
 23 июля исполнилось 60 лет Александру Степановичу Носкову – доктору технических наук, профессору, крупному специалисту в области технологии каталитических процессов и математического моделирования каталитических реакторов.
23 июля исполнилось 60 лет Александру Степановичу Носкову – доктору технических наук, профессору, крупному специалисту в области технологии каталитических процессов и математического моделирования каталитических реакторов.
В 1976 году А.С. Носков окончил Томский политехнический университет (ТПУ) и в течение двух лет работал в отраслевой лаборатории Миннефтехимпрома СССР при ТПУ. Дальнейшая трудовая деятельность Александра Степановича связана с Институтом катализа им. Г.К. Борескова СО РАН, где он прошел путь от аспиранта до заместителя директора по науке (с 1995 г. по настоящее время).
С 1985 г. по 1992 г. А.С. Носков возглавлял научную программу работ Сибирского отделения РАН по каталитическим методам очистки от токсичных примесей (оксиды азота, углеводороды), которая завершилась созданием промышленного производства установок очистки газов. Было построено и введено в эксплуатацию более 20 установок на химических, машиностроительных предприятиях и заводах военно-промышленного комплекса.
Работы А.С. Носкова по использованию методов вычислительной гидродинамики при разработке и эксплуатации каталитических реакторов позволили разработать оптимальные способы формирования зернистых структур в неподвижных слоях катализатора, показать возможность повышения производительности промышленных реакторов на 12-15% и обеспечить применение этих методов более чем в 60 агрегатах в азотной промышленности и нефтепереработке.
Под руководством А.С. Носкова разработаны научные основы селективного окисления аммиака в закись азота и впервые в мире создан в пилотном масштабе реактор получения закиси азота путем окисления аммиака в кипящем слое катализатора.
За время работы в Институте катализа под руководством А.С. Носкова выполнен ряд крупных научных и инновационных проектов. В течение 2003-2006 гг. А.С. Носков обеспечил в качестве исполнительного директора проекта успешное выполнение важнейшего государственного инновационного проекта по созданию и промышленному освоению катализаторов получения моторных топлив, включая новое поколение катализаторов глубокой гидроочистки дизельного топлива до норм Евро-5. С 2005 по 2008 гг. он был руководителем крупного инновационного проекта по созданию технологической базы для модернизации отечественного производства полиолефинов.
В период 2009-2013 гг. под руководством А.С. Носкова успешно реализуется научный проект по разработке и освоению нового поколения теплогенерирующих систем на основе каталитического сжигания твердых топлив. К настоящему времени создано и эксплуатируется в промышленности пять таких систем, позволивших снизить расход твердого топлива в 2-2,5 раза.
Под руководством А.С. Носкова за 2007-2013 гг. выполнено 12 государственных контрактов в рамках Федеральных целевых программ “Национальная технологическая база”, “Исследования и разработки по приоритетным направлениям развития научно-технологического комплекса России”, “Кадры”.
Научные результаты А.С. Носкова опубликованы в ведущих российских и международных журналах (более 500 публикаций, 3 монографии, 70 российских и 15 зарубежных патентов).
Александр Степанович Носков является признанным экспертом в области химической технологии и входит в состав ряда Научных Советов Российской академии наук: по катализу, по теоретическим основам химической технологии, по ископаемому сырью. Он является заместителем главного редактора журнала “Катализ в промышленности”, входит в состав редакционной коллегии нескольких российских журналов и международного журнала “Reviews in Chemical Engineering”. С 2001 г. под руководством А.С. Носкова проводятся Международные конференции по химическим реакторам (в Финляндии, Германии, Греции, на Мальте, в Австрии), которые объединяют ведущих российских и зарубежных ученых в области теоретических основ создания каталитических реакторов и процессов.
Большое внимание Александр Степанович уделяет подготовке научно-инженерных кадров – в 1998 г. он основал и по 2006 г. возглавлял кафедру “Инженерные проблемы экологии” Новосибирского государственного технического университета. За годы существования кафедры на ней подготовлено более 150 инженеров-экологов, которые востребованы предприятиями ТЭК, машиностроения и научной сферы.
С 2006 г. Носков А.С. входит в состав экспертного совета Высшей аттестационной комиссии России. Заслуги А.С. Носкова отмечены государственной (Орден Почета, 2007 г.) и региональной (медаль за особый вклад в развитие Кузбасса, 2009 г.) наградами.
Научный совет по катализу ОХНМ РАН и редакция
Каталитического бюллетеня
сердечно поздравляют Александра Степановича с юбилеем,
желают ему крепкого здоровья и новых творческих достижений!
В период с 24 по 27 июня 2013 года в поселке Бурдугуз Иркутской области проходила работа III Российско-Германского семинара по катализу. Этот уже традиционный семинар, проводящийся раз в три года, организуется Институтом катализа СО РАН (Новосибирск, Россия) и Институтом Фрица Хабера Общества Макса Планка (Берлин, Германия). В этот раз семинар был посвящен проблемам использования альтернативных и возобновляемых источников энергии. Значительная часть работ была ориентирована на развитие водородной энергетики (поиск новых источников водорода, его очистка, хранение и использование). Кроме того, традиционно в рамках семинара были освещены последние работы в области исследования механизмов гетерогенных каталитических реакций в режиме in situ с применением современных поверхностно-чувствительных методов.

В работе III Российско-Германского семинара приняли участие более 40 ученых: 16 из Германии и 27 из России, в их числе 19 студентов, аспирантов и молодых преподавателей. Наиболее многочисленные делегации прибыли из Института катализа СО РАН, Института Макса Планка и Института Фрица Хабера. В научную программу семинара были включены 6 пленарных лекций и 30 устных докладов.
В пленарной лекции профессора Л.М. Кустова из Института органической химии им. Н.Д. Зелинского (Москва, Россия) был приведен подробный анализ вопросов производства и хранения водорода. Особое внимание уделялось проблеме получения водорода из возобновляемого природного сырья. Кроме того, в лекции были детально рассмотрены каталитические процессы паровой конверсии и парциального окисления метана. Д.х.н. А.В. Романенко из Института катализа СО РАН в своей пленарной лекции изложил подходы, в том числе используемые в Институте катализа,
 по целенаправленному синтезу анодных и катодных электрокатализаторов низкотемпературных топливных элементов (ТЭ), эффективных в окислении водорода и низших спиртов. Был представлен сравнительный анализ методик приготовления высокодисперсных катализаторов с содержанием платины 40–80 мас.%.
Профессор А.А. Хасин из Института катализа СО РАН сделал пленарную лекцию о природе активных мест катализаторов гидрирования. Особое внимание в лекции было уделено катализаторам на основе кобальта для реакции Фишера-Тропша. На ряде примеров был продемонстрирован эффект сильного взаимодействия металл-носитель, оказывающий влияние на активность катализаторов.
по целенаправленному синтезу анодных и катодных электрокатализаторов низкотемпературных топливных элементов (ТЭ), эффективных в окислении водорода и низших спиртов. Был представлен сравнительный анализ методик приготовления высокодисперсных катализаторов с содержанием платины 40–80 мас.%.
Профессор А.А. Хасин из Института катализа СО РАН сделал пленарную лекцию о природе активных мест катализаторов гидрирования. Особое внимание в лекции было уделено катализаторам на основе кобальта для реакции Фишера-Тропша. На ряде примеров был продемонстрирован эффект сильного взаимодействия металл-носитель, оказывающий влияние на активность катализаторов.
В пленарной лекции профессора М.Ю. Синева из Института химической физики им. Н.Н. Семенова (Москва, Россия) была всесторонне рассмотрена проблема каталитической переработки легких алканов, являющихся основной составляющей природного и сопутствующего газов. В ходе лекции были сформулированы основные проблемы и возможные пути их решения в развитии каталитических технологий, ориентированных в первую очередь на развитие малой энергетики.
