Al2O3 с оптимальной структурой. Данный катализатор обеспечивает уровень конверсии бутиленов не менее 85%, селективность – выход С5+, превращенных в олефины – не менее 90%; олигомеризация с катализатором Ga-ZSM-5/Al2O3 проходит при температурах на 20-25°C ниже, чем на промышленном аналоге; ресурс работы разработанного катализатора примерно в 2,5 раза больше по сравнению с промышленным образцом БАК-70У. Проведены квалификационные испытания полученного олигомеризата, которые показали, что Ga-ZSM-5/Al2O3 позволяет получить высокооктановую добавку к моторным топливам с октановыми характеристиками не менее 91 пунктов (ИОЧ).
Доклад на тему «Неокислительная конверсия метана в ароматические углеводороды на металлсодержащих цеолитах» был представлен научным сотрудником Института химии нефти СО РАН, к.х.н. В.В. Козловым. В работе было отмечено, что в последние годы, помимо традиционных процессов переработки нефти, особое внимание уделяется новым технологиям получения практически важных продуктов из нетрадиционного сырья. В ИХН СО РАН разработан эффективный способ получения катализаторов путем сухого механического смешения цеолита с наноразмерным порошком соответствующего элемента-модификатора с последующей активацией приготовленной смеси. Для модифицирования используются нанопорошки различных металлов, полученные методом электрического взрыва проводника в инертной среде. Приготовленные катализаторы обладают большей активностью и стабильностью по сравнению с системами, полученными традиционными способами введения промотирующих добавок: импрегнирование металлов из растворов соответствующих солей, ионный обмен, введение металлов на стадии гидротермального синтеза цеолита.
Доклад начальника отдела перспективного развития инженерно-технического центра ООО «Газпром переработка»
Директор Центра переработки газа и жидких углеводородов ООО «Газпром ВНИИГАЗ» С.А. Сиротин представил доклад «Создание новой экспериментальной базы и результаты испытаний кобальтового катализатора синтеза Фишера-Тропша». Разработка технологии производства синтетических жидких топлив (СЖТ) является одним из приоритетных направлений деятельности института в области адсорбционных и каталитических технологий. Для этих целей была разработана опытно-экспериментальная база для отработки процесса получения СЖТ. Также была сконструирована опытная установка, отвечающая следующим параметрам и задачам: разработка и изготовление новой реакционной системы с объемом загрузки катализатора до 200 мл; реализация системы охлаждения реакционной зоны высокотемпературным теплоносителем для поддержания изотермического режима; обеспечение «on-line» контроля состава отходящего газа и периодического анализа жидких продуктов синтеза; организация рециркуляции непрореагировавших газов для увеличения степени конверсии сырья; проведение модернизации системы регулирования и контроля технологических параметров. В настоящий момент основными направлениями НИР и ОКР по совершенствованию процесса производства СЖТ являются: разработка более высокопроизводительных (>100 г C5+/л-ч) катализаторов; разработка новых методов контроля температуры в реакторе для высокопроизводительных катализаторов; разработка и оптимизация технологического процесса синтеза и переработки его продуктов с целью снижения энерго- и ресурсозатрат.

Доклад ведущего инженера-технолога лаборатории гетерогенного селективного окисления Института катализа им. Г.К. Борескова СО РАН И.А. Золотарского «Новая технология получения муравьиной кислоты и формиатных буровых растворов из метанола» вызвал большой интерес у слушателей. Ежегодное мировое потребление муравьиной кислоты составляет приблизительно 550 тыс. тонн. А сферы ее потребления варьируются от кожевенной и текстильной промышленности до химии и фармацевтики. Существующие технологии производства муравьиной кислоты капиталоемки и энергозатратны, что приводит к высокой себестоимости конечного продукта. Новая технология получения муравьиной кислоты из метанола была разработана в ИК СО РАН и успешно опробована на пилотной установке.

Важной особенностью стендовой сессии стало проведение флэш-презентации работ, в ходе которой авторы имели возможность предложить слушателям краткие визуальные сообщения, отражающие основные идеи представленных докладов. Участниками был отмечен высочайший уровень стендовых докладов.
