
Тел.: +7 (383) 330-67-71, факс: +7 (383) 330-80-56, E-mail: bic@catalysis.ru
630090, Россия, Новосибирск, пр-т Ак. Лаврентьева, 5

Тел.: +7 (383) 330-67-71, факс: +7 (383) 330-80-56, E-mail: bic@catalysis.ru
630090, Россия, Новосибирск, пр-т Ак. Лаврентьева, 5
Promising plastics recycling method relies on simple catalysts
Waste polyethylene and polypropylene can be turned into useful propene and isobutene
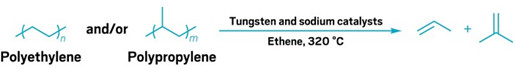
A pair of robust and inexpensive catalysts can help recycle mixtures of polyethylene (PE) and polypropylene (PP), two of the most common and intractable types of plastic waste (Science 2024, DOI: 10.1126/science.adq7316). The catalysts chop up the polymers and combine them with ethene to produce propene and isobutene, which can be used to make new plastics.
Although the lab-based process still faces many hurdles before becoming a practical industrial system, it shows how chemical recycling could address some of the most difficult challenges in tackling waste plastic, says John F. Hartwig of the University of California, Berkeley, who led the team.
“This is a pretty big step forward,” says Susannah L. Scott of the University of California, Santa Barbara, who works on similar polymer recycling processes and was not involved in the study. “The fact that they have simple catalysts that could potentially be robust in a realistic process is very important.”
Various forms of PE and PP account for roughly half of global polymer resin production. Their durability makes them incredibly useful but also persistent in the environment, and they are difficult to separate from one another, which poses a major recycling challenge. Pyrolysis can convert PE and PP into oils that feed into industrial crackers, but these hightemperature processes are energy intensive, produce complex mixtures of products, and potentially generate a lot of climatewarming CO2 and methane. Many researchers are instead working on ways to break down polymer chains into their original monomers, but PE and PP have been particularly tough to unpick.
Hartwig’s team has now achieved this using pressurized ethene in conjunction with two catalysts: tungsten oxide on silica and sodium on alumina, both of which are already used in the chemical industry. The process starts with catalytic cracking, which cleaves the polymer into shorter chains containing C=C double bonds. Next, a metathesis reaction with ethene cleaves these double bonds, leaving smaller fragments with terminal C=C groups.
An isomerization reaction then shifts the double bond one position along the chain. Finally, another ethene metathesis reaction slices through the C=C bond, freeing a small molecule from the end—propene from PE or a blend of propene and isobutene from PP. This catalytic isomerization-metathesis cycle repeats over and over again, nibbling away at the polymer chains to release the products.
The reactions run at 320 °C and produce a mix of ethene, propene, and isobutene, which could be teased apart by standard industrial cryogenic separations. It takes about 90 minutes to convert 1 g of mixed plastic with a yield of around 90% and works with real plastic waste such as bread bags, milk jugs, and centrifuge tubes.
But, the sodium catalyst loses about half of its activity with each batch run, and contamination with other polymers such as PET or PVC caused conversion yields to plummet. The researchers now plan further studies to understand exactly how the catalysts are working in the reaction and how the deactivation occurs. “Our high priority right now is to figure out what’s happening with that and how to regenerate it,” Hartwig says. They will also explore other basemetal catalysts that could avoid deactivation and lower the temperature of the process.
Hartwig’s team has previously converted PE into propene using soluble catalysts based on expensive metals such as iridium and palladium, which were fragile and could not handle PP (Science 2022, DOI: 10.1126/science.add1088). At the same time, Scott’s team had independently published a similar method (J. Am. Chem. Soc. 2022, DOI: 10.1021/jacs.2c07781) based on earlier theoretical modeling led by her collaborator Damien Guironnet at the University of Illinois Urbana-Champaign (J. Phys. Chem. A 2020, DOI: 10.1021/acs.jpca.0c01363). “The fact that these catalysts were fragile was a problem because the contaminants in real plastic waste tend to deactivate them very quickly,” Scott says. “This new set of catalysts are likely to be far more compatible with real, dirty, ugly, plastic waste.”