Профессор К.Ж.Ж. Майхофер из Института Макса Планка (Дюссельдорф, Германия) в своей пленарной лекции рассказал о природе размерного эффекта частиц платины, входящих в состав активного компонента электрокатализаторов низкотемпературных ТЭ. В лекции обсуждалась связь между исследованиями модельных и реальных катализаторов. Пленарная лекция профессора М. Мюллера из Университета г. Бохум (Германия) была посвящена вопросам синтеза, функционализации и применения углеродных нанотрубок для задач гетерогенного катализа. В качестве примера в лекции были приведены результаты тестирования наночастиц железа на поверхности O- и N-содержащих углеродных нанотрубок в качестве катализаторов процесса Фишера-Тропша. Показано, что такие катализаторы отличаются стабильностью и обладают высокой селективностью в синтезе олефинов.
Ряд устных докладов был посвящен проблемам электрокатализа. Доктор А. Кноп-Герике из Института Фрица Хабера представил результаты in situ исследований поверхности платинового электрода в условиях электролиза воды. Тему продолжил доктор С. Ранджан из Института Макса Планка (Мангейм, Германия), подробно рассказав о поведении оксидов переходных металлов в условиях электрокатализа. Доктор Е. Сантос из Института теоретической химии (Ульм, Германия) представила результаты теоретических исследований электрокаталитического восстановления кислорода. Доклад доктора С. Массию из Института Фрица Хабера был посвящен исследованию смешанных Mn-Sn катализаторов разложения воды. К.х.н. Е.Н. Грибов из Института катализа СО РАН рассказал о свойствах платиновых катализаторов для низкотемпературных топливных элементов. А. Топалов из Института Макса Планка в своем докладе представил разработанные ими методы исследования в режиме in situ активности и стабильности электрокатализаторов для ТЭ. Доктор В.П. Черепанов из Института физической химии (Байрез, Германия) представил новые данные по использованию ультразвука в технологиях синтеза катализаторов.
Значительное количество работ было посвящено топливным элементам, генераторам синтеза водорода и синтез-газа. Доктор М.В. Патракеев из Института химии твердого тела (Екатеринбург, Россия) рассказал о термической стабильности каталитических мембранных реакторов, разрабатываемых для парциального окисления метана. К.х.н. А.С. Федотов из Института нефтехимического синтеза (Москва, Россия)
 представил результаты тестирования каталитических систем конверсии метана в синтез-газ на основе пористых керамических мембран.
Профессор В.А. Собянин из Института катализа СО РАН рассказал о катализаторах синтеза водорода из диметоксиметана. Доктор А. Тарасов из Института Фрица Хабера в своем докладе подробно рассмотрел проблему зауглероживания катализаторов на основе никеля в условиях каталитической конверсии метана.
представил результаты тестирования каталитических систем конверсии метана в синтез-газ на основе пористых керамических мембран.
Профессор В.А. Собянин из Института катализа СО РАН рассказал о катализаторах синтеза водорода из диметоксиметана. Доктор А. Тарасов из Института Фрица Хабера в своем докладе подробно рассмотрел проблему зауглероживания катализаторов на основе никеля в условиях каталитической конверсии метана.
Несколько докладов было посвящено проблемам переработки биоресурсов. К.х.н. И.В. Делий из Института катализа СО РАН рассказала о природе никель-фосфорных катализаторов облагораживания бионефти. К.х.н. М.О. Казаков из Института проблем переработки углеводородов СО РАН (Омск, Россия) представил результаты тестирования платиновых катализаторов для одностадийного производства дизельного топлива из растительных масел. Доклад Н.В. Мезенцевой из Института катализа СО РАН был посвящен проблемам каталитической конверсии биомассы в синтез-газ и водород. В.О. Родина, сотрудница ИК СО РАН, сделала сообщение об особенностях применения различных катализаторов в производстве биодизеля. Ю.А. Зайцева, аспирант ИК СО РАН, представила доклад, посвященный развитию технологий производства биодизеля из растительного сырья. Три доклада молодых ученых, М.В. Бухтияровой, С. Буллер и С. Рейше, были посвящены исследованиям, проводимым в Институте Макса Планка (Мангейм, Германия) по созданию новых катализаторов на основе углеродных материалов.

К.ф.-м.н. В.В. Каичев из Института катализа СО РАН представил результаты in situ исследований механизмов каталитического разложения и окисления метанола на поверхности платины и палладия. К.х.н. Е.А. Козлова из Института катализа СО РАН представила доклад “Фотокаталитическое выделение водорода из водных растворов органических и неорганических доноров электронов под действием видимого излучения”, посвященный важной проблеме аккумулирования солнечной энергии. И.В. Токарева, аспирант ИК СО РАН, сделала сообщение о синтезе и областях применения бинарных систем на основе MgO и оксидов переходных металлов. Г. Вовсник, аспирант из Института Фрица Хабера, в своем выступлении подробно рассмотрел природу каталитического действия интерметаллических соединений на основе палладия и галлия.
Анализ представленных на семинаре научных докладов позволяет сделать вывод о том, что как в России, так и в Германии интенсивно ведутся исследования по разработке и совершенствованию альтернативных источников энергии, в частности, топливных элементов. При этом следует отметить, что в области разработки и исследования физико-химических свойств катализаторов для ТЭ участники семинара выступают как равноправные партнеры, тогда как исследования по электрохимическому тестированию катализаторов в материалах российских ученых представлены слабо из-за отсутствия экспериментальной базы. В докладах наших иностранных коллег этому сегменту исследований уделяется большое внимание. Из представленных работ также видно, что в Германии особое внимание уделяется теоретическим исследованиям и изучению механизмов электрокаталитических реакций в режиме in situ. С другой стороны, на семинаре российскими участниками были широко представлены исследования по переработке возобновляемого сырья для синтеза биотоплива. При этом в обеих странах традиционно уделяется повышенное внимание проблемам глубокой переработки природного и сопутствующего газов.

На семинаре происходил плодотворный обмен идеями в области разработки и исследования электрокатализаторов для ТЭ, производства водорода и водород-содержащих газов на основе каталитических технологий переработки биометанола, природного и сопутствующего газов, а также воды. В заключительной дискуссии участники семинара единодушно высказались за то, что проведение подобного рода мероприятий содействует укреплению и дальнейшему развитию сотрудничества российских и немецких специалистов как в области фундаментальных исследований, так и в сфере развития промышленно важных каталитических технологий. Возникновение новых контактов в рамках семинара, обмен идеями и мнениями, несомненно, найдут свое отражение в проведении совместных исследований, что, в свою очередь, будет способствовать повышению квалификации молодых российских ученых и расширению географии их научных стажировок в Германии.
Материал подготовили
В.В. Каичев, Е.А. Козлова, А.В. Романенко
(Институт катализа СО РАН)

| II Международная конференция |
22-28 июля 2013 года в старинном университетском городе Лунд, находящемся на юге Швеции, состоялась 2-я Международная конференция "Катализ для переработки возобновляемого сырья: топливо, энергия, химические продукты (CRS-2)". Первая конференция, CRS-1, прошла в 2010 году в пригороде Санкт-Петербурга, городе Пушкин (Царское Село), и имела большой резонанс как мероприятие, продемонстрировавшее высокий интерес к чрезвычайно актуальному и развивающемуся направлению в науке и технологии. Конференция 2010 года собрала около 200 участников, половину которых представляли иностранные специалисты. Наибольший интерес на тот момент был направлен на выступления бразильской делегации, состоявшей из 13 человек, поскольку Бразилия являлась безусловным лидером этого направления, ни одна страна мира не могла сравниться с ней по уровню развития альтернативной топливной промышленности.