Полный перечень представленных тезисов докладов, а также программы заседания можно найти на странице http://www.catalysis.ru/resources/institute/Publishing/Report/2014/Program-Abstracts-Gazprom-2014.pdf
Для участников заседания была организована обзорная экскурсия по Институту катализа им. Г.К. Борескова СО РАН, в ходе которой они имели возможность осмотреть ряд лабораторий и ознакомиться с научно-технологическим отделом.

Результатом проведения заседания стал обмен опытом и накопленными знаниями между ведущими представителями науки и промышленности, а также определение дальнейших направлений развития отрасли. Участники отметили творческую и доброжелательную атмосферу прошедшей встречи, которая, по их мнению, стала действительно актуальным событием, призванным эффективно реализовать намеченный комплекс задач.
Материал подготовили
С. Логунова, Т. Замулина, Л. Исупова, А. Носков, В. Собянин
(Институт катализа СО РАН, Новосибирск)
Фото: А. Спиридонов
(Институт катализа СО РАН, Новосибирск)
Organic Synthesis: Strategies make visible-light-driven reactions easier
Perylene diimide catalyst Using visible light to drive catalytic reactions offers a simple, inexpensive, and green approach to functionalizing complex molecules under mild reaction conditions. Two new catalytic strategies could finally make visible-light photocatalysis practical for commercial use, particularly in the fine chemicals and pharmaceutical industries.
Using visible light to drive catalytic reactions offers a simple, inexpensive, and green approach to functionalizing complex molecules under mild reaction conditions. Two new catalytic strategies could finally make visible-light photocatalysis practical for commercial use, particularly in the fine chemicals and pharmaceutical industries.
In one report, Eric Meggers of Philipps University, in Marburg, Germany, and coworkers coupled low-intensity visible light and the inherent chirality of an iridium complex to drive the enantioselective α-alkylation of carbonyl compounds with benzyl or phenacyl groups (Nature 2014, DOI: 10.1038/nature13892). The tasks of photoactivating the substrate and controlling the stereochemistry of the product are typically shared by two separate catalysts. Meggers and coworkers showed that their chiral catalyst can perform both tasks at the same time, via proposed enolate and radical intermediates.
In a second report, Burkhard Konig and coworkers of the University of Regensburg, in Germany, describe a perylene diimide organocatalyst that absorbs more light energy than typical photocatalysts. The catalyst can therefore functionalize less-reactive chemical bonds, a feat that until now has required using higher-energy ultraviolet light or harsher reaction conditions (Science 2014, DOI: 10.1126/science.1258232). Konig’s team found that the perylene diimide undergoes a two-photon electron-transfer process to form an excited radical anion with enough energy to reduce aryl halides. The aryl radicals that form can then be trapped by hydrogen atom donors such as amines to form aryl derivatives or pyrroles to form substituted aryl derivatives.
 In the past, chemists relied on single catalysts, UV light, auxiliary reagents, and special equipment to pull off light-driven enantioselective reactions, note Kazimer L. Skubi and Tehshik P. Yoon of the University of Wisconsin, Madison, in a commentary accompanying the Meggers paper. Chemists have more recently discovered dual chiralphotoredox catalyst systems that use lower-energy visible light supplied by simple household lighting for the same reactions, they add. “The discovery of a single transition-metal catalyst that fulfills both roles is a crucial conceptual step forward.”
In the past, chemists relied on single catalysts, UV light, auxiliary reagents, and special equipment to pull off light-driven enantioselective reactions, note Kazimer L. Skubi and Tehshik P. Yoon of the University of Wisconsin, Madison, in a commentary accompanying the Meggers paper. Chemists have more recently discovered dual chiralphotoredox catalyst systems that use lower-energy visible light supplied by simple household lighting for the same reactions, they add. “The discovery of a single transition-metal catalyst that fulfills both roles is a crucial conceptual step forward.”
Chemical & Engineering News
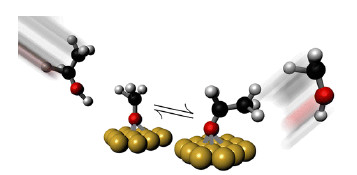
Self-Assembly: Simple electrochemical reactions at microsized metal disks drive microparticles to form crystalline structure
A microscale pump uses a simple chemical reaction to coax silica beads in a fluid to self-assemble into a crystalline structure (Langmuir 2014, DOI: 10.1021/la503118t). Such a device could provide a tiny motor for nanomachines, pull contaminants out of a fluid, or place coatings on nanoscale devices.