One drawback is that a lot of the carbon contained in the product gases comes from ethene added during the process rather than from the waste polymers. “That means the carbon efficiency is not super,” Scott says. “Figuring out how to do this so that most of the carbon in your product came from the polymers—that would be the next big breakthrough, I think.”
New techniques use visible light to destroy PFAS
Novel light-activated catalysts tear apart carbonfluorine bonds in the forever chemicals
Toxic per- and polyfluoroalkyl substances (PFAS) persist forever in the environment; even when they’re removed, it’s difficult to keep them from eventually winding up back in the environment. Annihilating PFAS is the only sure way to keep them out of drinking water and our bodies. In recent years, researchers and start-ups have devised several techniques to destroy these forever chemicals.
Two independent research groups now report for the first time that visible light can break apart PFAS into benign by-products (Nature 2024, DOI: 10.1038/s41586-024-08179-1 and 10.1038/s41586-024-08327-7). Both teams have developed photocatalysts that, when excited by purple light, energetically lob electrons at PFAS to sever the stubborn carbon-fluorine bonds that make the substances resistant to heat and water.
It’s still early, but the advances hint at a low-cost, large-scale route to destroy PFAS directly in water. “We use light as the energy source, and the sun could give this light,” says Yan-Biao Kang, a chemist at the University of Science and Technology of China. “Our dream is to put our catalyst, immobilized on some material, in water, where it would slowly destroy PFAS.”
Four years ago, Kang, Jian-Ping Qu of Nanjing Tech University, and colleagues made a carbazole-based catalyst that, under purple light, efficiently ruptured the carbonfluorine bond in fluorobenzene. The researchers then set their sights on PFAS, but that catalyst wasn’t great at cleaving all the C–F bonds in the compounds.
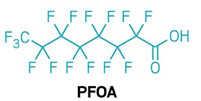
The team has now designed a version of the catalyst with a twisted carbazole ring and more electrons to donate. The researchers tested the new photocatalyst on various PFAS, including polytetrafluoroethylene (PTFE), known as Teflon, as well as multiple longchain PFAS with eight or more carbons, including perfluorooctane sulfonic acid and polyfluorooctanoic acid (shown).
They mix each PFAS separately in a solvent containing the photocatalyst and potassium formate as an electron donor to replenish the electrons that the photocatalyst sends off to cleave the carbonfluorine bond. They then shine a purple lightemitting diode (LED) on the mixtures. The PTFE breaks down to give amorphous carbon and reusable potassium fluoride; more than 95% of the fluorine is converted into the fluoride salt. The longchain PFAS are degraded and transformed into carbonate, formate, oxalate, and trifluoroacetate as the end products.
Chemists Garret M. Miyake and Robert S. Paton at Colorado State University, Niels H. Damrauer at the University of Colorado Boulder, and colleagues have followed a trajectory similar to that of the group in China. About 8 years ago, the Colorado team developed a benzoperylenebased photocatalyst; they have now tweaked and tamed its reactivity so it can shear carbon-fluorine bonds in PFAS when excited by purple light.
The researchers use tetrabutylammonium fluoride as the electron donor. Their reaction gives benign hydrocarbons and fluoride ions as the major byproducts.
Neither catalyst system is close to practical right now. The reactions are sluggish in water, and both Kang and Miyake say the first step will be getting their respective catalyst to work efficiently on PFAS dissolved in water.
“From a practical perspective there are a lot of grand challenges,” Miyake says. But an efficient system that uses LEDs, and maybe sunlight, offers a tantalizing economical route to destroy PFAS, he says.
Jinyong Liu, a chemical engineer at the University of California, Riverside, who wrote a commentary about the research for Nature, calls these ad-vances “exciting.” Although the photocatalysts have complicated structures and might not be immediately ready for realworld application, he says, these two studies pave the way for safe, lowenergy technologies to demolish PFAS compared with incineration.
Several low-temperature technologies—ultraviolet light combined with photocatalysts, plasma destruction, and electrochemical oxidation—are also being scaled up. “Each roomtemperature process has limitations in terms of what kind of PFAS structure they can destroy effectively,” Liu says. “Cur-rently, we expect to have an integrated system that combines the advantages of different technologies to achieve thorough destruction of PFAS.”
Chemical & Engineering News