Выбор места проведения конференции CRS-2 был обоснован интенсивным развитием биохимических технологий в Скандинавии, желанием кроме научной программы мероприятия ознакомиться с объектами по переработке возобновляемого сырья в Швеции и других, близко расположенных странах. Соорганизатором конференции стал Университет Лунда, сотрудники факультета химической технологии Университета активно участвовали в научной программе CRS-2.
Конференция была посвящена обсуждению фундаментальных подходов к осуществлению процессов каталитической переработки возобновляемого сырья растительного происхождения. Эта тематика направлена на решение жизненно важных и особенно актуальных на сегодняшний день проблем комплексной и глубокой переработки растительного сырья с получением ценных химических продуктов и топлива. Использование возобновляемого растительного сырья в энергетических целях позволяет не только снизить рост потребления традиционных ископаемых энергоносителей, но и уменьшить техногенную нагрузку на окружающую среду, в том числе и по выбросам углекислого газа. Большое внимание на конференции было уделено обсуждению процессов пиролиза и газификации, ферментативным технологиям для производства биотоплива. При этом акцент был сделан на каталитические методы решения этих вопросов.
 В конференции CRS-2 приняли участие около 100 специалистов из 28 стран мира. Научная программа конференции включала 7 пленарных лекций, 5 ключевых презентаций, 38 устных и около 30 стендовых докладов. Пленарную сессию открыл известный специалист в области химической технологии из Швеции, профессор Jan Brandin, Linnaeus University. Он представил обзорную лекцию по современному состоянию развития новых технологий в области производства энергии из возобновляемого сырья, процессов глубокой переработки растительного сырья в Швеции. Традиционно большое внимание было привлечено к лекции одного их ведущих ученых России в области биотехнологии, директора Института биохимической физики им. Н.М. Эмануэля, профессора С.Д. Варфоломеева. Он рассказал о современных подходах и симптоматике в решении технологических проблем переработки отходов в энергию и топливо. Лекция профессора Тапио Салми из Åbo Akademi University, Турку, Финляндия была посвящена применению законов и принципов химического инжиниринга при низкотемпературных превращениях биомассы. Интересные результаты продемонстрировал профессор Жан Карлос Серано-Руиз из компании Abengoa Research, Севилья, Испания. Было показано, что применение каталитических технологий, особенно при производстве энергии из возобновляемого сырья для решения энергетических и экологических проблем, может сыграть ключевую роль в развитии не только биоэнергетики, но также и нефтепереработки. Лекция профессора Владислава Садыкова из Института катализа им. Г.К. Борескова СО РАН (Новосибирск, Россия) была посвящена разработке структурированных нанокомпозитных катализаторов трансформации биотоплива в синтез-газ и водород.
В конференции CRS-2 приняли участие около 100 специалистов из 28 стран мира. Научная программа конференции включала 7 пленарных лекций, 5 ключевых презентаций, 38 устных и около 30 стендовых докладов. Пленарную сессию открыл известный специалист в области химической технологии из Швеции, профессор Jan Brandin, Linnaeus University. Он представил обзорную лекцию по современному состоянию развития новых технологий в области производства энергии из возобновляемого сырья, процессов глубокой переработки растительного сырья в Швеции. Традиционно большое внимание было привлечено к лекции одного их ведущих ученых России в области биотехнологии, директора Института биохимической физики им. Н.М. Эмануэля, профессора С.Д. Варфоломеева. Он рассказал о современных подходах и симптоматике в решении технологических проблем переработки отходов в энергию и топливо. Лекция профессора Тапио Салми из Åbo Akademi University, Турку, Финляндия была посвящена применению законов и принципов химического инжиниринга при низкотемпературных превращениях биомассы. Интересные результаты продемонстрировал профессор Жан Карлос Серано-Руиз из компании Abengoa Research, Севилья, Испания. Было показано, что применение каталитических технологий, особенно при производстве энергии из возобновляемого сырья для решения энергетических и экологических проблем, может сыграть ключевую роль в развитии не только биоэнергетики, но также и нефтепереработки. Лекция профессора Владислава Садыкова из Института катализа им. Г.К. Борескова СО РАН (Новосибирск, Россия) была посвящена разработке структурированных нанокомпозитных катализаторов трансформации биотоплива в синтез-газ и водород.
 С интересом отнеслась аудитория к лекции профессора Симони Менегетти из Federal University of Alagoas, Бразилия. Материалы ее доклада относились к олео-химии, ориентированной на получение биотоплив. Они касались технологий переработки растительных масел и жиров в биодизель и биокеросин.
Доклад содержал много интересных фактов и привлек внимание слушателей, тем более учитывая, что бразильские ученые и специалисты являются одними из лидеров в области производства биотоплива. Заключительную пленарную лекцию представил профессор Эрик Хеерес из Университета Гронингена, Голландия. Он уделил большое внимание количественной и качественной оценке применения эффективных катализаторов для интенсификации биохимических технологий.
С интересом отнеслась аудитория к лекции профессора Симони Менегетти из Federal University of Alagoas, Бразилия. Материалы ее доклада относились к олео-химии, ориентированной на получение биотоплив. Они касались технологий переработки растительных масел и жиров в биодизель и биокеросин.
Доклад содержал много интересных фактов и привлек внимание слушателей, тем более учитывая, что бразильские ученые и специалисты являются одними из лидеров в области производства биотоплива. Заключительную пленарную лекцию представил профессор Эрик Хеерес из Университета Гронингена, Голландия. Он уделил большое внимание количественной и качественной оценке применения эффективных катализаторов для интенсификации биохимических технологий.
Ключевые лекции, которые являлись обзорами к последующим выступлениям устных докладчиков в секционных сессиях, были представлены профессором Христианом Халтебергом (Университет Лунда, Швеция), профессором Марио Менегетти (Federal University of Alagoas, Бразилия), профессором Франческо Фрустери (CNR-ITAE “Nicola Giordano”, Мессина, Италия), профессором Борисом Кузнецовым (Институт химии и химической технологии СО РАН, Красноярск, Россия) и профессором Мани Субраманиан (Центр Биокатализа и Биотехнологий Университета штата Айова, США).
Сессии устных докладов в соответствии с секционной программой конференции проходили в очень активной атмосфере, сопровождались большим количеством вопросов и дискуссий. Они были посвящены каталитическим методам получения ценных продуктов из биомассы; применению катализа для получения чистого синтез-газа и чистого водорода, когенерации тепловой и электрической энергии в процессах газификации и горения биомассы; процессам каталитической переэтерификации и гидрокрекинга липидов для получения биотоплив; каталитическим подходам к производству жидких биотоплив из биомассы методом пиролиза; разработкам ферментативных и бактериальных процессов.
С большим интересом отнеслись участники к стендовой сессии. По инициативе авторов стендовых докладов и при поддержке программного комитета в рамках сессии было представлено несколько флэш-презентаций, которые были встречены аудиторией с большим вниманием.


Значительную и интересную часть программы конференции CRS-2 составляли поездки в разные районы Швеции на объекты – заводы по производству электроэнергии, топлива путем глубокой переработки растительного и другого возобновляемого сырья, отходов производств. Эту часть программы курировала компания Radscan Intervex, Швеция. В рамках посещений объектов участникам предлагались презентации специалистов предприятий с демонстрацией технологических особенностей процессов переработки возобновляемого сырья, бытовых отходов в энергоносители. Представитель компании Dr. Jozef Neterowicz рассказывал о деятельности компании и комментировал представленные презентации. Кроме этого, были проведены экскурсии по цехам, где можно было наблюдать за процессом сжигания отходов, увидеть действующие реакторы и аппараты, посмотреть всю технологическую линию.
 В результате участники получили исчерпывающую информацию о возможностях экологически чистой утилизации отходов. Из отходов предварительно отделяют металлический мусор.