The catalytic pump consists of a gold anode and a platinum cathode. Maria Jose Esplandiu of the Autonomous University of Barcelona and her team placed 20- to 50-µm-diameter platinum disks on top of gold films sitting on a wafer. They subjected the pumps to one minute of oxygen plasma cleansing to remove any residual contamination and activate the surface then placed a gasket on top of the patterned pumps. They then mixed negatively charged 1.5-µm-wide silica spheres in a solution of hydrogen peroxide and added the mixture onto the pumps.
Electrochemical reactions between hydrogen peroxide and a platinum-gold micropump (large gray disk) cause negatively charged silica spheres to first cluster at a distance from the pump (top). But as the spheres’ charge is neutralized by the chemical reactions, they begin to gather around the disk (middle) then arrange themselves into a crystalline structure (bottom). The disk is about 20 µm in diameter.
 The hydrogen peroxide reacted with the metals, creating an electrical field oriented from the gold toward the platinum. This field triggers electroosmosis, causing the fluid containing silica spheres to flow toward the platinum disk. But because the spheres are negatively charged, the electrical field repels them, and they stop about 20 µm from the edge of the platinum. Gradually, however, protons produced by the chemical reactions bind to the spheres and neutralize their charge. The particles then begin to clump together, move toward the disk, and eventually build up into a crystalline structure surrounding the platinum.
The hydrogen peroxide reacted with the metals, creating an electrical field oriented from the gold toward the platinum. This field triggers electroosmosis, causing the fluid containing silica spheres to flow toward the platinum disk. But because the spheres are negatively charged, the electrical field repels them, and they stop about 20 µm from the edge of the platinum. Gradually, however, protons produced by the chemical reactions bind to the spheres and neutralize their charge. The particles then begin to clump together, move toward the disk, and eventually build up into a crystalline structure surrounding the platinum.
Esplandiu says translating chemical energy into mechanical energy could be useful in nanofabrication, both by providing a power source and by guiding materials to self-assemble. She’d like to try replacing one of the metals in the pump with a semiconductor, which could absorb photons to create even stronger electrical fields.
Chemical & Engineering News
Catalysis: Graphitic carbon nitride looks promising as a catalyst to harness sunlight to produce hydrogen fuel from water
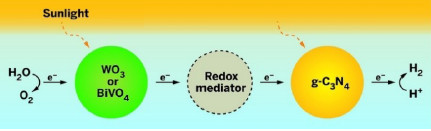
TWO-STEPPING
In a water-splitting system inspired by photosynthesis, first sunlight activates a metal oxide photocatalyst (WO3 or BiVO4), which oxidizes water into O2 and hydrogen ions (left). The catalyst transfers electrons absorbed during the reaction to graphitic carbon nitride (g-C3N4) through a redox mediator. Sunlight excites the now electronrich g-C3N4, which catalyzes the formation of H2 from hydrogen ions.
Inexpensive water-splitting catalysts could lower the costs of generating hydrogen for fuel cells. Towards that end, scientists in England now have integrated a stable, lowcost organic catalyst into a water-splitting system (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja506386e). The researchers made the catalyst, graphitic carbon nitride, from abundant, cheap urea.
Water-splitting techniques usually mimic plant photosynthesis, using photocatalysts to drive a two-step process to generate O2 and H2 from water. First, light activates a catalyst, such as a metal oxide, which then oxidizes water into O2 and hydrogen ions. The metal oxide absorbs electrons in the process and then transfers them to a redox mediator, such as sodium iodide, dissolved in the water. This mediator transfers the electrons to a second photocatalyst. Light excites the electrons in the second catalyst, which then reduces hydrogen ions to produce H2.
Junwang Tang of University College London says the trick lies in finding the right material to use as a catalyst in each half of the reaction. Organic semiconductors have high photocatalytic activity, he says, but they tend to be a lot less physically stable than inorganic semiconductors. But other researchers recently found that graphitic carbon nitride (g-C3N4), an organic semiconductor, remains stable in both acid and alkaline conditions.