Бытовые отходы, представляющие собой смесь из органических веществ различного происхождения, являются ничем иным как высококалорийным топливом, не уступающих по энергетике традиционному бурому углю. Особенностью данного завода по утилизации бытовых отходов является глубокая очистка отходящих газов от вредных примесей. Очищенная вода в виде пара подается повторно в печь, способствуя тем самым достижению полного выгорания органической компоненты ТБО. Получаемое тепло используется для отопления жилых домов и производственных зданий. При получении энергии из мусора одновременно решается проблема его утилизации и использования дешевой, практически бесплатной тепловой энергии.
В результате участники получили исчерпывающую информацию о возможностях экологически чистой утилизации отходов. Из отходов предварительно отделяют металлический мусор.
Бытовые отходы, представляющие собой смесь из органических веществ различного происхождения, являются ничем иным как высококалорийным топливом, не уступающих по энергетике традиционному бурому углю. Особенностью данного завода по утилизации бытовых отходов является глубокая очистка отходящих газов от вредных примесей. Очищенная вода в виде пара подается повторно в печь, способствуя тем самым достижению полного выгорания органической компоненты ТБО. Получаемое тепло используется для отопления жилых домов и производственных зданий. При получении энергии из мусора одновременно решается проблема его утилизации и использования дешевой, практически бесплатной тепловой энергии.
В целом из возобновляемого сырья можно получать не только энергию, но и газообразное (биогаз, содержащий метан), твердое и жидкое топливо. Использование возобновляемого топлива позволяет не только повысить конкуренцию в ТЭК и тем самым стабилизировать цены на основные энергоносители, но и положительно влиять на окружающую среду путем снижения выбросов СО2 и утилизации отходов.
На закрытии конференции состоялось вручение дипломов за лучшие доклады молодых ученых (лучшим был признан доклад Фатимы Пардо, аспирантки Королевского Института технологии Стокгольма), за лучшие презентации на стендовой сессии. От участников поступило несколько предложений о месте проведения следующей конференции, свои страны предлагали представители Испании, Италии, Румынии. Было выработано общее мнение, что конференция должна продолжать свое существование, что она является ценной площадкой для планирования дальнейшей совместной работы, укрепления уже существующего международного сотрудничества, а также для установления новых полезных контактов.
Материал подготовили
В.А. Яковлев, Т.В. Замулина
(Институт катализа СО РАН)
15-19 августа 2013 года в поселке Листвянка (Иркутская область) состоялась II Международная школа-конференция “Прикладная нанотехнология и нанотоксикология” (2nd International School-Conference “Applied Nanotechnology and Nanotoxicology”). Организаторами мероприятия выступили Институт катализа им. Г.К. Борескова СО РАН, Дальневосточный федеральный университет, Институт проблем переработки углеводородов СО РАН, Институт цитологии и генетики СО РАН, Иркутский государственный технический университет, Байкальский музей СО РАН, ЦК “Катализаторы для энергоэффективных технологий” и Научный совет по катализу ОХНМ РАН. В работе конференции приняли участие около 100 специалистов из США, Ирландии, Германии, Англии, Финляндии, Румынии и России.
 Основная цель конференции состояла в создании междисциплинарной площадки, обеспечивающей контакт и обмен информацией ученых-нанотехнологов, занимающихся созданием новых наноразмерных материалов, с учеными-нанотоксикологами, являющимися специалистами по вопросам безопасности массового использования этих материалов.
Основная цель конференции состояла в создании междисциплинарной площадки, обеспечивающей контакт и обмен информацией ученых-нанотехнологов, занимающихся созданием новых наноразмерных материалов, с учеными-нанотоксикологами, являющимися специалистами по вопросам безопасности массового использования этих материалов.
На секции по нанотехнологии основное внимание было уделено вопросам синтеза новых наноструктурированных материалов, исследованию их уникальных физико-химических свойств, наноразмерным эффектам, а также применению нанотехнологий для зеленой химии и экологически чистой энергии.
Проф. S. Vajda (в пленарной лекции Design of catalytic materials in the subnanometer and nanometer size range: from the understanding of size, composition an support effects via in situ and ex situ studies, to optimizing performance) затронул проблемы контроля за методами формирования наноматериалов для обеспечения желаемого размера частиц и их химического состава.
 Доктор М.С. Мельгунов в ключевой лекции (Pilling-Bethworth ratio and the Periodic Table) осветил основные тенденции в изменении текстуры при топохимических трансформациях материалов.
Доктор М.С. Мельгунов в ключевой лекции (Pilling-Bethworth ratio and the Periodic Table) осветил основные тенденции в изменении текстуры при топохимических трансформациях материалов.
 Е. Бессуднова (устный доклад Low temperature synthesis and characterization of nanoscale rutile TiO2) описала новый способ приготовления наноразмерного рутила при низких температурах. Г. Чурилов в устном докладе (The nanoparticles with core-shell grain created by plasma of HF discharges) описал физические методы синтеза материалов с морфологией “ядро-оболочка”.
Е. Бессуднова (устный доклад Low temperature synthesis and characterization of nanoscale rutile TiO2) описала новый способ приготовления наноразмерного рутила при низких температурах. Г. Чурилов в устном докладе (The nanoparticles with core-shell grain created by plasma of HF discharges) описал физические методы синтеза материалов с морфологией “ядро-оболочка”.
 В ключевых лекциях И.В. Мишакова (Aerogel technology for preparation of nanoscale oxides), О.Б. Бельской (Synthesis of nanostructured mixed oxides M(II)M(III)Ox as supports for platinum catalysts), устных докладах Н.Н. Леонтьевой (Investigation of dehydrated, oxide and hydrated phases derived from Mg-Al and Mg-Ga hydrotalcites by different X-ray diffraction approaches), С. Лазаревой (Synthesis and stabilization of nanosized silicon dioxide) были затронуты вопросы формирования наноразмерных оксидов как катализаторов и носителей катализаторов. Вопрос формования оксидных наноматериалов в макроскопические изделия был освещён в устном докладе Е.А. Мельгуновой (The pathway to the self-supported mesoporous mesophase materials). Т. Диденко в устном докладе (The modified carbon-mineral sorbent for platinum (IV) selective sorption) описала новый углерод-минеральный сорбент для Pt(IV).
В ключевых лекциях И.В. Мишакова (Aerogel technology for preparation of nanoscale oxides), О.Б. Бельской (Synthesis of nanostructured mixed oxides M(II)M(III)Ox as supports for platinum catalysts), устных докладах Н.Н. Леонтьевой (Investigation of dehydrated, oxide and hydrated phases derived from Mg-Al and Mg-Ga hydrotalcites by different X-ray diffraction approaches), С. Лазаревой (Synthesis and stabilization of nanosized silicon dioxide) были затронуты вопросы формирования наноразмерных оксидов как катализаторов и носителей катализаторов. Вопрос формования оксидных наноматериалов в макроскопические изделия был освещён в устном докладе Е.А. Мельгуновой (The pathway to the self-supported mesoporous mesophase materials). Т. Диденко в устном докладе (The modified carbon-mineral sorbent for platinum (IV) selective sorption) описала новый углерод-минеральный сорбент для Pt(IV).
 Вопросы приготовления и применения магнитоотделяемых наноматериалов были затронуты в пленарной лекции проф. V.I. Parvulescu (Fine tuning the properties of magnetic nanoparticle catalysts for selective transformation of cellulose to value-added chemicals) и ключевой лекции доктора M. Forrester (The dynamics and control of nanomagnet cluster formation in a flow for medical applications). В пленарной лекции проф. F. Kusmartsev (The design of nanomagnets for medical applications) затронуты проблемы синтеза анизотропных наномагнитов для медицинских применений.
Вопросы приготовления и применения магнитоотделяемых наноматериалов были затронуты в пленарной лекции проф. V.I. Parvulescu (Fine tuning the properties of magnetic nanoparticle catalysts for selective transformation of cellulose to value-added chemicals) и ключевой лекции доктора M. Forrester (The dynamics and control of nanomagnet cluster formation in a flow for medical applications). В пленарной лекции проф. F. Kusmartsev (The design of nanomagnets for medical applications) затронуты проблемы синтеза анизотропных наномагнитов для медицинских применений.
 Широкое обсуждение получила тема формирования и применения углеродных нановолокон (УНВ) и нанотрубок (УНТ). Так, Д.В. Красников (устный доклад Towards optimization of carbon nanotube properties via in situ studies of growth mechanism) затронул вопросы оптимизации синтеза УНТ применительно к их определённым свойствам на основе детальных исследований механизма их формирования. Особый интерес вызвал устный доклад Ю. Баумана (New catalyst for synthesis of carbon nanofibers from chlorinated hydrocarbons) по синтезу УНВ из хлоросодержащих углеводородов (к которым можно отнести отравляющие вещества и отходы химической промышленности). Такая технология позволила бы перерабатывать трудноутилизируемые отходы в полезные продукты.
Широкое обсуждение получила тема формирования и применения углеродных нановолокон (УНВ) и нанотрубок (УНТ). Так, Д.В. Красников (устный доклад Towards optimization of carbon nanotube properties via in situ studies of growth mechanism) затронул вопросы оптимизации синтеза УНТ применительно к их определённым свойствам на основе детальных исследований механизма их формирования. Особый интерес вызвал устный доклад Ю. Баумана (New catalyst for synthesis of carbon nanofibers from chlorinated hydrocarbons) по синтезу УНВ из хлоросодержащих углеводородов (к которым можно отнести отравляющие вещества и отходы химической промышленности). Такая технология позволила бы перерабатывать трудноутилизируемые отходы в полезные продукты.
 В устном докладе И.В. Токаревой (Nanostructuring of carbon microfiber surface) отражены вопросы формирования углерод-углеродных композитов на основе PAN-волокон и УНТ/УНВ, полученных в результате каталитического разложения углеводородов. Это позволяет получать прочные материалы для широкого спектра применений. В пленарной лекции проф. K. Savolainen (Rigid rod-like carbon nanotubes induce signs of allergic asthma) затронуты вопросы применения УНТ для исследования аллергической астмы.
В устном докладе И.В. Токаревой (Nanostructuring of carbon microfiber surface) отражены вопросы формирования углерод-углеродных композитов на основе PAN-волокон и УНТ/УНВ, полученных в результате каталитического разложения углеводородов. Это позволяет получать прочные материалы для широкого спектра применений. В пленарной лекции проф. K. Savolainen (Rigid rod-like carbon nanotubes induce signs of allergic asthma) затронуты вопросы применения УНТ для исследования аллергической астмы.
 Следует отметить каталитические аспекты применения наноматериалов. Т.В. Чернышова в устном докладе The research of nitrogen compounds effect on the activity of cracking catalysts рассказала о влиянии азотсодержащих компонентов на активность катализаторов крекинга. А.А. Курохтина (Study of selectivity of the Suzuki-Miyaura reaction with aryl bromides using Pd on micro- and mesoporous activated carbons: evidences for nanocatalysis) с соавторами исследовала реакцию Сузуки на палладиевых наночастицах, нанесённых на различные углеродные носители. М.Н. Ефимов (Metal nanoparticles supported by nanodiamonds as novel catalysts for steam ethanol reforming reaction) привёл результаты по использованию металлических наночастиц, нанесённых на наноалмазы в реакции парового риформинга этанола.
Следует отметить каталитические аспекты применения наноматериалов. Т.В. Чернышова в устном докладе The research of nitrogen compounds effect on the activity of cracking catalysts рассказала о влиянии азотсодержащих компонентов на активность катализаторов крекинга. А.А. Курохтина (Study of selectivity of the Suzuki-Miyaura reaction with aryl bromides using Pd on micro- and mesoporous activated carbons: evidences for nanocatalysis) с соавторами исследовала реакцию Сузуки на палладиевых наночастицах, нанесённых на различные углеродные носители. М.Н. Ефимов (Metal nanoparticles supported by nanodiamonds as novel catalysts for steam ethanol reforming reaction) привёл результаты по использованию металлических наночастиц, нанесённых на наноалмазы в реакции парового риформинга этанола.
 Важным аспектом стало применение физических методов для синтеза и исследования наноматериалов: ключевая лекция М.В. Тренихина Electron microscopy investigation of carbon nanomaterials under the influence of high energy beams, устный доклад Н. Торопова Influence of silver nanoparticles on the conformational changes of cyanine dye thin films under laser irradiation, стендовые доклады В. Егорушкина DOS and electrical resistivity in disordered graphene at low temperatures, А.А. Кирсанкина Electron traps in nanostructured oxides of tin and zinc, В.А. Харитонова Organoboron nanoparticles: size, geometry, electronic structure and chemical properties on the example of interaction with ND3.
Важным аспектом стало применение физических методов для синтеза и исследования наноматериалов: ключевая лекция М.В. Тренихина Electron microscopy investigation of carbon nanomaterials under the influence of high energy beams, устный доклад Н. Торопова Influence of silver nanoparticles on the conformational changes of cyanine dye thin films under laser irradiation, стендовые доклады В. Егорушкина DOS and electrical resistivity in disordered graphene at low temperatures, А.А. Кирсанкина Electron traps in nanostructured oxides of tin and zinc, В.А. Харитонова Organoboron nanoparticles: size, geometry, electronic structure and chemical properties on the example of interaction with ND3.
На секции по нанотоксикологии обсуждались вопросы токсикологии наночастиц, токсичности очищенных и неочищенных наноматериалов, окислительный стресс и антиоксиданты, ответная реакция живых тканей на контакт с наночастицами, распознавание и выведение наночастиц, индивидуальная сопротивляемость и восприимчивость к наноинфицированию, животные модели в нанотоксикологии.
В лекции проф. Л.М. Фатхутдиновой (Казанский государственный медицинский университет) и докладе А.А. Гусева (Тамбовский государственный университет) была представлена токсикологическая характеристика МУНТ марки “Таунит”. Особое внимание было уделено оценке потенциального риска здоровью работников и оценке потенциальной экотоксичности МУНТ. Были представлены результаты изучения экспозиционных сценариев на рабочих местах предприятий-производителей МУНТ: доказано присутствие агломератов МУНТ в воздухе рабочей зоны, определены максимальные разовые и среднесменные концентрации аэрозоля МУНТ. Установлено, что нативные (неочищенные) МУНТ в дозах, релевантных реальной производственной экспозиции, вызывали развитие локального воспаления, индукцию оксидативного стресса и разрастание соединительной ткани (фиброз) в легких мышей. Выявлен профиброгенный эффект МУНТ в группе экспонированных работников. Предложены биомаркеры (TGF-β, KL-6) и биологические среды (кровь, индуцированная мокрота) для осуществления медицинского наблюдения за лицами, работающими в контакте с аэрозолем МУНТ. Данное исследование является одним из первых в мире панельных биомаркерных исследований, оно организовано с учетом оценки реальных производственных экспозиций и подбора биомаркеров в ходе предварительных экспериментов in vivo и in vitro. Исследована токсичность МУНТ “Таунит” в отношении бактерий, водных организмов, высших растений. Генотоксичность МУНТ “Таунит” оценивалась, среди прочих наночастиц, в НИИ экологии человека и охраны окружающей среды. Результаты данных исследований были представлены в докладе проф. Л.П. Сычевой.