So Tang’s team decided to test g-C3N4 as a catalyst on the hydrogen side of the process. To make g-C3N4, Tang’s group thermally decomposed urea, an inexpensive compound owing to its abundance. This approach, he says, increased the degree of polymerization of the g-C3N4 units. Greater polymerization means more surface sites for the reduction reaction, allowing electron transfer to take place quickly and easily.
The researchers tested the g-C3N4 with two metal oxides previously developed for the oxidation process, bismuth vanadate (BiVO4) and tungsten trioxide (WO3), and found they got the best results with the tungsten.
The reaction is still not very efficient; they reduced 200 mL of water in 10 hours, consuming less than 1% of the total water in their system but producing an ideal hydrogen to oxygen ratio of 2:1. Tang hopes the efficiency can be improved. For example, reducing the particle size of the WO3 would provide more surface area for reactions to take place.
Tang’s group is also working to increase the polymerization of the carbon nitride material.
Kazunari Domen of the University of Tokyo says carbon nitride is indeed a promising photocatalyst for largescale use because its elements are so abundant. He says Tang’s work provides a good example of how carbon nitride can be used, though the researchers should aim at improving the material’s efficiency at turning absorbed photons into usable electrons, “which appears to be considerably low for practical use.”
Chemical & Engineering News
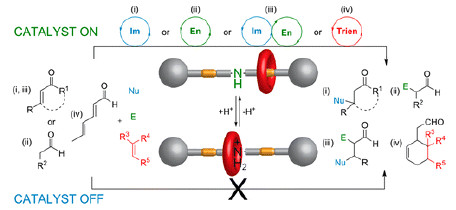
Switchable catalysts are those whose activity can be turned on and off, or whose stereoselectivity can be reversed, as a result of structural changes triggered by external stimuli, such as chemicals and light. Although a number of switchable catalysts have been synthesized, few can go beyond the scope of one specific type of reaction.
Following their recent discovery of a rotaxane-based switchable organocatalyst for the thiol-Michael addition, David A. Leigh and colleagues continue to explore its capability in activating carbonyl compounds (J. Am. Chem. Soc., 2014, DOI: 10.1021/ja509236u).
The catalyst masks and exposes an amine catalytic center through a macrocycle by acid and base modulation, respectively, and shows distinct on/off activity in iminium, enamine, tandem iminium–enamine, and trienamine catalyses.
The researchers have for the first time demonstrated multiple activation modes of a switchable catalyst, providing valuable insights into behaviors of its different states in various types of reactions. This study also opens avenues for the design of advanced switchable catalytic systems, where multistep reaction sequences may be choreographed by programming the “on” and “off” states of catalysts.Journal of the American Chemical Society
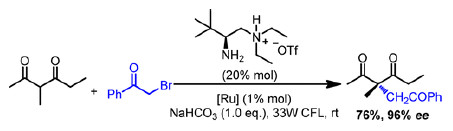
The construction of acyclic all-carbon quaternary stereogenic centers is an ongoing challenge for synthetic chemists. One of the most common strategies is the chiral α-alkylation of carbonyl compounds, usually entailing coupling between electronrich enolate or enamine-based intermediates and electrophiles, but this strategy has disadvantages that must be overcome.
Now, Sanzhong Luo and coauthors report a creative modification to this approach by combining organocatalysis with radical addition, instead of conventional nucleophilic substitution (J. Am. Chem. Soc., 2014, DOI:10.1021/ja508605a). The transformation proceeds via a tandem primary aminephotoredox catalytic process where photogenerated openshell acyl radicals are added to β-ketocarbonyl-derived chiral enamine intermediates with high stereoselectivity. Even more impressively, spiro-γ-lactams with two well-defined nonadjacent quaternary stereocenters can be synthesized from appropriate substrates in single operations.
As a facile and powerful path to asymmetric α-alkylation, this method expands the tools available for the construction of chiral carbon centers. By facilitating access to molecular motifs that are otherwise intractable, it demonstrates potential utility in overcoming some of the challenges facing pharmaceutical and natural product chemistry.
Journal of the American Chemical Society
The manufacture of many chemicals and fuels depends on heterogeneous catalysis on a metal surface. Additionally, chemical production is often a process requiring a significant energy input. Therefore, an important step in the future of heterogeneous metal catalysis is the development of highly selective catalysts that can function at lower energies, ideally using sustainable resources.
In order to create a catalyst that has high selectivity for a particular reaction, researchers must be able to determine the relative concentrations of intermediates at the surface, which may depend on the relative binding strengths of the intermediates. A team led by Cynthia Friend and Robert Madix has found a way to better predict these binding strengths, using both experimental and computational methods to take into account the van der Waals interactions between the reactants and the surface (J. Am. Chem. Soc., 2014, DOI: 10.1021/ja506447y).
The authors find that, although these associations are weak, they affect the relative stability of the intermediates and, therefore, have an important impact on the conditions required for peak selectivity. The methods used are highly relevant to many catalytic applications. They give insight into the significant role of weak interactions in more complex chemical processes, and may lead to more efficient manufacture of important chemicals in the future.
Journal of the American Chemical Society
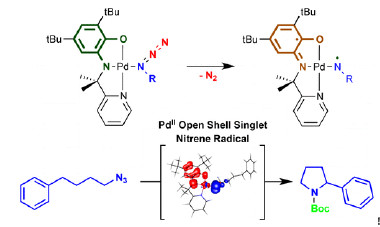
Redox-active ligands are useful compounds in catalysis, bond activation, and other metalloenzymatic transformations where they donate electrons to metal centers to which they are attached. However, ligands that can activate the substrate while leaving the metal unaffected are rare. This type of transformation could allow selective substrate activation through a controlled radical-type mechanism, combined with favorable metal–substrate coordination.
Now Jarl Ivar van der Vlugt and colleagues have synthesized such a ligand, and they present ligand-to-substrate single-electron transfer without metal redox changes (J. Am. Chem. Soc., 2014, DOI: 10.1021/ja502164f). The ligand binds with palladium(II) to form a paramagnetic complex that bears a ligand-centered radical, as evidenced by both experimental and computational data. Once reduced, the complex creates a diamagnetic Pd–Co complex that can bind an organic azide. Activation by ligand-induced electron transfer leads to H-atom abstraction and cyclization of the resulting Pd-bound radical to produce pyrrolidine.
The reaction is rare in that it goes through a radical-type pathway with a metal that ordinarily goes through two-electron processes, and it also proceeds very cleanly. “This concept is likely more broadly applicable with group 8–10 metals, including for cooperative bond activation processes and catalysis,” the authors say.
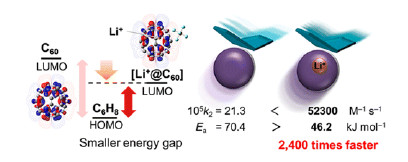
One well-known approach for speeding up a Diels–Alder reaction involves Lewis acid catalysis, which requires that the dienophile bear a heteroatom to serve as a coordination site. But the addition of a Lewis acid catalyst introduces steric effects, which makes it impossible to determine the exact contribution of the electronic effects to the rate increase. In order to tease apart these contributions, the team compares [60]fullerene (C60) with its Li+-encapsulated counterpart (Li+@C60) on the basis of each molecule’s ability to undergo a cycloaddition reaction with 1,3-cyclohexadiene.
Because the Lewis acid, Li+, is contained inside the soccer-ball-shaped molecule, its presence does not affect the molecule’s size, so any observed changes in reaction rate can be attributed fully to electronic effects. In this first-of-its-kind study, the team determines that the entrapped Li+causes the reaction to proceed 2400 times faster, a finding confirmed by both experimental and theoretical studies.