 Токсикологическая характеристика металлических наночастиц была представлена в докладе проф. М.П. Мошкина. На базе Института цитологии и генетики СО РАН и GLP вивария (совместно с сотрудниками Института катализа СО РАН и ряда других институтов) проводится серия исследований по нейрональным эффектам наночастиц марганца при интраназальной экспозиции. Показана возможность прямого транспорта наночастиц марганца из носовой полости в мозг подопытных животных, функциональные и поведенческие нарушения при длительной (3 недели) экспозиции. Исследование поддержано грантом РФФИ и грантом СО РАН.
Токсикологическая характеристика металлических наночастиц была представлена в докладе проф. М.П. Мошкина. На базе Института цитологии и генетики СО РАН и GLP вивария (совместно с сотрудниками Института катализа СО РАН и ряда других институтов) проводится серия исследований по нейрональным эффектам наночастиц марганца при интраназальной экспозиции. Показана возможность прямого транспорта наночастиц марганца из носовой полости в мозг подопытных животных, функциональные и поведенческие нарушения при длительной (3 недели) экспозиции. Исследование поддержано грантом РФФИ и грантом СО РАН.
Из других металлических наночастиц интенсивно изучаются наночастицы оксида железа, серебра и некоторые другие. Группа под руководством проф. Б.А. Кацнельсона (г. Екатеринбург) установила, что интенсивность фагоцитарного ответа, цитотоксичность и системная токсичность (включая генотоксичность) зависят скорее от химического состава (оксиды железа, серебро, золото, медь), нежели от размера частиц.
 Исследования транспорта металлических наночастиц в клетки, результаты которых были представлены в докладе проф. Е.И. Рябчиковой (Институт химической биологии и фундаментальной медицины СО РАН), подтвердили, что наночастицы могут воздействовать на клетку химическим путем, т.е. повторять “поведение” макромолекул. Однако наночастицы могут быть более токсичными, т.к. для них не действует барьер эндоцитоза, и частицы могут повреждать внутриклеточные элементы. В физико-химических исследованиях показана высокая активность металлических наночастиц (цинк, никель, алюминий, медь, оксид алюминия, оксид цинка) в физиологических растворах. Исследования поддержаны грантом Минобрнауки РФ.
Исследования транспорта металлических наночастиц в клетки, результаты которых были представлены в докладе проф. Е.И. Рябчиковой (Институт химической биологии и фундаментальной медицины СО РАН), подтвердили, что наночастицы могут воздействовать на клетку химическим путем, т.е. повторять “поведение” макромолекул. Однако наночастицы могут быть более токсичными, т.к. для них не действует барьер эндоцитоза, и частицы могут повреждать внутриклеточные элементы. В физико-химических исследованиях показана высокая активность металлических наночастиц (цинк, никель, алюминий, медь, оксид алюминия, оксид цинка) в физиологических растворах. Исследования поддержаны грантом Минобрнауки РФ.
Научное мероприятие способствовало обмену мнениями между представителями различных российских и зарубежных научных школ, определению перспективных направлений исследований и планированию совместных работ. Было отмечено, что российские ученые в некоторых разделах прикладной нанотоксикологии (изучение влияния МУНТ на здоровье человека, нейроповеденческие эффекты наномарганца, транспорт наночастиц в клетки) занимают ведущие научные позиции на мировом уровне. Работу конференции отличал высокий уровень дискуссии, который не всегда приводил к консенсусу, но всегда характеризовался дружелюбным отношением спорящих сторон. Встреча в рамках одного научного мероприятия представителей разных дисциплин из разных стран имеет огромную ценность для развития таких мультидисциплинарных направлений фундаментальных и прикладных исследований, как нанотехнология, нанобиология и нанотоксикология.

Было принято решение организовать III Международную школу-конференцию “Прикладная нанотехнология и нанотоксикология” в Казани. Право быть главным организатором следующей школы-конференции было передано Казанскому государственному медицинскому университету.

Материал подготовили
А.А. Ведягин, М.С. Мельгунов
(Институт катализа СО РАН),
Л.М. Фатхутдинова
(Казанский государственный медицинский университет)
Ironing Out Nitrogen Fixation
Bioinorganic Chemistry: Iron catalyst could aid search for im-proved ammonia process
Ammonia maker
Tris(phosphine)borane-supported Fe-N2 complex catalyzes NH3 formation at low temperature and atmospheric pressure, using an inorganic acid (H+) and electron (e-) source.
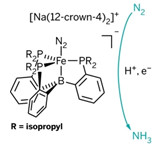 Conversion of dinitrogen to ammonia is a process that’s both essential to life on Earth and industrially important in fertilizer production. Researchers have long studied the enzymes that living things use to promote the reaction, but details of their catalytic mechanisms have remained elusive.
Conversion of dinitrogen to ammonia is a process that’s both essential to life on Earth and industrially important in fertilizer production. Researchers have long studied the enzymes that living things use to promote the reaction, but details of their catalytic mechanisms have remained elusive.
Now, Caltech scientists have created an iron complex that catalyzes this conversion in a manner that may help reveal the way nitrogenases do it. The iron catalyst and earlier molybdenum versions could also help lead to improved catalysts for reducing N2 to NH3 industrially.
Bacteria make nitrogenases and use them to convert N2 in air to NH3, a process called nitrogen fixation. The process is a primary source of nitrogen in proteins, nucleic acids, and other biomolecules. The Haber-Bosch process, which was developed in the early-20th century and uses a solid iron catalyst for adding H2 to N2 at high temperatures and pressures to form NH3 for fertilizer production, is now also a primary source of fixed nitrogen.
Most nitrogenases have an active-site cofactor—a molybdenum-iron cluster—but researchers don’t yet know whether it’s Mo or Fe that coordinates and reduces N2. In 2003, Richard R. Schrock’s group at MIT developed the first Mo complex that catalyzes N2 to NH3 conversion under mild conditions. Yoshiaki Nishibayashi of the University of Tokyo and coworkers reported another such Mo complex two years ago. Both Mo complexes are inefficient catalysts and break down quickly, but they work. That could not be said for any Fe-based complex, favoring the idea that nitrogenase’s Mo is the active metal.
John S. Anderson, Jonathan Rittle, and Jonas C. Peters at Caltech have now come up with the first Fe-based complex that directly catalyzes nitrogen fixation to NH3 under mild conditions (Nature 2013, DOI: 10.1038/nature12435).
The tris(phosphine)borane-supported Fe-N2 complex’s catalytic activity is slightly lower than the Mo catalysts’, and it likewise breaks down quickly. But its supporting ligand “can be varied systematically to improve the catalyst, so there are good prospects for future improvement,” notes Patrick L. Holland of Yale University, who specializes in Fe-based cleaving reactions.
The Fe complex tips the balance of evidence back toward the idea that nitrogenase catalysis may be Fe-based. The study “provides favorable information for Fe claimants, but further study is necessary,” Nishibayashi says. One such claimant, Brian M. Hoffman of Northwestern University, says, “You can no longer use the argument that Mo must be it because it’s the only one shown to do the job.”
Peters and coworkers propose that a flexible Fe-boron interaction in their complex may play a key catalytic role and that a carbon-Fe interaction in nitrogenase may work similarly. “Through analogies like this, synthetic compounds can help chemists to gain insight into the mechanisms of enzymes,” Holland says. Nishibayashi also believes the result “provides useful and significant information to elucidate the reaction mechanism of the MoFe cofactor in nitrogenase.” But Schrock says, “It’s a stretch to say it tells us much, if anything, about the mechanism of reduction by various nitrogenases.”
The Fe-based and Mo-based complexes are possible first steps toward developing nitrogen fixation routes that are more economical than the energyintensive Haber-Bosch process. “The work shows that chemistry just has a much wider range of elements and structures to choose from than nature had during evolution and that mechanistically there may be quite a few possibilities for efficient N2-fixing complexes,” says bioinorganic chemist Oliver Einsle of the University of Freiburg, in Germany.
Method Adds Metals To Frameworks Selectively
Atomic layer deposition bypasses pitfalls of other common deposition techniques
A new procedure for tailoring metal-organic framework (MOF) compounds is poised to make this highly popular class of useful solids even more useful (J. Am. Chem. Soc.2013, DOI: 10.1021/ja4050828). MOFs are porous crystals composed of metal ions or clusters connected by organic linkers. The materials’ extreme surface areas and porosities have led to numerous record-breaking demonstrations and some commercialization in gas separation and storage as well as catalysis. Tailoring MOFs’ internal surfaces with chemically active metal ions can enhance the materials’ performance. Yet most methods for adding metals call for solution-phase treatments that can plug the crystals’ pores with unwanted solvents and reagents. Gas-phase deposition could eliminate the need to purify MOFs, but it provides little control over the deposited metals’ distribution. Northwestern University chemists Omar K. Farha and Joseph T. Hupp and coworkers have shown that these problems can be sidestepped by using atomic layer deposition. In this process, sequentially pulsed precursors react to form one atomic layer of products per pulse sequence. The team used diethylzinc and trimethylaluminum to decorate microscopic MOF channels with Zn and Al, respectively. They showed that unlike native MOFs, the metal-decorated versions are active catalysts for modified aldol condensations.
Nanostructures That Heal Themselves
Materials Science: Tiny patterns imprinted on the surface of the polymer Nafion can recover from repeated damage
 The iridescence of a butterfly’s wing and the stickiness of a gecko’s foot derive their properties from nanoscale structures on their surfaces. Unlike with those natural materials, nanostructures engineered from synthetic materials often lack the ability to heal themselves when damaged. Now a Singaporean team has imprinted nanopatterns into a shape-memory polymer and found that the patterns can repeatedly recover from damage (Langmuir 2013, DOI: 10.1021/la401621j).
The iridescence of a butterfly’s wing and the stickiness of a gecko’s foot derive their properties from nanoscale structures on their surfaces. Unlike with those natural materials, nanostructures engineered from synthetic materials often lack the ability to heal themselves when damaged. Now a Singaporean team has imprinted nanopatterns into a shape-memory polymer and found that the patterns can repeatedly recover from damage (Langmuir 2013, DOI: 10.1021/la401621j).
Hong Yee Low, a polymer chemist at the Singapore University of Technology & Design, and her team molded a nanopattern into Nafion, a well-studied fluoropolymer-copolymer. They heated Nafion film at 310 °C for 10 minutes and used a mold to imprint rows of pillars, ranging from 500 nm to 5 μm wide, onto the material. After cooling the polymer to room temperature, the researchers bent the pillars by smudging them with a finger, blasting them with a focused electron beam, or squishing them with a diamond stylus. When the team heated the material to 140 °C, past Nafion’s glass transition temperature, the ruined structures regained their original shape. The structures withstood this damage-and-repair process 25 times. Towards a possible application, Low is now working to mimic the complex nanopatterns on gecko feet with a polyurethane-based polymer that can heal itself. The materials could lead to a new type of dry adhesive.
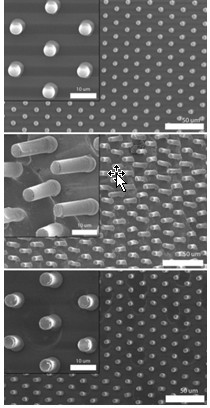
Polymer revival
Scanning electron micrographs show 5-µm-wide pillars molded into Nafion (left). In the center image, researchers deformed the pillars by rubbing the polymer surface with a finger. At the bottom, the pillars have returned to their original shape after heating. Scale bars are 10 µm for the closeups and 50 µm for the wide views.
Probing Gold’s Catalytic Prowess
The unusually strong Au–C bond in gold alkynyl complexes provides insight on the precious metal’s catalytic activity in cross-coupling reactions
Once thought to be mostly chemically inert, gold has been shown in recent years to display surprising catalytic activity. An international research team studying how gold binds to carbon has now discovered a further surprise behind gold’s catalytic prowessan inverse correlation between bond strength and the multiplicity of Au–C bonds in organogold complexes (Nat. Commun. 2013, DOI: 10.1038/ncomms3223). Gold is a good catalytic activator of alkynes for C–C cross-coupling reactions. To understand why, Brown University’s Lai-Sheng Wang, Tsinghua University’s Jun Li, and coworkers took a close look at Au–C bonding. The researchers used AuI and HC≡CMgCl to create a series of complexes by electrospray ionization in a mass spectrometer and then probed them via photoelectron spectroscopy. They further modeled the complexes and compared the experimental and computational results. The most interesting observation, Wang says, is that the Au–C bond in ClAu–C≡CH–, which one might expect to form during a cross-coupling reaction, is stronger than the double and triple bonds in ClAu=CH2 and ClAu≡C species. The strong Au–C single bond explains gold’s ability to readily form the necessary alkyne intermediates in catalytic cross-couplings, the researchers conclude.
Element 115 Detected Again
Building Blocks: Findings should bolster the case for a spot on the periodic table
Confirmatory evidence for the existence of element 115 has been reported by an international research team that successfully used X-ray detection methods for the first time. The new work should bolster the case for adding the element to the periodic table almost a decade after it was first spotted.
Nuclear physicist Dirk Rudolph of Lund University, in Sweden, led the team. The scientists did the experiment at the GSI heavy ion accelerator center in Darmstadt, Germany. A paper of the work has been accepted by Physical Review Letters.
Element 115 was first observed by physicists at Russia’s Joint Institute for Nuclear Research working with scientists from Lawrence Livermore National Laboratory. An international committee of chemists and physicists will decide whether to add the element to the periodic table.
Rudolph’s team created element 115 by aiming a beam of calcium ions at an americium target. Sifting through the jumble of photons, particles, and atoms that results from such an experiment, the researchers detected particle decay chains consistent with isotopes of element 115 decaying to isotopes of dubnium. They also identified X-ray emissions from the decay chain, a long-sought goal of the nuclear physics community because it can be another piece of supporting evidence.
Interference from other emissions makes the X-rays very hard to detect, says Dawn A. Shaughnessy, an LLNL chemist who was part of the first team to observe element 115.
“The fact that they pulled it off and got these measurements is really phenomenal,” she says of the new report.
Chemistry On Cloth
Catalysis: Modified textiles offer new method for organocatalysis
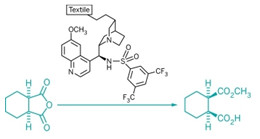 Organocatalysts have become fashionable in chemistry circles during the past decade. Now, chemists are taking a cue from fashion by immobilizing these small, nonmetallic organic catalysts onto fabric (Science 2013, DOI:10.1126/science.1242196). The new organotextile catalysts offer a general, inexpensive, and practical approach to solid-supported organocatalysis, which aids catalyst recycling and could find use in the manufacture of pharmaceuticals and fine chemicals.
Organocatalysts have become fashionable in chemistry circles during the past decade. Now, chemists are taking a cue from fashion by immobilizing these small, nonmetallic organic catalysts onto fabric (Science 2013, DOI:10.1126/science.1242196). The new organotextile catalysts offer a general, inexpensive, and practical approach to solid-supported organocatalysis, which aids catalyst recycling and could find use in the manufacture of pharmaceuticals and fine chemicals.
“The difficulty with any catalyst that is not supported on a heterogeneous material is that the recovery from homogenous phase requires an additional operation,” explains Benjamin List, of Germany’s Max Planck Institute for Coal Research, who spearheaded the organotextile research. Heterogeneous catalysts can be easily recovered by filtration, for example.
“One inherent advantage of organocatalysts, as compared to enzymes or metal-based catalysts, is that they can be easily and covalently attached to solid supports,” List adds. Chemists realized this advantage early on, he says, but so far the catalytic activity, selectivity, and recyclability of solidsupported organocatalysts have been inferior to those of homogeneous ones.