Journal of the American Chemical Society
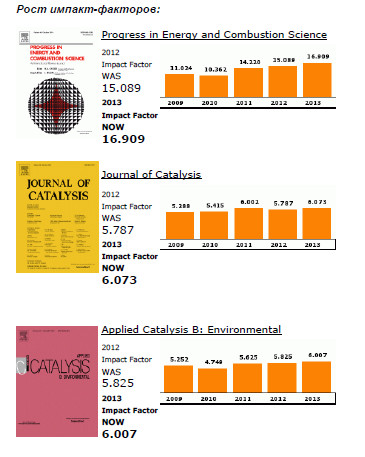
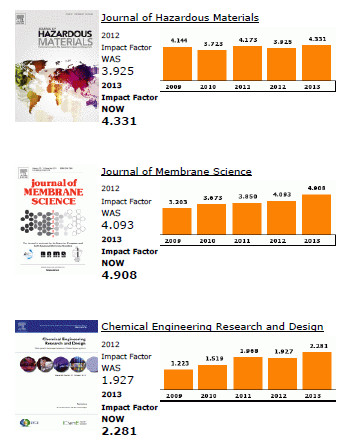
Elsevier publishes 15 out of the top 20 journals within the ISI Catego-ry, Engineering, Chemical, both with regards to Impact Factors and total citations.
The top ranking Elsevier journals in terms of Impact Factor are Progress in Energy and Combustion Science (1st), Journal of Catalysis (4th), Applied Catalysis B: Environmental (5th), Applied Energy (6th), and Journal of Membrane Science (7th).
In terms of total citations, Journal of Membrane Science, Journal of Catalysis and Chemical Engineering Science rank respectively 2nd, 3rd and 4th.
Also noteworthy for their increase in Impact Factor are Desalination (+ 94% since 2009) and Chemical Engineering Research and Design (+ 86% since 2009).
| Journal title | 2013 Impact Factor |
| Applied Energy | 5.261 |
| Chemical Engineering Journal | 4.058 |
| Proceedings of the Combustion Institute | 3.828 |
| Combustion and Flame | 3.708 |
| Journal of Molecular Catalysis. A, Chemical | 3.679 |
| Applied Catalysis A, General | 3.674 |
| Dyes and Pigments | 3.468 |
| Fuel | 3.406 |
| Catalysis Communications | 3.320 |
| Catalysis Today | 3.309 |
| Microporous and Mesoporous Materials | 3.209 |
| Separation and Purification Technology | 3.065 |
| Fuel Processing Technology | 3.019 |
| Reactive and Functional Polymers | 2.822 |
| Journal of Molecular Catalysis. B, Enzymatic | 2.745 |
| Journal of Aerosol Science | 2.705 |
| Journal of the Taiwan Institute of Chemical Engineers | 2.637 |
| Chemical Engineering Science | 2.613 |
| Journal of Food Engineering | 2.576 |
| The Journal of Supercritical Fluids | 2.571 |
| Computers and Chemical Engineering | 2.452 |
| Biochemical Engineering Journal | 2.368 |
| Food and Bioproducts Processing | 2.285 |
| Powder Technology | 2.269 |
| Hydrometallurgy | 2.224 |
| International Journal of Adhesion and Adhesives | 2.216 |
| Journal of Process Control | 2.179 |
| Journal of Industrial and Engineering Chemistry | 2.063 |
| Chemical Engineering & Processing: Process Intensifi-cation | 1.959 |
| Process Safety and Environmental Protection | 1.829 |
| Minerals Engineering | 1.714 |
| Particuology | 1.648 |
| Advanced Powder Technology | 1.642 |
| Chinese Journal of Catalysis | 1.552 |
| International Journal of Mineral Processing | 1.461 |
| Journal of Loss Prevention in the Process Industries | 1.347 |
| Journal of Bionic Engineering | 1.333 |
| Chinese Journal of Chemical Engineering | 0.872 |
Продолжается подготовка к проведению XII Европейского конгресса по катализу (30 августа – 4 сентября 2015 г., Казань, Россия).
Ведущие специалисты в области катализа подтвердили свое согласие выступить с пленарными и ключевыми лекциями.
Пленарные лекторы
Butlerov honorary Lecture
Prof., Dr. Valentine Ananikov
Catalysis in organic chemistry: from Butlerov to these days
Zelinsky Institute of Organic Chemistry RAS, Moscow, Russia
http://nmr.ioc.ac.ru/val/index.html
François Gault Lectureship Award (2013)
Prof., Dr. Johannes A. Lercher
The interactions and transformations of molecules on solid catalysts: an insight through physicochemical and kinetic analysis
Technical University of Munich, Munich, Germany
http://www.tc2.ch.tum.de/index.php?id=77
Dr. Giuseppe Bellussi
Fossil and renewable energy: the turning point of the liquid fuels production
Eni SpA, Refining & Marketing Division, San Donato Milanese, Italy
http://www.eni.com/en_IT/home.html
Prof. Joseph T. Hupp
Mesoporous metal-organic frameworks as platforms for single-site hetrogeneous catalysts
Northwestern University, Evanston, USA
http://chemgroups.northwestern.edu/hupp/
Prof. Graham Hutchings
Redox reactions catalysed by gold-containing catalysts
Cardiff Catalysis Institute, Cardiff, UK
http://www.cardiff.ac.uk/chemy/contactsandpeople/academicstaff/
hutchings-graham-overview_new.html
Prof., Dr. Robert Schlögl
Generation of solar hydrogen and its use in CO2 reduction
Fritz Haber Institute of the Max Planck Society, Berlin, Germany
Max Planck Institute for Chemical Energy Conversion, Mülheim a.d. Ruhr, Germany
http://www.fhi-berlin.mpg.de/acnew/department/pages/director.ht
ml
Prof. Magnus Skoglundh
Fundamental studies of metal exchanged zeolites for selective catalytic reduction of nitrogen oxides in oxygen excess
Chalmers University of Technology, Göteborg, Sweden
https://www.researchgate.net/profile/Magnus_Skoglundh
Ключевые лекторы
Prof. Gabriele Centi
University of Messina, Messina, Italy
http://ww2.unime.it/catalysis/peoples.htm
Prof. Claude Descorme
IRCELYON, Lyon, France
http://www.ircelyon.univ-lyon1.fr
Dr. Stig Helveg
Haldor Topsoe Research Labs., Lyngby, Denmark
www.topsoe.com
Prof. Xile Hu
Ecole Polytechnique Fédérale de Lausanne (EPFL), Institute of Chemical Sciences and Engineering, Lausanne,
Switzerland
http://lsci.epfl.ch/hu
Prof. Christopher W. Jones
Georgia Institute of Technology, Atlanta, USA
http://jones.chbe.gatech.edu
Prof. Andrei Y. Khodakov
UCCS, USTL-ENSCL-EC Lille, VIlleneuve d’Ascq, France
http://uccs.univ-lille1.fr/index.php/catalyse-heterogene/energie
Prof. Burkhard Koenig
University of Regensburg, Regensburg, Germany
http://www-oc.chemie.uni-regensburg.de/koenig/
Prof. Walter Leitner
Institut für Technische und Makromolekulare Chemie, Lehrstuhl für Technische Chemie und Petrolchemie, RWTH Aachen, Aachen,
Germany
http://www.tc.rwth-aachen.de/aw/cms/TC/Zielgruppen/~vft/prof-leitner/?lang=de
Prof. Can Li
Dalian Institute of Chemical Physics, Dalian, Liaoning, China
http://www.canli.dicp.ac.cn
Prof. Scott McIndoe
University of Victoria, Victoria, Canada
http://web.uvic.ca/~mcindoe/index.html
Prof. In-Sik Nam
Department of Chemical Engineering/School of Environmental Science & Engineering, University of Science and Technology
(POSTECH), Pohong, Republic of Korea
http://pecl.postech.ac.kr
Prof. Konstantin M. Neyman
Institució Catalana de Recerca i Estudis Avançats ,
Barcelona, Spain
http://www.icrea.cat/Web/ScientificForm.aspx?key=292
Prof. Unni Olsbye
University of Oslo, Oslo, Norway
http://www.mn.uio.no/kjemi/personer/vit/unniol/index.html
Prof. Vasile Parvulescu