Researchers led by Klaus Opwis, of the German Textile Research Center, recently discovered a practical method for modifying textiles, such as nylon, with organic molecules simply by using ultraviolet light.
So List and Opwis teamed up to modify textiles with organocatalysts. To date they’ve made catalyst cloths modified with a Lewis base, a Brønsted acid, and a chiral organocatalyst. The textiles possess excellent stability, activity, and recyclability for various organic transformations. In one example, the researchers noted the high enantioselectivity was maintained for more than 250 cycles of asymmetric catalysis, an unusually high number of catalytic turnovers.
“What excited me most about this work was the incredibly high number of reaction cycles,” comments Franco Cozzi, an organocatalysis expert at the University of Milan, in Italy. “Generally, a supported organocatalyst after a few cycles is totally or at least partially inactivated, but this seems not to be the case in the present work.”
Bioinspired Filter Captures Carbon
ACS Meeting News: Microchannel-filled material based on avian anatomy might one day help remove CO2 from smokestacks
To reduce greenhouse gas emissions, industrial plants filter carbon dioxide from smokestacks by passing flue gas through a column filled with water and nitrogen-containing compounds such as monoethanolamine (MEA). Although it’s widely used, this carbon-capture technique isn’t energy efficient, so researchers have been looking for alternative filtration materials capable of trapping large amounts of CO2.
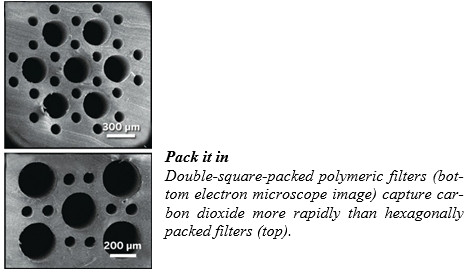
One research team’s efforts to find such materials has yielded a high-surface-area capture membrane inspired by one of nature’s most efficient gas exchangers: a bird’s lungs. The work, by Aaron P. Esser-Kahn of the University of California, Irvine, and colleagues, was reported at the American Chemical Society national meeting in Indianapolis.
To fly, birds need a lot of energy, so they’ve evolved intricate structures in their lungs to rapidly exchange CO2 for oxygen. “We wanted to be able to match what nature can create,” Esser-Kahn said in a session sponsored by the Division of Polymeric Materials: Science & Engineering.
The chemist and his team produced their birdlung mimics by stretching polylactic acid fibers of two different diameters between a pair of brass plates. They then filled polydimethylsiloxane into a mold around them. After the PDMS set, the team heated the assembly in vacuum to degrade the tightly packed fibers and leave behind microchannels of two sizes.
When the researchers filled the smalldiameter channels with MEA and flowed CO2 through the larger ones, they observed the gas diffuse through the walls of the polymer structure and react with MEA. Surprisingly, Esser-Kahn told the audience, the greenhouse gas transferred most rapidly when the channels were packed into a not-found-in-nature “double square” pattern, rather than a dodecagonal or hexagonal pattern that more closely simulates a bird’s airways.
The Esser-Kahn group’s membrane production method may enable the manufacture of high-surface-area three-dimensional architectures not accessible via standard approaches, says Abraham Dun-can Stroock, a chemical engineer at Cornell University. This “newsworthy” work, he adds, “has taken seriously the challenge of recreating the extraordinary rates of mass transfer that are seen in the vascular systems of animals.”
With these preliminary results in hand, Esser-Kahn’s team will next try to optimize the porosity of its filters and filter material, which can break down over time with exposure to MEA.
Chemical & Engineering News
 |
II Российский конгресс по катализу “РОСКАТАЛИЗ” |
II Российский конгресс по катализу “РОСКАТАЛИЗ” будет проведен под эгидой Российской академии наук
со 2 по 5 октября 2014 г. в Самаре.
Заявки на участие и тезисы докладов
Регистрация заявок на участие в Конгрессе и тезисов докладов
доступна на сайте http://conf.nsc.ru/RUSCATALYSIS-2014 до 15 апреля 2014 г.
 |
XXI International Conference on Chemical Reactors "CHEMREACTOR-21" |
We are pleased to announce the XXI International Conference on Chemical Reactors "CHEMREACTOR-21"
to be held
on September 22-25, 2014 in Delft, The Netherlands. You are cordially invited to participate.
Registration
To register for the conference, please fill in on-line application form at
http://conf.nsc.ru/CR-21-2014
|
October 21-24, 2013 |
|
|
21-25 октября 2013 г. |
|
|
21-25 октября 2013 г. |
|
|
22-24 октября 2013 г. |
|
|
22-25 октября 2013 г. |
|
|
October 22-25, 2013 |
|
|
23-25 октября 2013 г. |
|
|
October 24-25, 2013 |
|
|
October 28-30, 2013 |
|
|
28-29 октября 2013 г. |
|
|
28 октября – 2 ноября 2013 г. |
http://www.misis.ru/crystalxxi/tabid/3251/language/ru- |
|
29-30 октября 2013 г. |
|
|
29-30 октября 2013 г. |
|
|
October 30-31, 2013 |
|
|
Energy Summit Berlin, Germany |
http://v11.vuturevx.com/exchange- |
|
1-2 ноября 2013 г. |
http://www.nanometer.ru/2013/06/09/ |
|
2-9 ноября 2013 г. |
|
|
November 6-7, 2013 |
|
|
November 8-10, 2013 |
|
|
11-14 ноября 2013 г. |
|
|
14-15 ноября 2013 г. |
proekt@plastpolymer.com |
|
November 18-20, 2013 |
http://www.omicsgroup.com/conferences/petrochemist |
|
18-21 ноября 2013 г. |
|
|
19-20 ноября 2013 г. |
|
|
November 19-22, 2013 |
|
|
19-22 ноября 2013 г. |
http://www.nas.gov.ua/conferences/nansys2013/ |
|
November 20-21, 2013 |
|
|
21-22 ноября 2013 г. |
|
|
3-4 декабря 2013 г. |
|
|
3-5 декабря 2013 г. |
|
|
December 4-5, 2013 |
|
|
December 8-11, 2013 |
|
|
December 12-13, 2013 |
|
|
December 18-20, 2013 |
|
|
December 19-20, 2013 |
|
|
|
|
|
January 1-3, 2014 |
|
|
January 20-22, 2014 |
|
|
January 24-25, 2014 |
|
|
February 16-20, 2014 |
http://ceramics.org/meetings/materials-challenges-in- |
|
February 19-21, 2014 |
|
|
March 18-20, 2014 |
|
|
1-4 апреля 2014 г. |
|
|
21-25 апреля 2014 г. |
|
|
22-26 апреля 2014 г. |
|
|
May 4-7, 2014 |
|
|
20-22 мая 2014 г. |
|
|
May 26-31, 2014 |
|
|
June 1-6, 2014 |
|
|
June 8-12, 2014 |
|
|
4th International Colloids Conference “Surface Design & Engineering” Madrid, Spain |
|
|
June 15-19, 2014 |
|
|
June 23-26, 2014 |
|
|
June 26-30, 2014 |
|
|
July 6-10, 2014 |
|
|
July 6-11, 2014 |
|
|
July 13-18, 2014 |
|
|
July 15-18, 2014 |
|
|
August 23-27, 2014 |
|
|
September 7-10, 2014 |
|
|
September 7-14, 2014 |
|
|
September 8-11, 2014 |
|
|
8-12 сентября 2014 г. |
|
|
September 22-25, 2014 |
|
|
September 22-26, 2014 |
|
|
2-5 октября 2014 г. |
|
|
October 11-13, 2014 |
|
|
|
|
|
June 15-18, 2015 |
|
|
August 30 – September 4, 2015 |
|
|
October 27-30, 2015 |
|