University of Bucharest, Bucharest, Romania
http://www.unibuc.ro/prof/parvulescu_v/
Prof. Yuriy Roman
Department of Chemical Engineering, Massachusetts Institute
of Technology, Cambridge, USA
http://www.romangroup.mit.edu/
Prof., Dr. Günther Rupprechter
Institute of Materials Chemistry, Vienna University of Technology, Vienna, Austria
http://www.imc.tuwien.ac.at/pers_pid_d.php?pid=3174381
Prof. Kazuhiro Takanabe
King Abdullah Univ Sci & Technol, KAUST Catalysis Ctr KCC,
Div Phys Sci & Engn, Thuwal, Saudi Arabia
http://catec.kaust.edu.sa/Pages/Home.aspx
Prof. Takashi Tatsumi
Tokyo Institute of Technology, Tokyo, Japan
http://www.titech.ac.jp/english/about/overview/board/tatsumi.html
Dr. Aleksey Yezerets
Cummins Inc., Columbus, USA
http://www.cummins.com
Увеличилось число сателлитных конференций:
III International School-Conference on Catalysis
“Applied Nanotechnology and Nanotoxicology” (ANT-2015),
August 27-29, 2015, Kazan, Russia
XI International Symposium of Heterogeneous Catalysis
September 5-9, 2015, Varna, Bulgaria
IV International School-Conference on Catalysis for Young Scientists “Catalyst Design. From Molecular to Industrial level”
September 5-6, 2015, Kazan, Russia
III International Conference “Catalysis for Renewable Sources: Fuel, Energy, Chemicals (CRS-3)
September 6-11, 2015, Catania, Italy
IV Symposium on Modeling of Exhaust-Gas After-Treatment (MODEGAT IV)
September 13-15, 2015, Bad Herrenalb/Karlsruhe, Germany
Регистрация участников проводится на сайте Конгресса: http://www.europacat2015.com
|
30 ноября – 6 декабря 2014 г. |
|
|
2-3 декабря 2014 г. |
тел: (+994 12) 490 24 43 |
|
2-4 декабря 2014 г. |
(846) 332-34-96, 8-927-905-0941 Ученыйсекретарь |
|
December 7-9, 2014 |
|
|
December 7-10, 2014 |
|
|
December 8-9, 2014 |
|
|
December 9-12, 2014 |
|
|
10-12 декабря2014 г. |
|
|
December 26-28, 2014 |
|
|
2015 |
|
|
February 25-27, 2015 |
|
|
March 9-13, 2015 |
|
|
March 14-15, 2015 |
|
|
March 29 – April 1, 2015 |
|
|
April 14-16, 2015 |
|
|
April 14-16, 2015 |
|
|
April 14-16, 2015 |
|
|
April 25-28, 2015 |
|
|
May 3-7, 2015 |
|
|
May 11-15, 2015 |
|
|
19-22 May, 2015 |
|
|
26-29 May, 2015 |
|
|
June 11-14, 2015 |
|
|
June 15-18, 2015 |
|
|
June 16-20, 2015 |
|
|
June 21-25, 2015 |
|
|
June 22-25, 2015 |
|
|
June 22-26, 2015 |
|
|
June 28 – July 2, 2015 |
|
|
29 июня – 3 июля 2015 г. |
|
|
June 30 – July 3, 2015 |
|
|
30 июня – 4 июля 2015 г. |
|
|
July 4-11, 2015 |
|
|
July 5-8, 2015 |
|
|
July 5-9, 2015 |
|
|
July 5-9, 2015 |
|
|
July 7-10, 2015 |
|
|
July 12-15, 2015 |
|
|
July 12-16, 2015 |
|
|
July 20-24, 2015 |
http://www.rsc.org/ConferencesAndEvents/ |
|
July 26-30, 2015 |
|
|
July 27-30, 2015 |
|
|
August 6-13, 2015 |
|
|
August 23-28, 2015 |
|
|
August 27-29, 2015 |
http://www.europacat2015.com/index.php/ |
|
August 30 – September 4, 2015 |
|
|
August 31 – September 4, 2015 |
|
|
September 5-6, 2015 |
|
|
September 5-9, 2015 |
http://www.europacat2015.com/index.php/ |
|
September 6-9, 2015 |
|
|
September 6-11, 2015 |
http://www.europacat2015.com/index.php/ |
|
8-12 сентября 2015 г. |
|
|
September 13-15, 2015 |
http://www.itcp.kit.edu/deutschmann/1746.php
|
|
September 14-18, 2015 |
|
|
14-19 сентября 2015 г. |
|
|
September 20-24, 2015 |
|
|
September 27 – October 1, 2015 |
|
|
October 27-30, 2015 |
|
|
December 6-10, 2015 |
|
|
2016 |
|
|
April 4-6, 2016 |
http://www.rsc.org/ConferencesAndEvents/ |
|
July 3-8, 2016 |
|


